1 Introduction
The genus Tityobuthus appears as a particular element within the Malagasy scorpion fauna. Despite being an endemic element to Madagascar, Tityobuthus has a close relationship with other genera of the Ananterinae, or ‘Ananteris Group’ [1], which are only present in quite distinct geographical regions of the world. The most marked example is the genus Ananteris Thorell, 1891, an element of the Neotropical scorpion fauna, which, however, clearly appears as quite distinct from the other buthid elements of this fauna.
Tityobuthus was established by Pocock [2] for Tityobuthus baroni (Pocock, 1890) previously included in the genus Rhoptrurus Karsch, 1886, because this name was already preoccupied by that of a snake genus, Rhoptrura Peters, 1858 [3]. Confusion existed about the genera Tityobuthus Pocock, Pseudobuthus Pocock and Odonturus Karsch until they were revised by Vachon [3], who finally included only two species in the genus Tityobuthus: Tityobuthus baroni (Pocock, 1890) and Tityobuthus gracilis (Fage, 1946). The latter was originally described in the genus Babycurus Karsch 1886. T. gracilis was finally accommodated in its own genus by Lourenço [4] as Troglotityobuthus gracilis. Subsequently, several other species of Tityobuthus have been described from the island [5].
In this current paper, we bring some new insights into the biogeographic patterns presented by the genus Tityobuthus in Madagascar. One new species is also described, attesting to the high levels of diversity presented by this genus in Madagascar [5]. For further historical details about this genus and its increasing species richness, see Lourenço [5].
2 The Ananterinae or ‘Ananteris Group’; history, composition and known distribution
As outlined in recent papers [1,6], the position of subfamilies within the family Buthidae has always been a controversial matter [7,8]. The subfamily Ananterinae was proposed by Pocock [9] to accommodate the genus Ananteris Thorell. Pocock wrote: ‘I propose to eliminate from this subfamily (Buthinae) the isolated Neotropical genus Ananteris, which differs strikingly from the rest of the family in the structure of the pectines. The subfamily Ananterinae may be created for its reception’. Kraepelin [10] added Ananterinae, previously described by Pocock [9] and the Tityinae, within the Buthidae family. The definition of a subfamily Ananterinae, or of one ‘Ananteris group’, remains to be decided. For more details, see Lourenço [1,6].
A number of genera may be accommodated within the Ananterinae or ‘Ananteris Group’. Some are strongly speciose, as Ananteris, Tityobuthus or Lychas C.L. Koch, and can be distributed over broad geographical ranges, whereas others may be represented by only one or two species such as Lychasioides Vachon or Troglotityobuthus Lourenço and are endemic to rather narrow ranges. The global extant distribution of the Ananteris group is tropical in South America, tropical and subtropical in Asia, Oceania and Sub-Sahara Africa, in climatic conditions encompassing humid, dry and arid ones [11] (Fig. 1).
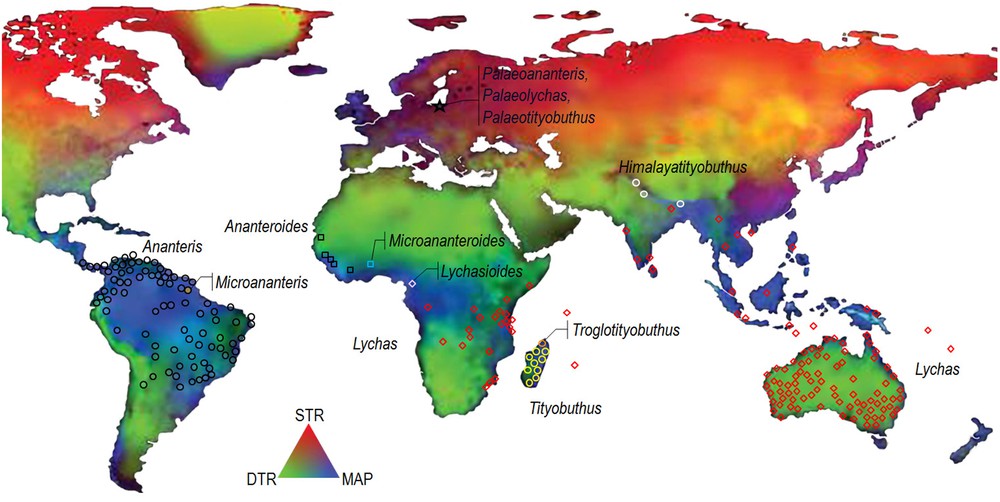
Distribution of the nine genera in the Ananteris group, as well as the three fossil Cenozoic genera on the global map of climate variability modified from Chan et al. [11]. (STR: seasonal temperature range; MAP: mean annual precipitation; DTR: diurnal temperature range).
