The reproductive organs of flowering plants are key elements for both plant survival and genetic diversification. They also give rise to seeds, which are critical elements in human nutrition and contributed to the emergence of most human civilizations. Nonetheless, the developmental biology of reproductive organs tends to be less studied, and thus less well understood than other plant organs, especially in the current “image and omics-based” era. This is in part because of their compositional and genetic complexity, and in part because of their physical inaccessibility. A similar imbalance, in terms of scientific focus, can also be observed within each organ. Anthers are male reproductive organs inside which multiple pollen grains are produced (Figure 1). Each pollen grain is a tiny haploid organism containing two sperm cells (gametes) encased into a larger haploid cell (vegetative cell), which is, in turn, covered by a tough protective external pollen wall that ensures prolonged pollen survival. At maturity, pollen grains are released from the anthers and deliver their sperm cells to the ovules that are buried within the female reproductive organs (the carpels) (Figure 1), triggering fertilization. Because of their critical role in fertilisation, the development of pollen grains inside anthers has received considerable attention. Moreover, the fertilisation of the egg cell within the female ovule by one of the sperm cells delivered by the pollen leads to the development of the embryo. The fertilised ovule will develop into a seed inside which the embryo will grow and finally emerge as a new plant, thus assuring the future generation (Figure 1). Furthermore, embryos often store energy reserves (lipids, proteins and carbohydrates) at seed maturity, which ensure their survival upon germination, but are also of critical importance as human and animal food, and as inputs to multiple industries. As a result, embryo development in seeds, like pollen development in anthers, has received considerable attention.
Sexual reproduction in Arabidopsis thaliana. The organs and tissues highlighted in this review are illustrated.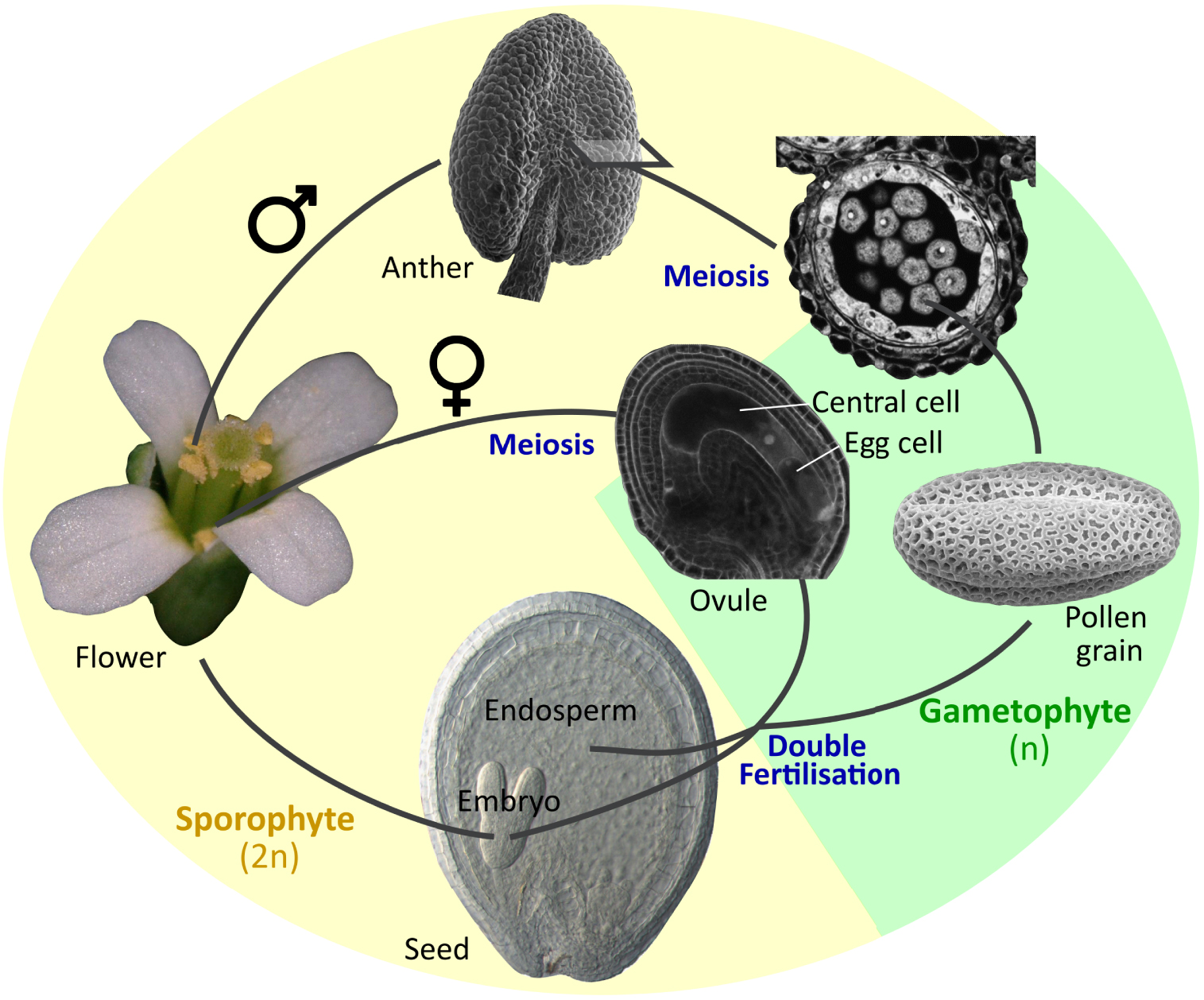
However, the embryo and the pollen grain would be unable to complete their development in the absence of “supporting” tissues that surround and nurture them during their genesis inside the seed and the anthers. These critically important tissues, the endosperm and the tapetum respectively, whilst being amongst the most poorly understood tissues in terms of developmental biology in plants, are also two of the most fascinating, and peculiar. Furthermore, despite their very different genetic and developmental origins, their analogous functions appear to have led them to develop a suite of shared cellular and developmental attributes. This apparent convergence will form the subject of this review. For the sake of simplicity, we will focus principally on current knowledge of the model Arabidopsis thaliana, although reference will be made to other species when pertinent.
