1 Introduction
Kin recognition developed as a major research subject as soon as Hamilton (1963) introduced the notion of inclusive fitness leading to kin selection, i.e. an increasing fitness through the breeding of relatives [1,2]. The relationship between kin recognition (an internal process) and kin discrimination (observable kin bias in behaviour) is a complex one. First, although in many cases kin bias has been proved to be linked to recognition, kin bias does not necessarily involve kin recognition. Second, lack of kin discrimination does not imply a failure to recognise kin, which can be revealed only by appropriate experimentation [3].
Ability for individual discrimination has been demonstrated within numerous species [4–6]. Many studies have focused on the underlying mechanisms of kin discrimination. These mechanisms are diverse, but they can be divided into two main categories. Kin discrimination by conspecifics cues [7] occurs through the detection of phenotypic similarities in the absence of previous experience. When prior experiences are required, kin discrimination arises via direct or indirect familiarisation (self-matching or allo-matching) [8]. Indeed, only indirect familiarisation allows the animal to recognise an unfamiliar kin. However, as put forward by Waldam [9], the extent of mutual exclusion between some of these mechanisms remains far from clear.
Many functions of kin recognition have been described previously. These include care of offspring, helping siblings (allo-grooming, alarm…), cooperation, development of effective bonds, communal breeding, helpers at the nest [10,11], escaping from cannibalism [12], mate choice and avoidance of inbreeding [13,14]. Hamilton [15] also emphasised that kin selection theory could also be applied to aggressive behaviour. In the 1980s, studies on spiny mice [16], primates such as Macaca nemestria [17], ground squirrels [18], or golden hamsters [19] improved our understanding of kin recognition in mammals. Holmes and Sherman [18] demonstrated that sisters, in contrast to brothers, displayed less aggressive behaviour among themselves, even if they were separated at birth (unfamiliar kin), than when confronted with non-kin females.
Most studies on kin recognition have examined social species where individuals are linked by social bonds throughout their life. However, in many species, including mammals, bonds are not long lasting; they are limited to mother-offspring and sibling ties. Adult male–female relationships are often restricted to reproductive periods. In polecats (Mustela putorius), male and female territorial boundaries are defined by scent marking [20], which limits direct confrontation between individuals. This solitary or individualistic characteristic of the polecat [21,22] is expressed by aggressive encounters, including between males and females [23–26]. During reproductive periods, behavioural modifications lead to short-lived tolerance between male and female adults [26,27]. Despite their individualistic way of life, communal activities have been observed in some mustelids, including foraging and sharing of prey [28,29].
That solitary carnivores show mechanisms for kin discrimination may be addressed. Recently, Tang-Martinez [30] hypothesized whether kin discrimination may derive from other, non-specialized abilities of animals. Determining the mechanisms favouring recognition is hence a fundamental question. The issue is especially to distinguish kin versus familiarity effect. The aim of this research was first to develop an experimental design in order to detect kin discrimination in the polecat and to specify the mechanism underlying this discrimination. Second, I analysed the ontogeny of interactions at the time of active discovery of the environment, i.e. 50 days after birth and just before dispersal when they were 70 days old.
2 Methods
The study was carried out on five litters (a, b, c, d and e) of laboratory-bred polecats (10 males and 9 females, Authorisation DPN, ‘Direction de la protection de la Nature’ and Capacity Certificate). Litters ‘a’, ‘b’, ‘c’, and ‘d’ were identified by a coloured mark. Newborn animals were separated from their mothers 10 days after birth and divided into four groups:
- – group 1, kin, familiar – related animals (siblings, i.e. brothers and sisters) were raised by their biological mother;
- – group 2, kin, unfamiliar – related animals (siblings) were raised by two different ‘mothers’;
- – group 3, non-kin, familiar – these animals were born of different parents, but raised together by the same unrelated ‘mother’;
- – group 4, non-kin, unfamiliar – unrelated animals were raised by different ‘mothers’ – litter size varied from 3 to 5.