Ananteris Thorell, 1891 – Almost all the Neotropical region.
Ananteroides Borelli, 1911 – Africa; Guinea Bissau, Guinea, Ivory Coast, Mauritania, Sierra Leone.
Himalayotityobuthus Lourenço, 1997 – India and Nepal.
Lychas C.L. Koch, 1845 – Africa, India, Southeast Asia, Australia, Indian Ocean and Western Pacific Ocean islands.
Lychasioides Vachon, 1974 – Africa; Cameroun.
Microananteris Lourenço, 2003 – French Guiana.
Microananteroides Rossi & Lourenço, 2015 – Africa; Ghana.
Tityobuthus Pocock, 1893 – Madagascar.
Troglotityobuthus Lourenço, 2000 – Madagascar (cave species).
†Palaeolychas Lourenço & Weitschat, 1996 – Cenozoic fossil, Samland Peninsula, Baltic coast.
†Palaeoananteris Lourenço & Weitschat, 2001 – Cenozoic fossil, Samland Peninsula, Baltic coast.
†Palaeotityobuthus Lourenço & Weitschat, 2000 – Cenozoic fossil, Samland Peninsula, Baltic coast.
Among the several genera of Cenozoic fossil scorpions described from the Samland Peninsula along the Baltic coast (today Poland and Kaliningrad in Russia), most are related to the Ananterinae [12]. Some of these fossil specimens remain rather incomplete and their morphology cannot be globally known. Nevertheless, their affinities with the extant Ananterinae genera are well supported by a number of characters [12–15].
Several recent studies [4,5,16,17] attest that Tityobuthus shows closer affinities with the genus Ananteris than to the other Ananterinae genera. Another genus that shows affinities with Tityobuthus is Himalayotityobuthus, endemic to the Himalayas of India and Nepal [18]. The association of all these different genera within the subfamily Ananterinae clearly indicates a Gondwanian pattern of distribution for this undoubtedly ancient lineage of buthid scorpions. The discovery of the fossil elements from Baltic amber brought further evidence to support the biogeographic model of past distribution and the present observed pattern of distribution of the Ananterinae, which can be attributed to a model of panbiogeography [19,20].
It is interesting to notice that the genus Lychas is encountered in the Seychelles, on satellite islets of Mauritius in the Indian Ocean, and in Eastern and Southern Africa, but not in Madagascar. Most of the Seychelles, the so-called granitic Seychelles, were part of the Gondwana, while the coral portions are of much younger age [21]. Mauritius is a volcanic island. The endemic species only known from the satellite islets of Mauritius, Round Island and Gunner's Quoin, is extremely rare and currently of unknown affinities.
3 Methods
Illustrations and measurements were produced using a Wild M5 stereomicroscope with a drawing tube and an ocular micrometer. Measurements follow Stahnke [22] and are given in millimetres. The trichobothrial notations follow Vachon [23], and the morphological terminology mostly follows Vachon [24] and Hjelle [25].