Because of space constraints, we have also chosen not to review pre-meiotic and pre-fertilisation functions of the tapetum and endosperm precursor (the central cell), although intriguing similarities are also observed at these timepoints, particularly regarding the provision of small RNAs to the germinal and egg cells [1, 2]. We have also chosen not to discuss other reproductive support tissues such as the female nucellus, which shows strong analogies to the endosperm and tapetum. The nucellus, which is in fact more analogous to the tapetum than is the endosperm [3] (in purely ontogenetic terms), has been even more strongly neglected by the scientific community.
1. The endosperm: a developmental overview
The endosperm in the strictest sense is an innovation of the angiosperms, which undergo a unique double fertilization event during which a pollen tube delivers two genetically identical sperm cells to the multicellular female gametophyte within the ovule. In Arabidopsis, one sperm cell fuses with the egg cell to give rise to a diploid zygote (the baby plant), whilst the other fuses with the central cell (which is double haploid). Central cell fertilization triggers the growth and nuclear proliferation of the resulting triploid endosperm cell. Initially, nuclear division occurs in the absence of cytokinesis, giving rise to a coenocyte containing multiple nuclei in a single cytoplasm that surrounds a large central vacuole. The initial dramatic expansion of the coenocytic endosperm corresponds to a phase of limited embryo growth. Subsequently, endosperm growth slows and the endosperm progressively cellularizes, starting from the zone surrounding the developing embryo (the micropylar endosperm) and progressing towards the point of ovule attachment to the mother plant (the chalaza). Cellularization is closely followed by an acceleration in the growth of the embryo, which invades the endosperm. This process is accompanied by a controlled cell separation, breakdown and elimination of the endosperm cells, which are progressively replaced by the expanding embryo so that at seed maturity, only a single layer of endosperm cells remains[4, 5] (Figure 2A). Although the development of the endosperm is very variable across angiosperms, a basic pattern of fertilisation, expansion, invasion by the embryo, and controlled elimination is generally observed. Indeed, even in gymnosperms, in which only the egg cell is fertilized, and in which female gametophyte proliferation is not strictly fertilisation dependent, the relative behaviours of the female gametophyte tissues surrounding the developing embryo (which are progressively eliminated), and the fertilized embryo (which invades them) appear to be analogous to those of the angiosperm endosperm and embryo [6].