Fifty-four dyads were tested: 13 were male–male, 10 were female–female, and 31 were male–female. Animals of each dyad were introduced simultaneously into a 16-m2 neutral enclosure just at the dusk because of twilight and of the nocturnal habits of the species [31]; they were observed using a red light. Interactions were studied during these dyadic encounters that lasted 10 minutes, but some were interrupted before intense aggression was displayed. Between tests, animals were isolated from their real or adoptive mother and their littermates during two consecutive days.
The results of each encounter were classified into one of four behavioural categories. The first three were defined according to the degree of tolerance observed: (1) negative interactions characterised by more or less pronounced aggression, (2) intermediate interactions – displays of intimidating behaviour –, (3) positive interactions – tolerance, investigation of the other, play. As during some encounters, there were no direct interactions, we added a fourth category of behaviour: exploration of surroundings, self-grooming, etc. This last category was labelled non-interactive behaviour. Encounters between a given dyad were replicated five times. Confrontations were staged every other day.
Two types of data analysis were made. Firstly, individuals were tested as separate units (). Secondly, each encounter was considered a unit and data are expressed as proportions of encounters in each behavioural category (). A first series of dyadic encounters were carried out on polecats aged 48–55 days. A second series of dyadic encounters, following the same protocol, were carried out when the animals were 70 days old. Results were analysed by non-parametric statistical tests adapted to the type of data, taking into account related and independent values (H Kruskal–Wallis, U Mann–Whitney, τ Wilcoxon).
3 Results
A comparison between the four groups revealed differences, both for positive interactions or negative interactions (Table 1). For intermediate interactions, only female–female and male–female encounters showed significant variations among groups and only male–female encounters significantly varied among groups for non-interactive behaviour. This study aimed at detecting the contribution of four variables – sex, age, kinship, and familiarity – to these variations.
Differences among types of interactions in the European polecat
| Differences | Male–male | Female–female | Male–female |
| Positive interactions | KW = 22.6 | KW = 16.7 | KW = 49.2 |
| p=0.0001 | p=0.0001 | p=0.0001 | |
| Negative interactions | KW = 20.1 | KW = 17.1 | KW = 39.5 |
| p=0.0001 | p=0.0001 | p=0.0001 | |
| Intermediate interactions | KW = 2.12 | KW = 13.1 | KW = 16.8 |
| Not significant | p=0.004 | p=0.001 | |
| Non-interactive behaviours | KW = 4.69 | KW = 1.02 | KW = 12.4 |
| Not significant | Not significant | p=0.01 |
3.1 Sex effect
Males were found more aggressive than females (, , ). Analysis of male–male and female–female encounters showed that male–male encounters were characterised by more negative interactions (48.5%) than female–female ones (21%, , , ). The contrary was true for non-interactive behaviour, which accounted for 14% of the female–female and 3.1% of the male–male encounters (, , ). There were no significant differences between data for male–male and for female–female encounters for the other categories of interactions (Fig. 1).
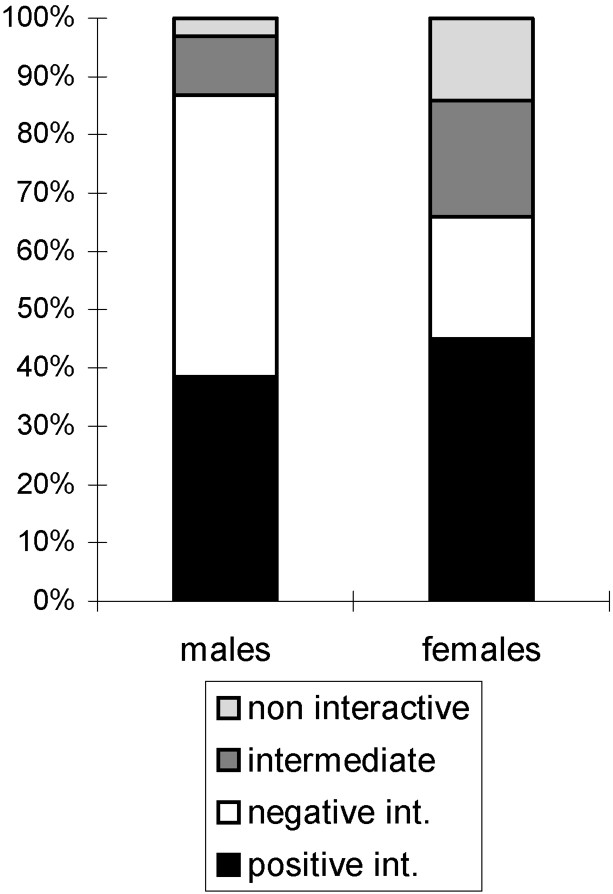
Sex effect. Respective proportions of different interactions in the European polecat depending on the sex of individuals.