4 Taxonomic treatment
Family Buthidae C.L. Koch, 1837
Subfamily Ananterinae Pocock, 1900
Genus Tityobuthus Pocock, 1890
Tityobuthus lokobe sp. n. (Fig. 2)
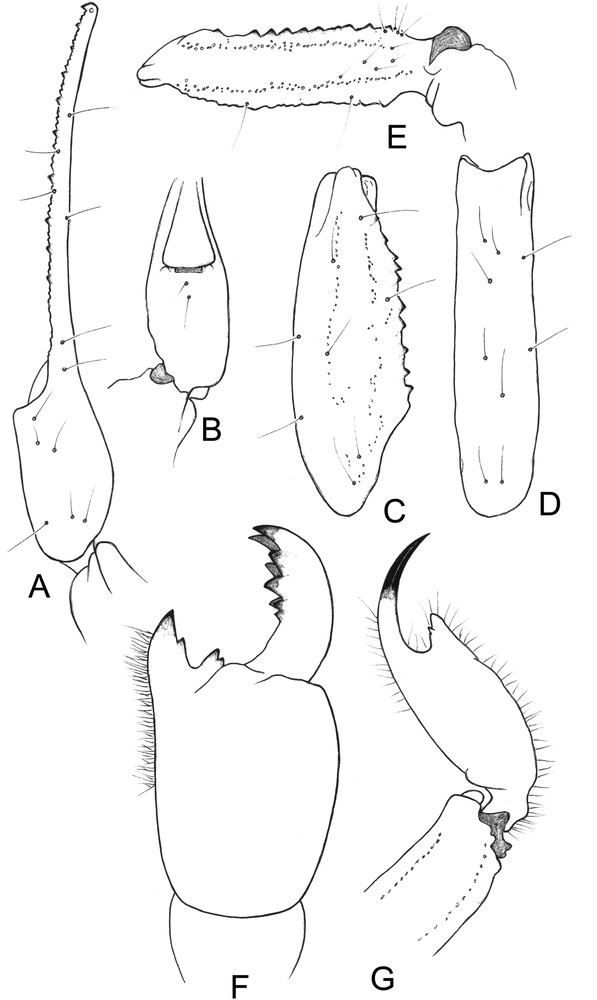
Tityobuthus lokobe sp. n. Female holotype. A–E. Trichobothrial pattern. A–B. Chela dorso-external and ventral aspects. C–D. Patella, dorsal and external aspects. E. Femur, dorsal aspect. F. Chelicera, dorsal aspect. G. Metasomal segment V and telson, lateral aspect.
Type material. Madagascar, Nosy-Be, R.N. Lokobe. 1 female holotype with 5 juvenile paratypes, 4 males & 1 female (one juvenile was removed by M. Vachon), IX/1947 (J. Millot leg.), battage (RS-1428), deposited in the Muséum national d’Histoire naturelle, Paris.
Etymology. The specific name is a noun in apposition to the generic name and refers to the locality where the new species was collected.
Diagnosis. Scorpion of moderate size, with respect to the genus, and with 31–32 mm in total length. General coloration pale yellow throughout, both the body and the appendages with some spots on the carapace, tergites, legs, and partially on pedipalps and metasomal segments. Carapace with a moderate concavity. Pectines large and less separate than in females of other species with 14 to 15 teeth; fulcra reduced. Telson moderately elongated, with a short and curved aculeus; subaculear tooth strong, between rhomboid and spinoid, with two subaculear granules. Internal face of the patella with 9–10 spinoid granules. Tibial spurs strongly reduced on leg IV, absent on leg III. Pedipalp fixed and movable fingers with 8–9 slightly oblique rows of granules. Trichobothrial pattern of type A-α (alpha) – orthobothriotaxic.
Relationships. From its general morphology and by the pattern of pigmentation, Tityobuthus lokobe sp. n. seems to be associated with Tityobuthus darainensis Lourenço & Goodman, 2002, described from the ‘Forêt de Binara’, located in Daraina. These two sites are situated in the extreme north of Madagascar at a similar latitude. The two species can be distinguished by a number of features:
- • the new species shows only 14 to 15 teeth on pectines vs. 16–19 in T. darainensis;
- • smaller global size of the new species;
- • the new species has reduced fulcra in pectines;
- • in the new species tibial spurs are reduced on leg IV and absent on leg III; these are present on legs III and IV of T. darainensis.
4.1 Description based on female holotype and paratypes
Coloration. Ground colour yellow throughout the body and appendages. Carapace yellowish with diffused dark spots, between median and lateral eyes, and with some dark zones on the lateral and posterior margins; eyes surrounded by black pigment. Mesosoma yellow, with three diffuse longitudinal dark stripes; the central is separated by a yellow band. Metasomal segments I to V yellowish; all segments with blackish spots, better marked on IV and V; telson yellow with the tip of aculeus reddish. Venter yellowish. Chelicerae yellowish, with variegated pigmentation at the base of fingers; fingers intensely spotted with blackish; teeth reddish. Pedipalps yellowish, with dark spots on the femur and the patella; chela yellowish; fingers brown to blackish, with darker zones on their base. Legs yellowish, with diffuse dark spots.