Seed and anther development in Arabidopsis thaliana. (A) Arabidopsis seed development. The endosperm (highlighted in orange) progresses from a large multinucleate coenocyte (first panel, globular stage embryo), undergoes cellularization (second panel, heart-torpedo stage embryo) and then undergoes a progressive elimination process as the embryo expands (third and fourth panels). (B) Arabidopsis anther development. The tapetum (highlighted in orange) surrounds the post meiotic tetrads (first panel) and the released microspores (pollen grain precursors, second panel). As the pollen wall is deposited, the tapetum starts to shrink (third panel) and is ultimately eliminated (fourth panel).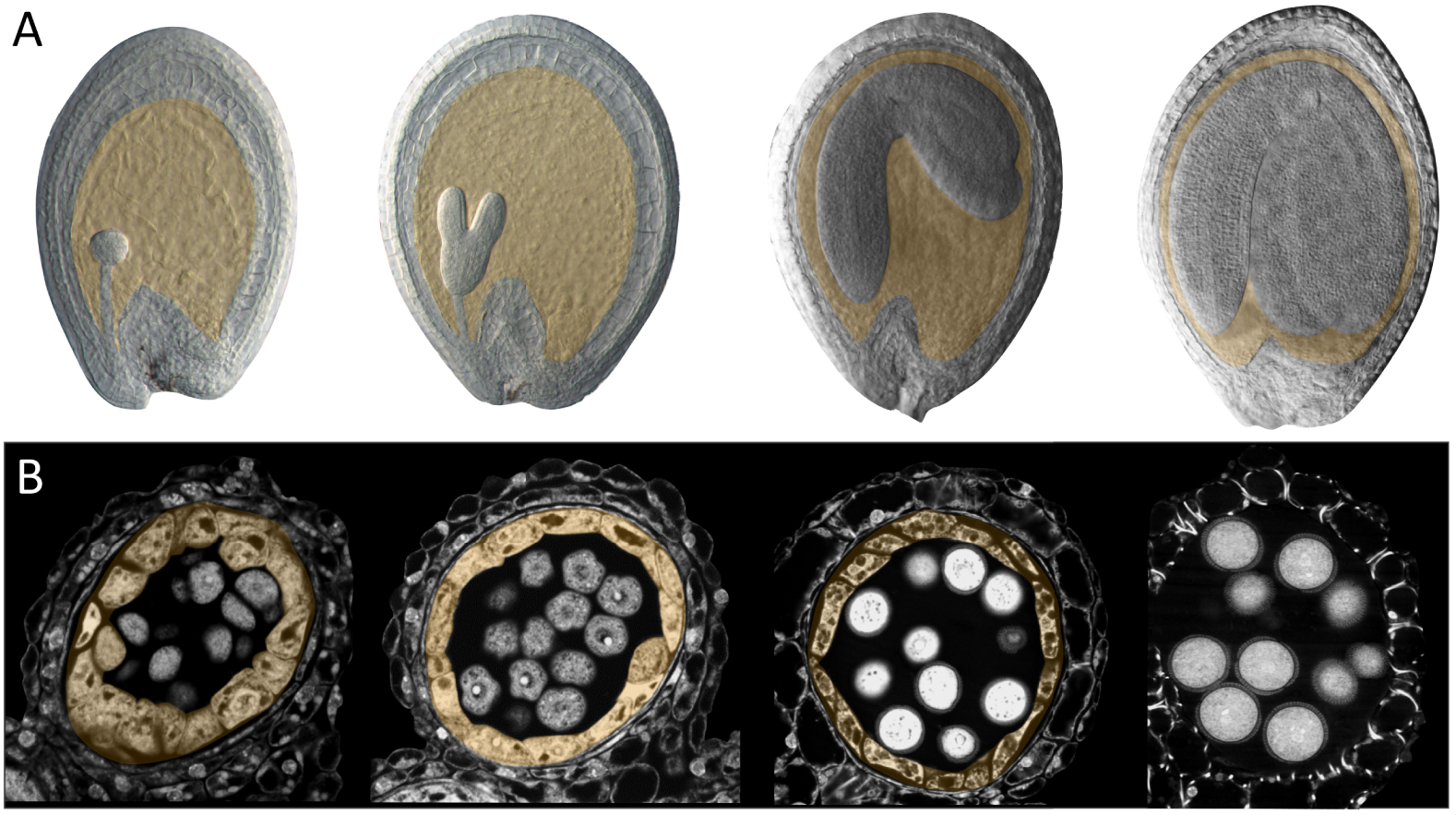
2. The tapetum: a developmental overview
The tapetum is the diploid cell layer located next to the developing pollen inside the anthers (Figure 2B). The tapetum arises inside the young anther together with other diploid cell layers, including the centrally located precursors of the future pollen. During differentiation and maturation, the tapetum cells become binuclear and are enriched with complex endomembrane-derived structures, vacuoles, and tapetum-specific organelles [7]. The tapetal cells, although initially surrounded by detectable cell walls, undergo dramatic cell wall remodelling as they develop. The loss of cell wall material has been proposed to facilitate secretion of materials from the tapetum cells into the surrounding space. This remodelling coincides with an apparent widening of cytoplasmic connections between the tapetal cells and consequently the tapetum cells form a highly interconnected