3.2 Age effect
Male–male, female–female, and male–female encounters were analysed, yielding 270 encounters for each age series. Comparisons between data for the two age groups studied (i.e. 50 and 70 days) highlighted the fact that incidences of negative interactions tended to increase with age (Fig. 2, , ), and so did non-interactive behaviour (, ). In contrast, incidences of intermediate and positive interactive behaviour decreased significantly with age (, ; , ).
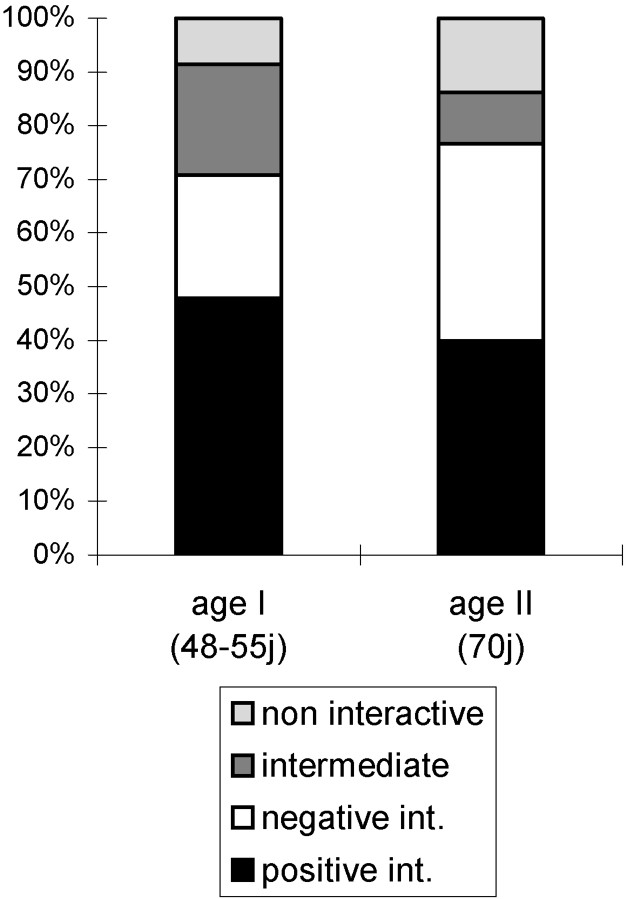
Age effect. Respective proportions of different interactions in the European polecat depending on the age of individuals.
3.3 Kin effect
In groups 2 and 4, all animals were unfamiliar, but were either kin (group 2) or non-kin (group 4). Considering all types of encounters (male–male, male–female, female–female for both age series), the proportion of positive interactions between unfamiliar kin did not differ significantly from that observed between unfamiliar non-kin (, , ). These results were not significant as well for male–male encounters, as for female–female or for male–female encounters (respectively, , , , ). There were no differences in the proportion of negative interactions between unfamiliar kin and unfamiliar non-kin (, , ). There were no significant differences regarding every category of encounters (male–male , female–female , male–female , ).
In groups 1 and 3, all animals were familiar, but differed in kinship. No significant differences were noted between animals from groups 1 and 3 as regards the proportion either of negative or of positive interactions (respectively, , , and , , ). These findings showed no differences regarding every category of encounters (positive interactions: male–male , , female–female , , male–female , ; negative interactions: male–male , , female–female , , male–female , ).
As no significant kin effect could be evidenced, data were pooled for encounters between, on the one hand, familiar animals (groups 1 and 3), and, on the other hand, unfamiliar animals (groups 2 and 4). Therefore, data for interactions between familiar animals (groups 1 and 3) could be compared to those for interactions between unfamiliar animals (groups 2 and 4).
3.4 Familiarity effect
Familiarity influenced significantly the proportion of positive and negative interactions observed during male–male and female–female encounters (familiar/unfamiliar: , for every encounter, Fig. 3).