Morphology. Carapace weakly to moderately granular; anterior margin with a moderately pronounced median concavity. Anterior median and posterior median carinae weak to obsolete; furrows moderate to weak. Median ocular tubercle distinctly anterior to the centre of the carapace; median eyes large in size, separated by about one ocular diameter. Three pairs of lateral eyes. Sternum subtriangular. Mesosoma: tergites weakly granular. Median carina moderate to weak on all tergites; absence of other carinae. Tergite VII pentacarinate. Venter: genital operculum divided longitudinally, each half being semi-oval in shape. Pectines large in size; pectinal tooth count 14 to 15; basal middle lamellae not dilated; fulcra present but reduced. Sternites smooth, excepted for VII, which shows some minute granulations; spiracles small and elongate. Metasomal segments I and II with 10 carinae, crenulate; segments III and IV with 8 carinae, crenulate. Intercarinal spaces weakly granular. Segment V rounded, with five vestigial carinae and weakly granular. Telson with a pear-like shape and totally smooth; presence of some setations; aculeus moderately curved; subaculear tooth strong and from rhomboid to spinoid with two basal granules. Cheliceral dentition characteristic of the family Buthidae [26]; basal teeth of movable fingers reduced but not fused; ventral surfaces of finger and manus with long setae. Pedipalps: femur pentacarinate and crenulate; patella with some carinae, weakly crenulate; chela rounded and smooth; internal face of patella with 9–10 spinoid granules; all faces weakly granular; fixed and movable fingers with 8–9 almost linear rows of granules. Trichobothriotaxy; orthobothriotaxy A-α (alpha) [23,27]. Legs: tarsus with numerous fine median setae ventrally. Pedal spurs moderate; tibial spurs reduced on leg IV, absent on leg III.
Distribution and ecology. The species is only known from its type locality, the ‘Parc National de Lokobe’ on the Island of Nosy-Be, where rainforest formations predominate. Some authors consider the forests encountered on Nosy-Be as dry forests [28], but the forest in Lokobe is an evergreen rainforest, and subhumid forest would better qualify the vegetation of this small reserve. The bioclimate is subhumid, with an average of six months of hydric deficit per annum, a mean annual rainfall above 2000 mm and mean temperatures of the coldest month between 16 °C and 18 °C [29] (see also Fig. 3).
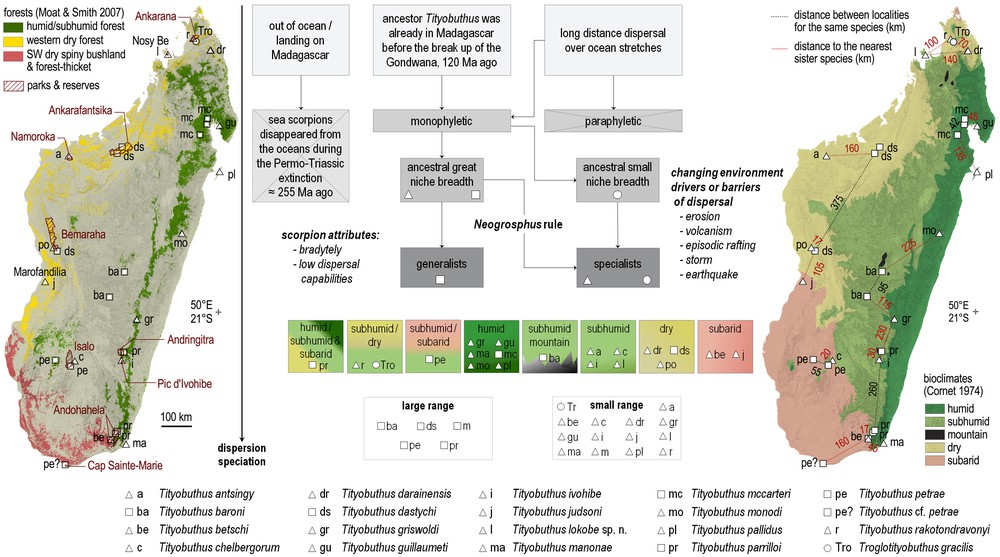
Distribution of the Madagascar species of the Ananteris group according to forest cover (left) [28] and simplified bioclimates (right) [29], and logical flow chart summarizing the evolution of the group in Madagascar (center).
Morphometric values (in mm) of female holotype. Total length (including telson) 31.8. Carapace: length 3.5; anterior width 2.4; posterior width 3.4. Mesosoma length 9.2. Metasomal segment I: length 2.4, width 1.8; II: length 2.6, width 1.6; III: length 2.8, width 1.6; IV: length 3.2, width 1.5; V: length 4.3, width 1.4, depth 1.5. Telson length 3.8. Vesicle: width 0.5, depth 0.6. Pedipalp: femur length 3.3, width 0.5; patella length 4.3, width 0.7; chela length 6.4, width 0.5, depth 0.4; movable finger length 4.7.