population. The mature tapetum thus represents a symplastic continuum of highly metabolically active secretory cells. As the tapetum cell layer develops, the neighbouring diploid pollen precursor cells undergo meiosis and produce haploid microspores (the direct precursors of the future pollen). At this point the tapetum cells start to secrete large quantities of material, particularly towards the developing microspores as they grow and start to assemble their complex external pollen wall. Subsequently the tapetum is totally eliminated, releasing more material towards the pollen that is incorporated within the pollen surface. Again, although tapetum development is variable across angiosperms, a basic pattern of specification, endoreduplication, cell wall remodelling, secretion, and cell elimination is observed in most species [8].
3. Functional analogies between the endosperm and the tapetum
Both the endosperm and the tapetum play a supporting/nursing role, that is seminal for the development and survival of the embryo and the pollen grains respectively. In each case, this role can be split into three potentially distinct, but likely overlapping, activities: (1) transfer of energy and metabolic building blocks from surrounding tissues to the developing embryo/microspore; (2) ensuring the formation and integrity of the embryo/microspore surface; (3) controlled cell elimination permitting the recycling of cellular material (and space).
Here each of these functions will be addressed in turn, with reference to the apparent developmental and physiological strategies that each tissue deploys to ensure a satisfactory outcome (Figure 3).