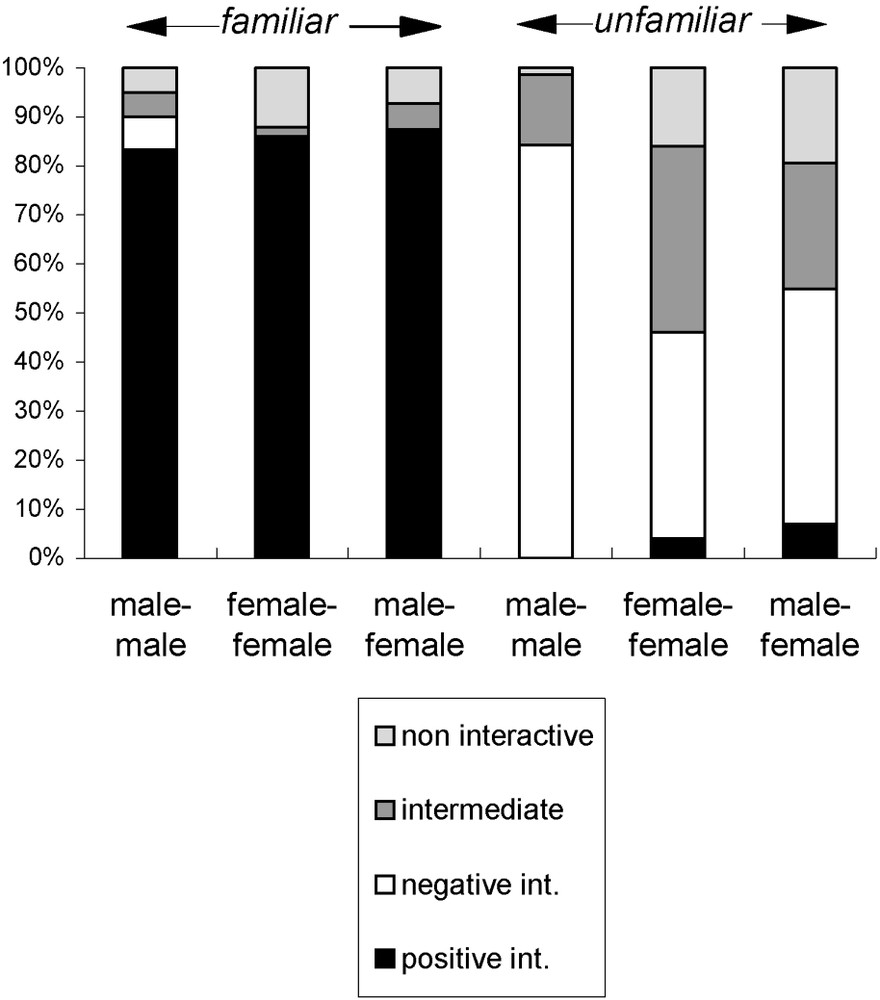
Familiarity effect. Respective proportions of different interactions in the European polecat depending on the familiarity of individuals.
Familiarity also influenced significantly the proportions of all four categories of interactions in male–female encounters (positive , , , negative , , , intermediate , , , non-interactive , , ).
Familiarity influenced significantly the proportion of intermediate interactions, but only in female–female encounters (, , Fig. 3).
4 Discussion
This study raised several issues for kin recognition.
- (1) The reactions of females clearly differed from those of males, who were always more aggressive. This aggression has a functional significance in terms of reproductive strategy by favouring a male to assert his territoriality [32,33]. The lower level of aggressiveness in interactions between females could be conveyed by asserting territoriality less than males.
- (2) Non-interactive behaviour as well as aggression increased with age, thereby reinforcing the polecat's individualistic tendency [25,27].
- (3) Kinship did not influence significantly the behaviour of polecats when they were raised under similar conditions; sibling separated soon after birth behaved like separated non-kin. Therefore, there is no clear behavioural discrimination of kin in polecats, thus excluding the presence of any mechanism of kin recognition without prior experience. However, although they were not statistically significant, differences tend to emerge between related and unrelated groups concerning the proportion of positive and negative interactions, and the possibility that, under different conditions, kinship may influence interactions more cannot be ruled out.