5 Ecology and population densities of Ananterinae
The ecology and biology of Ananterinae are poorly known, but detailed inventories carried out during the last 40 years have significantly increased the number of described scorpion species [30]; from 3 to more than 70 in Ananteris and from 1 to almost 20 in Tityobuthus. However, in most cases, the species are extremely rare and narrow endemic, with limited ranges.
A few species of Ananteris proved to be more common [31,32]. Recent ecological studies using pitfall and Winkler traps showed remarkable results and clearly suggested that at least some species are much more common than was originally suspected [32]. The collection efforts in Madagascar have been opportunistic and not systematic, thus not allowing us to assess the geographic range and distribution of the scorpion species. However, some species of Tityobuthus may have larger ranges, as T. dastychi or T. petrae, but more studies are needed to understand the species circumscription as well as their precise ranges. T. dastychi is encountered in the dry forests growing above marls in the Bemaraha, and also in the dry forests of Ankarafantsika some 375 km to the northeast. Several species occur in forests growing above sedimentary reliefs where humidity can sometimes be maintained. Species like T. rakotondravonyi, T. antsingy and T. pococki occur in the forests growing above marls or limestones of Ankarana, Namoroka, or Bemaraha, respectively, and T. chelbergorum occurs in the Isalo sandstones where subhumid forests grow in the deep canyons (Fig. 3).
A peculiarity of the museum collections of Ananteris and Tityobuthus is the frequent absence of juveniles. Collections have been based mainly on the overturning of rocks and the use of ultra-violet light and pitfall traps. Only the use of extraction methods such as Berlese, Winkler and Kempson ones have resulted in the collection of juvenile forms [33]. Extraction methods also led to the discovery and description of new humicolous species [34,35]. These methods have been used more in Madagascar than in the Neotropics and have resulted in the collection of juvenile forms of Tityobuthus [5], while there is still a big knowledge gap for the Ananteris [6]. The new species described here is based on one female collected together with a brood of five or six juveniles. Although almost nothing is known about the reproductive biology of the Tityobuthus species, these values seem to be in accordance with those already observed for other species of micro-buthoid scorpions [36].
6 Distribution of Tityobuthus species
The majority of the members of the genus Tityobuthus are encountered in lowland humid forests, with an elevation range from sea level to 900 m a.s.l., where there is no hydric deficit and mean annual precipitation can be up to 4000 mm. Only T. baroni is known from the central highlands at an elevation of 1500 m and 1650 m (Fig. 3). Other species are encountered in several mountainous areas, including Andringitra, Ivohibe, Andohahela, but only at low elevation. Some species from the eastern humid or subhumid forests are also occurring in the dry or subarid forests, e.g., T. parrilloi in Andohahela.
Few species of Tityobuthus are encountered in the subarid bioclimate of southern and southwestern Madagascar (Fig. 3). A notable exception is T. judsoni, which is only known from the type locality, the dry forest of Marofandilia. In the subarid southwest with its spiny forests and thickets, no Tityobuthus has been recorded to date. The only spiny forest hosting the genus is in the reserve of Cap Sainte-Marie. The Madagascar scorpion fauna has a high diversity, with most species being geographically extremely localized; only Cap Sainte-Marie harbours as many as six species (Grosphus feti Lourenço, 1996, Neogrosphus griveaudi (Vachon, 1969), Pseudouroplectes betschi Lourenço 1995, P. pidgeoni Lourenço & Goodman, 1999, Opisthacanthus luciennae Lourenço & Goodman 2006, Tityobuthus cf. petrae) [37,38]. Some authors have argued that the ‘chevrons’ deposits occurring above the southern region of the island and visible on satellite imagery could be traces of a past Holocene mega-tsunami [39,40]. Such extreme events would have had an impact on the fauna and flora of the entire region that could still be visible nowadays. These historical conditions have to be carefully studied on the ground before any inferences can link past and current environmental settings to explain local scorpion fauna over the region in general, and Cap Sainte-Marie in particular [41].
Tityobuthus lokobe sp. n. is only known from the type locality in the subhumid forests protected on the satellite island of Nosy-Be (Fig. 3). Nosy-Be was connected to the mainland during several dry phases of palaeoclimate oscillations, when sea levels were at ca. 130 m below the current levels [42]. T. lokobe has not been recorded in the Sambirano region [38]. The absence of the genus in the Sambirano may be due to the paucity of inventories in the region, the conspicuous behaviour of some members of this group, the absence of suitable habitat, i.e. low elevation forests, or a combination of these factors [37: fig. 3].