Endosperm–embryo and tapetum–pollen interfaces in Arabidopsis thaliana. (A) Transmission Electron Micrograph of the endosperm–embryo interface at the torpedo stage of embryo development (during endosperm elimination). (C) Schematic diagram of the endosperm (yellow) and neighboring tissues (seed coat and embryo) prior to endosperm cellularization. Nutrient influx into and out of the coenocytic endosperm is shown by orange arrows. Nutrients are transiently stored (green spheres) or converted into molecules destined for deposition at the embryo surface (blue spheres). (E) Schematic diagram of the endosperm and neighboring tissues after endosperm cellularization and during elimination (top to bottom). Nutrients from maternal tissues can diffuse directly to the embryo though the progressively modified endosperm apoplast and can be remobilized after transient storage for transfer to the embryo. Components are secreted from the endosperm to contribute to embryo surface construction (the sheath, shown in blue). (B) Transmission Electron Micrograph of the tapetum–pollen interface during pollen wall deposition. (D) Schematic diagram of the tapetum (yellow) and neighboring tissues (anther wall and microspores/pollen grains) prior to microspore release. Nutrient influx into and out of the tapetum is shown by orange arrows. Nutrients can be transiently stored (green spheres) or converted into molecules destined for deposition in the pollen wall (blue spheres). Nutrients from maternal tissues can diffuse directly to the developing pollen between tapetum cells. (F) Schematic diagram of the tapetum and neighboring tissues (anther wall and microspores/pollen grains) after microspore release and during tapetum elimination (top to bottom). Nutrients from maternal tissues can diffuse directly to the developing pollen though the progressively modified tapetum apoplast and locular matrix and can be remobilized after transient storage for use in the synthesis of pollen wall components. Whether stored nutrients are remobilized and re-exported from the tapetum to fuel pollen grain development remains open to question. Specific components are secreted from the tapetum both prior to and during elimination to contribute to pollen wall construction (shown in blue).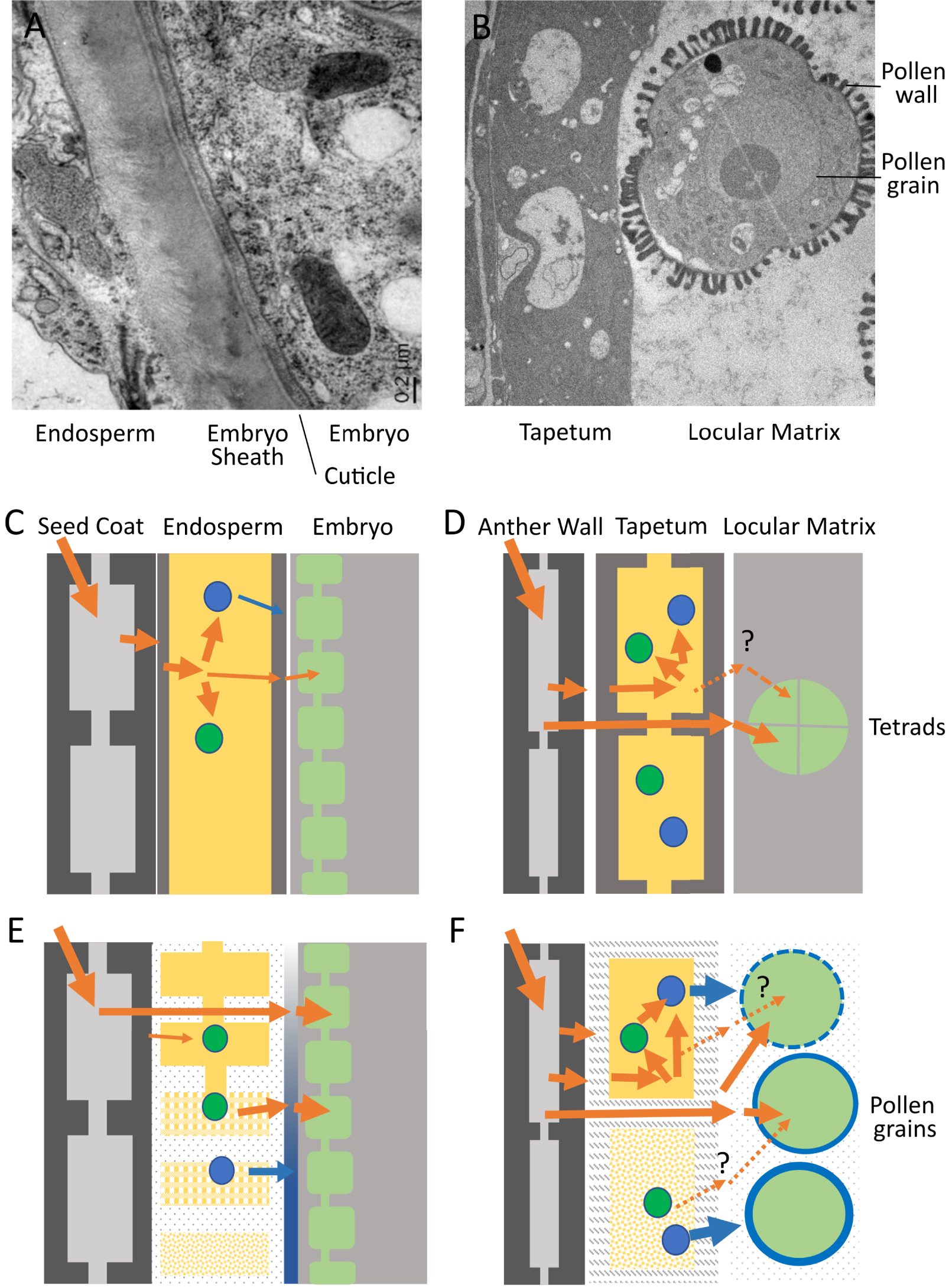
3.1. Transfer of energy and metabolic building blocks from surrounding tissues to the developing embryo/pollen grains