- (4) The behaviour of animals raised together clearly differed from that of animals raised separately. Animals raised together were more tolerant of each other in that they exhibited more positive interactions and less negative interactions compared to animals raised apart. There is therefore a familiarisation process that is not modulated, or only slightly, by kinship, since interactions between siblings raised together and those between non-siblings raised together did not differ significantly.
Young animals raised together learn to recognise one another; this recognition through prior experience implies familiarisation by allo-reference. This distinction between familiar versus unknown individual explains their subsequent behavioural discrimination (i.e. a particular behaviour according to the familiar/unknown status of the conspecifics encountered). Increasing the benefit of territoriality, individual recognition by familiarisation may allow reducing the intensity of agonistic encounters. The importance of experience acquired during the first encounters can be determining in mechanisms of kin recognition [34–36], and Taylor and Irwin [37] showed that altruism might be promoted by overlapping generations. Erhart et al. [38] suggested that social learning and social history are the most likely mechanisms for kin recognition. Other authors such as [39,40] or Aragon et al. [6] stressed that familiarisation mechanisms can play an important role in the social biology of a species, as does real kinship. Thus, coalition behaviour or cooperative reproduction (helpers at the nest) has been observed even in the absence of kinship, cooperative foraging being a key factor influencing social tolerance [29,41–44].
In social species, such as baboons [45] and vervets [46], unrelated animals show a sort of cooperation called ‘reciprocal altruism’. In the ferret, Mustela furo, scent molecules emitted by the anal gland differ between males and females [47]. Furthermore, differences in concentrations of constituents of these scents provide information about the identity of animals within a species, thereby allowing them to distinguish the sex of conspecifics and to find out whether or not they are familiar [47].
Mutual tolerance could be expressed at a very young age, thereby inhibiting, at least partially, antagonisms between animals that are familiar with one another. Because polecats' responses from kin versus non-kin did not differ, my results suggest that polecats referred to their own odour (self-referent phenotype matching). Analyzing kin recognition in golden hamsters, Heth et al. [19] and Mateo and Johnston [48] also concluded that self-referent phenotype matching is involved in specific recognition. Actually, some species such as baboons recognize kin only when they live in maternal association [38]. In polecat, familiarity may be used to recognise littermates, regardless they are kin or non-kin, and this discrimination may favour a kin facilitation effect for mate choice and territory acquisition in females or in competition in males. Juvenile polecats raised apart showed intolerance towards conspecifics during encounters. This aggressiveness is probably linked to the individualism of polecats [27]. A similar process was also evidenced in social species. In polycalic ants, the weak antagonism between neighbour colonies was attributed to their genetic relatedness [49], but Langlen et al. [50] argued that the decrease in aggressiveness mainly results from habituation effect acting as a ‘dear enemy’ effect [51]. The intraspecific aggression and the individualism may have an adaptive significance when animals compete for restricted resources. Because kin recognition allows one to discriminate between relatives [13,14], inbreeding avoidance is another significant benefit of specific recognition of littermates. This antagonism induces a territorial way of life: intrasexual territoriality accompanied by a temporary form of sexual segregation for habitat exploitation, females avoiding frequenting the same sites as males do [20,25]. Furthermore, since familiarity with conspecifics increases tolerance in polecats, one could expect that animals possessing close or adjoining territories would be more likely to come from the same litters. Thus, Allen and Sargeant [52] showed that red fox littermates tended to disperse in similar directions. This settling in close proximity would not be without consequences on the genetic structure and evolution of populations [2].
Kin selection theory provides successful explanations for a wide range of phenomena, but my results suggest that multiple mechanisms running simultaneously might be involved in social behaviours. It may be argued that recognition is chiefly based on familiarisation rather than constituting the evolution of a specialized kin recognition system. Familiarisation in polecat may act as a cognitive form of recognition supporting the Tang-Martinez's conjecture [30], asserting that kin discrimination results from an extension of other, non-specialized sensory and cognitive abilities of animals. Anyway, tolerance through familiarisation could be expressed even in a species where, until now, the individualistic character of animals has been emphasised, thus underlining that solitary species may provide significant information on social life.