7 Neogrosphus rule reapplied
The species of the genus Tityobuthus belong to a group of scorpions of ancestral age [1]. To date it remains unclear how and where the ancestral Ananterinae survived at the K–Pg 65 Ma, or at the beginning of the Cenozoic (Fig. 4). However, three related fossils of the scorpion group from the Baltic coast, Palaeoananteris, Palaeotityobuthus and Palaeolychas allow for a glimpse of the ancestral's total range.
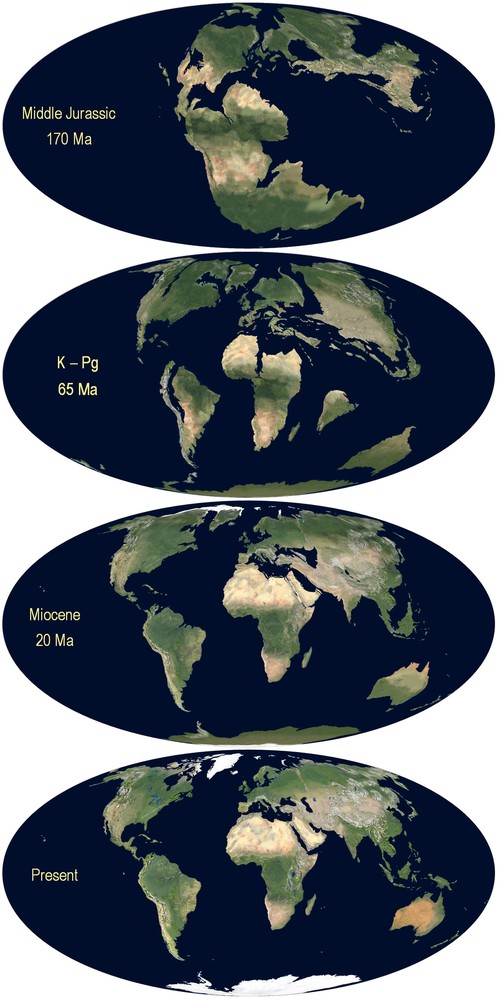
Gondawana: breakup of the Gondwana 170 Ma.
Modified from http://jan.ucc.nau.edu/rcb7/mollglobe.html [43]. ©Ron Blakey, Colorado Plateau Geosystems, used with permission.
We put forth two possible explanations of the group's dispersal and speciation in Madagascar:
- • the ancestor of Tityobuthus was already on Madagascar before it separated from the supercontinent;
- • the ancestor of Tityobuthus arrived later by means of long-distance dispersal (LDD) over ocean stretches (Fig. 3).
The two Madagascar endemic genera Tityobuthus and Troglotityobuthus are phylogenetically more closely related to one another than to any other genus of scorpion in the Ananteris group [4,5,16,17], hence the consideration as monophyletic. If the ancestor reached Madagascar by means of LDD, one single case of successful colonization could explain the entire group in Madagascar. The affinities of Tityobuthus and Himalayatityobuthus (Fig. 1) could also find an explanation in the Cretaceous when peninsular India was still attached to Madagascar before it drifted to the north some 80 Ma ago [43] (Fig. 4). Such a case could echo the Gondwana link of an Indian burrowing frog, a sister taxa of the family Sooglossidae only found on the granitic Seychelles of Gondwanan origin, but not in Madagascar [44]. The affinities between Tityobuthus and South-American Ananteris can be illustrated with another frog: the only Cretaceous frog known from Madagascar, Beelzebufo ampinga Evans et al., 2008, has a link with South America, but is unrelated to extant Madagascar frogs, or frogs found in the Seychelles or India [45]. South America and Indo-Madagascar have been separated before the Cretaceous (Fig. 4). The current distribution of the genus Lychas is best explained by LDD, in particular since the genus occurs on young volcanic islands. If LDD can be invoked to explain the presence of the genus in Africa, its absence in Madagascar is not understood, especially in context of its occurrence on the small islands of the Seychelles and the satellite islands of Mauritius. A southern Gondwana palaeo-distribution for Lychas cannot be excluded, but would be extremely ancient, since Antarctica was not connected to Africa and Australia anymore at the Early Cretaceous (Figs. 1 and 4).