Both the endosperm and the tapetum act as conduits through which nutrients required for the development of the embryo/microspores must pass. Both the embryo and the microspores store considerable quantities of energy as they grow and mature, in order to prepare them for their dormancy and ultimate germination. Energy, which is stored in the form of lipids, proteins, and carbohydrates (starch), enters the embryo and microspores in the form of sugars and amino acids, which either act as building blocks or provide the energy required for enzymatic conversion through respiration. How these molecules are transferred to the embryo and/or microspore has been a subject of considerable interest, not least because of the presence of symplastic barriers within plant reproductive organs [9, 10]. The cytoplasms of many neighbouring plant cells are connected through regulatable pores called plasmodesmata, generating so-called symplastic connections. Shortly after fertilisation, the Arabidopsis endosperm loses symplastic connections with both the developing embryo and surrounding maternal tissues [11]. Likewise, although the tapetum forms a symplastic continuum, it is symplastically isolated from both the developing microspores and the surrounding external anther cell layers from before the onset of meiosis [12]. The importance of this symplastic isolation for physiology, development and/or defence remains unclear. However, there are no cytoplasmic connections between the endosperm and either the maternal tissues or the embryo within the seed, or between the tapetum and either the surrounding anther cell layers or with the microspores during the most metabolically active phases of their development. This means that all the sugars, amino acids, and minerals entering the embryo and microspore must be absorbed across the plasma membrane from the surrounding apoplastic space (the cell wall). This implies one of two, non-exclusive, scenarios (Figure 3).
Scenario 1
Sugars and other metabolites from the phloem are released into the apoplast overlying the endosperm/tapetum and imported into the endosperm/tapetum cells. They are then re-exported (often after a period of temporary storage as starch or lipids, followed by remobilisation) by the endosperm or tapetum towards the embryo/microspores. This may occur in both the endosperm and the tapetum, but has only been conclusively demonstrated in the context of the endosperm, which can be considered a true transient energy storage tissue [13, 14] (Figure 3A). In the anther, the tapetum, like other cells of the anther wall, can contain starch, and also lipid bodies. However, it is not clear whether these compounds serve principally as a reserve for the frenetic biosynthetic and secretory activity that occurs within tapetal cells themselves (see below), or whether they can also be remobilised towards the microspores as sugars (Figure 3B).
During the early stages of seed development, the endosperm absorbs sugars and other osmolytes that drive its early rapid growth and can also be stored transiently, for subsequent remobilisation to fuel embryo development. The endosperm, like the tapetum is thus a major nutrient/energy sink in its own right. Indeed, it has been proposed that early, growth-driving nutrient uptake by the endosperm may be a target of parental conflict in Angiosperms due to the introduction of a male genome into the endosperm by double fertilisation, potentially placing the embryo and endosperm in direct conflict for maternally derived nutrients [15]. The work of Kohler and colleagues [16] has led to the proposition that endosperm cellularization, which is promoted by maternal genomes but repressed by paternal genomes in the endosperm, could provide a critical counterbalance within this conflict by favouring a second nutrient transport scenario, described below.
Scenario 2
Sugars and other metabolites from the phloem could be released into the apoplast overlying the endosperm/tapetum. They could then diffuse between the cells of these tissues, through the apoplast, and be taken up directly by the embryo/pollen grain. In this scenario the endosperm/tapetum can be effectively bypassed, and energy/metabolites can move directly to their ultimate sink, the embryo/microspores. Again, some evidence for this scenario exists in seeds, both based on metabolic tracing and the expression of sugar transporters [13, 14]. However, the question has been less extensively studied in anthers, where both spatially resolved gene expression data, and metabolic tracer studies are lacking. In the context of scenario 2, it is nonetheless intriguing to note that the apoplastic interfaces between tapetum cells and between the tapetum and microspores, as well as between endosperm cells and between the endosperm and the embryo, are significantly modified as the energy needs of the embryo/microspores increase [7, 17, 18]. In the seed, this corresponds to a thinning of endosperm cell walls, and in Arabidopsis at least, to the physical weakening and expansion of the apoplastic interface between the embryo and the endosperm involving the deposition of a glycoprotein-rich matrix [10] (Figure 3A). In the anther, it corresponds to an apparent loss of tapetum cell wall material, and to the degradation of the callose matrix surrounding the microspores and its replacement by the locular fluid [7] (Figure 3B). If, and if so how, these changes impact molecular diffusion within these intercellular spaces remains unclear.
3.2. Ensuring the formation and integrity of the embryo/microspore surface
In addition to acting as a conduit for basic metabolites to fuel the growth and synthesis of storage compounds in embryos and microspores, the endosperm and tapetum also synthesise and release specialised molecules required for the construction of the protective surfaces that cover embryos and microspores at maturity. Some of these molecules are involved in communication either between the endosperm and the embryo, or between the tapetum and the microspore, which is required for the timely and spatially organised construction of protective surfaces. For example, recent research has revealed that related peptide mediated dialogues are critical for ensuring the integrity of the diffusion-limiting cuticle and the anti-adhesive sheath, which protect the embryo both during development and at germination [17, 19, 20], and for the timely activation of the secretory activity of the tapetum at microspore release [21]. In both cases, inactive signalling peptide precursors from the CASPARIAN STRIP INTEGRITY FACTOR (CIF) family are released by the embryo in the seed (TWISTED SEED1) and by the tapetum in the anther (CIF3 and CIF4). They are subsequently activated by Subtilase family proteases released by the endosperm in the seed (including ABNORMAL LEAF SHAPE1), and by the microspores/developing pollen in the anther. The perception of the activated peptides by the GASSHO1 and GASSHO2 receptors leads to correct construction at the embryo cuticle and sheath, and to tapetum activation and successful pollen wall formation in the anther.