Generally, scorpions are known to be bradytelic, i.e. they evolve at a slow rate when compared to other groups of animals, in particular if belonging to an old group such as the Ananteris. The ancestor of Tityobuthus and Troglotityobuthus have been on Madagascar for millions of years during which the Madagascar plate experienced rifting towards the north (Fig. 4) and was undergoing major climatic changes [42,46,47]. The ancient population was not adapted to dry or subarid environments; these traits are of recent adaptation only (see, for example, the case of modern buthids in the Sahara desert [33,35]). However, this does not rule out an ancestral large niche breadth allowing for adaptation. In such a scenario, the modern population could have colonized and survived in a variety of different environments (Fig. 3). In a small niche breadth scenario, the modern species could only have survived in a localized environmental setting with characteristics of a climatic refuge [42,48], i.e. be specialized and showing a narrow distribution range.
We hypothesize that the monophyletic ancestor of the Madagascar clade must have had a great niche breadth. The bradytelic ancestor, as all Ananterinae scorpions, had low dispersal capabilities. The Neogrosphus rule [38] states that ‘the lower the species’ dispersal ability and the greater the niche breadth of the ancestor taxum, the higher the species richness in a changing environment producing geographical barriers, and vice-versa.’ Based on the extant distributions, we can infer that the ancestor of Troglotityobuthus had a small niche breadth. This monotypic cavernicolous genus is only known from its type locality, a cave in the sandstone formations of Northern Madagascar. The ancestor of Tityobuthus (Fig. 3) with its 19th species described here must have had a large niche breadth, with modern species occurring both on large ranges or narrow ranges, all over Madagascar in the various bioclimates, but mainly at lower elevations.
8 Conclusions
In the case that Tityobuthus would have kept ancestral features while most species are occurring in humid and subhumid forests, some species must have been able to cope with mesic environments. We can postulate a possible ‘Tityobuthus pattern’: The small ranged species of the group occur in every bioclimate, with the exception of mountains, while the large-ranged species occur also in mountains.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
Wei-Ping Chan and Sheng-Feng Shen, Academia Sinica in Taiwan, are acknowledged for the permission to use their climate map. We are thankful to Ron C. Blakey, Colorado Plateau Geosystems, for the permission to use the paleogeography figures.Funding: FACAD.
Appendix 1 Checklist for the Tityobuthus species known from Madagascar
Tityobuthus monodi Lourenço, 2000
Distribution: only known from the type locality; Prov. Toamasina, F.C. Andriantantely (18°41,7′S–48°48,8′E), 530 m, 7–10/12/1998 (H.J. Ratsirarson leg.).
Tityobuthus guillaumeti Lourenço, 1995
Distribution: only known from the type locality; Iaraka, ‘Baie d’Antongil’, 700 m, rain forest 17/11/1969 (J.-M. Betsch).
Tityobuthus mccarteri Lourenço et al., 2008
Distribution: Toamasina Province, Akirindro mountain, NW of Ambinanitelo, [presumed to be 15°17′3″S–49°32′9″E, cf. Heterick, 2006], 600 m, ‘forêt humide’, 03/1969 (J.-M. Betsch); Réserve Spéciale d’Ambatovaky, 450 m, rain forest, 31/I/2006 (L.R. de Roland); plateau de Makira, forêt de Vohitaly (site F), 5 km SE of the Anjiahely village (15°26′58″S–49°32′06″E), 540–680 m, pitfall vial marked “C9 site F Vohitaly”, no date (V. Andrianjakarivelo).
Tityobuthus rakotondravonyi Lourenço & Goodman, 2003
Distribution: only known from the type locality; Antsiranana Province, ‘Réserve spéciale d’Ankarana, Campement des Anglais’ (Anilotra), 7.5 km NW of Mahamasina, 12°54,5′S–49°06,6′E, 125 m (S.M. Goodman).
Tityobuthus judsoni Lourenço, 1996
Distribution: only known from the type locality; Mad. W, route de Morondava, Belo-sur-Tsiribihina, ‘forêt de Marofandilia, sous bois mort’, 8/12/1969 (J.-M. Betsch).
Tityobuthus pallidus Lourenço, 2004
Distribution: only known from the type locality; Sainte-Marie Island, Ambohidena Forest Station, 16°51′S–49°57′E, 27/12/1955 (IRSM leg.).
Tityobuthus manonae Lourenço, 2000
Distribution: only known from the type locality; Mandena, Fort Dauphin, littoral forest 10 km north of Fort Dauphin, 6–12/01/1999 (J.-B. Ramanamanjato).