In addition to these dialogues, both the endosperm and the tapetum secrete structural components that are integrated into the embryo and microspore surfaces respectively. In the seed, relatively little is known regarding this process, although the glycoproteins that form the embryo sheath clearly originate in the endosperm [17] (Figure 3A). Whether other surface components, such as cuticle monomers, could be derived from the endosperm remains unexplored. By contrast, it is clear that the tapetum is a major player in the formation of the complex pollen wall that assembles on the microspore surface [22]. The tapetum participates in the timely degradation of the callose layer which surrounds pre-meiotic microspores, a process that is critical to allow organised pollen wall assembly. It then synthesises many of the monomers involved in the assembly of the pollen wall, including the constituents of the highly resistant polymer sporopollenin [23, 24] (Figure 3B). These compounds are actively exported from the tapetum, together with potential molecular chaperones such as LIPID TRANFER PROTEINS [25], and other components of the locular matrix surrounding the developing microspores. The production of these unusual compounds, in the large quantities required by the developing microspores, presumably explains much of the apparently unique biology of the tapetum.
3.3. Controlled cell elimination and the recycling of cellular material (and space)
Both the endosperm and the tapetum are transient tissues that are destined, ultimately, to die. However, the cell death observed in these tissues, at least in Arabidopsis, is rather different to the senescence-associated cell death that occurs, for example, in autumn leaves, or to cell death pathways that give rise to hollow xylem vessels. Endosperm and tapetum cell elimination involves the total dismantling of the cells and the apparently complete recycling of their contents [7, 26]. This process appears to be necessary for reproductive success and is highly regulated in both the endosperm and the tapetum. In both systems, cell wall dismantling occurs well before cell elimination[7, 17, 18]. In the endosperm, cell wall softening and associated cell separation have been proposed to be important prerequisites for subsequent cell death, since they facilitate the physical crushing of the endosperm by the expanding embryo. Indeed, the death of the endosperm is delayed if embryo growth is defective, even when cell wall softening has occurred [18]. Cell elimination is tightly regulated, possibly in line with the metabolic requirements of the growing embryo. Cell wall derived sugars may be taken up directly by the developing embryo upon release from cell wall polymers, and act as primary metabolites. Meanwhile, cell contents appear to be broken down to sugars, amino acids, and essential minerals, and exported to the apoplast via a variety of transporters, presumably again prior to uptake by the embryo [27]. In Arabidopsis the process of endosperm elimination is regulated by heterodimers of the endosperm-specific ZHOUPI and the broadly expressed INDUCER OF CBP EXPRESSION 1, basic Helix–Loop–Helix transcription factors. This complex is necessary for the expression of numerous cell wall modifying enzymes. Consistent with this, endosperms lacking this complex develop and cellularize normally, but do not undergo cell wall thinning/modification or cell elimination, and cannot be invaded by the expanding embryo, the growth of which is thus very limited, and the viability of which is significantly compromised [18, 28, 29]. Although the endosperm of these mutants does eventually die as seeds desiccate upon maturation, the recycling of cell contents appears not to occur.
In the anthers, tapetum degradation serves to release a final wave of lipidic and proteinaceous substances which become integrated on the surface of the pollen wall [24, 30]. These substances appear to be important for pollen viability, as well as for interactions of pollen grains with receptive female surfaces [31]. Consistent with this, mutants defective in tapetum elimination show defective pollen wall deposition and reduced pollen viability [32].
In summary, the cells of the endosperm and of the tapetum fit the accepted definition of “nurse cells”, in that they provide nutrients, growth materials and physiological support to their neighbours. Despite their very different ontogenetic and evolutionary origins, tapetal cells and endosperm cells share striking similarities, both in terms of their functions (in nutrient provision and in the provision of other structurally important compounds to the developing microspores and embryo) and, possibly more surprisingly, in terms of the cellular/developmental strategies that they employ in achieving these functions. How similar molecular mechanisms have been co-opted in these tissues during angiosperm evolution remains unclear. Visualising molecular movement deep within plant organs and understanding how intercellular communication between the compartments of plant reproductive organs is controlled remain some of many unresolved technical challenges holding back the field. Rising to these challenges and answering these questions should provide fertile ground for cell, developmental and evolutionary biologists over the coming decades.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0