Tityobuthus baroni (Pocock, 1890)
Distribution: this species was the subject of numerous misidentifications. Consequently, many wrong citations remained in the literature until the final clarification about the identity and the status of this species was published by Lourenço et al. (2008). Refer to this publication for further details on the species and type material. The present known distribution of T. baroni can be summarized as follows: Madagascar, type material, Rev. R. Baron. BM 1888-12. Parc de l’Est, Antsirabe, 16/09/1973 (C. Duval leg.) [male holotype and female allotype of Tityobuthus lucileae]. Fianarantsoa, forêt d’Atsirakambiaty, 7.6 km WNW from Itremo, 1550 m (20°35′36″S-46°33′48″E), montane rainforest (Fisher, Griswold et al.), 22–26/01/2003.
Tityobuthus pococki Lourenço, 1995
Distribution: only known from the type locality; W. Bekopaka, Antsingy, 07/1970 (P. Griveaud).
Tityobuthus ivohibe Lourenço & Goodman, 1999
Distribution: only known from the type locality; Prov. Fianarantsoa, exterior northern limit of the ‘R. S. d’Ivohibe’, along the Hefitany River, 7.5 km ENE of Ivohibe (22°28,2′S–46°57,6′E), 900 m, 6–13/10/1997 (S. Goodman).
Tityobuthus griswoldi Lourenço, 2000
Distribution: only known from the type locality; Prov. Fianarantsoa, P.N. Ranomafana, Talatakely (21°15,3′S–47°25,9′E), 9–26/04/1998 (pitfall traps; C.E. Griswold, D.H. Kavanaugh, N.D. Penny & M.J. Raherilalao leg.).
Tityobuthus parrilloi Lourenço, 1996
Distribution: only known from the type locality; Madagascar Est, Prov. Fianarantsoa, 45 km south of Ambalavao (eastern bank of the Iantara River, along the Ambalamanenjana–Ambatomboay trail, edge of the ‘Réserve Naturelle Intégrale no 5 de l’Andringitra’), 22°13′40″S–47°00′13″E (720 m alt.), 19–20/11/1993 (S. Goodman).
Tityobuthus dastychi Lourenço, 1997
Distribution: this species was also the subject of a number of misidentifications. Consequently, a number of sites attributed to this species in the SW of Madagascar should now be disregarded (see Lourenço & Goodman, 2003). In fact, the populations in the SW of the island proved to be a distinct species yet to be described. Only the sites reported for the Province of Mahajanga should be retained for T. dastychi. Prov. Mahajanga, Ampijoroa, 03/1996 (P. Chouteau), [type material]; idem, 02/2001 (G. Garcia); ‘Parc National de Bemaraha’, southern bank of the Manambolo River, near Tombeau Vazimba, 3.5 km of Bekopaka, 19°08,4′S–44°49,7′E, 100 m, Tsingy forest (A. Raselimanana).
Tityobuthus petrae Lourenço, 1996
Distribution: south of Madagascar, Tuléar Province, Vohibasia, 10/1995 (S. Goodman); a few paratypes also from Prov. Tuléar, Tanjon’i Vohimena (Cap Sainte-Marie), ‘Réserve spéciale’.
The original type material was collected in two different sites; however, new elements led to believe that the material from Cap Sainte-Marie should be reconsidered as a possible distinct population. Only further material will allow the clarification of this situation.
Tityobuthus betschi Lourenço et al., 2008
Distribution: only known from the type locality; Toliara Province, ‘Parc National d’Andohahela’ parcel 2, ‘forêt de Manantalinjo’, 24°49′01″S–46°36′36″E, 150–300 m, V/1972 (J.-M. Betsch); ‘extraction dans forêt sèche’ (spiny bush forest).
Tityobuthus chelbergorum Lourenço et al., 2008
Distribution: only known from the type locality; Fianarantsoa Province, ‘Parc National d’Isalo’, 8–9 km north of Ranohira (22°28′59″S–45°27′38″E), 700–730 m, nearby ‘forêt galerie’, 8/11/2004 (W.R. Lourenço).
Tityobuthus darainensis Lourenço & Goodman, 2002
Distribution: only known from the type locality; Antsiranana Province, ‘Forêt de Binara, near Analamazava River, 7.5 km SW of Daraina (13°15,3′S–40°37,0′E), 325–600 m (S. Goodman).
Tityobuthus lokobe sp. n.
Distribution: only known from the type locality; Nosy-Be, Parc National (e.g., ‘Réserve Naturelle Intégrale’) Lokobe. DINA region (formerly Province of Antsiranana).


