1 Introduction
Citrus fruit trees present mainly diploid species with a chromosome haploid number of
Citrus rootstocks are propagated by polyembryonic seeds. Polyembryony arises from adventious embryogeny from nucellar cells of the ovule and results in partial apomixis. It was observed that in polyembryonic seeds, the frequency of spontaneous tetraploids varies from 1 to 3% [5]. Tetraploidy seems to be under genetic control and the rate of polyploids obtained can be modified by physiological and environmental factors [5]. Tetraploids are the result of a chromosome stock doubling of the nucellar tissues [6]. In spite of its aptitude to produce a tetraploid seedling with relatively high frequency, tetraploidy seems to have played a negligible part in the evolution of citrus fruits [7]. In citrus fruits, it is worth noting that autetraploid cultivars have a generally poorer yield, a thicker and an irregular fruit bark [8] and then present little interest for human consumption. However, such a phenotype affecting fruit quality is not observed in fruit of allotetraploid scion cultivars [9]. In the field, the use of tetraploid rootstocks is usually associated to a lower growth of the trees. However, the investigation of polyploids as a new source of rootstocks for biotic and abiotic stress tolerance is poorly documented. In ligneous plants such as plum tree and paper birch, it has been shown that tetraploid, and even pentaploid and hexaploid plants, are more tolerant to water deficiency than their corresponding diploid [10,11].
Citrus rootstock tolerance to salt has been investigated for decades. For instance, it has been observed in the field that yield reduction occurs at relatively low salinity levels [12–16]. Burnt leaf symptoms are usually associated with chloride leaf accumulation which causes nutritional imbalances and then senescence and leaf abscission [14,17]. Higher toxicity can also lead to defoliation, twig necrosis and canopy thinning. Salt stress will also decrease the size and the number of fruits that are produced [18]. In short, one can say that the salinity tolerance range for the mainly used rootstocks is the following: Cleopatra mandarin (Citrus reshni Hort. ex Tan.) > Rangpur lime (Citrus limonia Osbeck) > Citrus macrophylla (Citrus macrophylla Wester) > Sour orange (Citrus aurantium [L.]) > Sweet orange (Citrus sinensis [L.] Osbeck) = Swingle citrumelo (Citrus paradisi Macf × Poncirus trifoliata [L.] Raf.) > Citrus Volkameriana (Citrus volkameriana Ten) = Rough lemon (Citrus jamhiri Lush) > Carrizo citrange (Citrus sinensis [L.] Osbeck × Poncirus trifoliata [L.] Raf.) > Poncirus trifoliata (Poncirus trifoliata [L.] Raf.) [19]. Rangpur lime and Cleopatra mandarin appear to be chloride excluders [19–21] whereas Poncirus trifoliata and its hybrid appear to be sodium excluders [19,22]. It is important to note that citrus trees do not exclude sodium and chloride ions in the mean time [23] and regulatory mechanisms for uptake and transport of chloride and sodium appear to be different [24]. In the citrus industry, the main argument of the use of chloride rootstock excluders is that this criterium is heritable. Most of the time, it confers tolerance to the grafted variety; even so, the effectiveness of the tolerance will also depend of the physiological properties of the variety and the graft itself.
In this work, growth rate, sodium and chloride content of nucellar diploid and tetraploid citrus trees submitted to a salt stress were investigated.
2 Materials and methods
2.1 Plant material
Seedlings were provided by the INRA-CIRAD station of Corsica, France. Three different diploid rootstocks of Poncirus trifoliata (Poncirus trifoliata [L.] Raf.; SRA 1074), Carrizo citrange (Citrus sinensis [L.] Osbeck × Poncirus trifoliata [L.] Raf.); SRA 796), and Cleopatra mandarin (Citrus reshni Hort. ex Tan.; SRA 948) have been selected on the basis of their wide range of tolerance towards salinity. Hundreds of seeds of the different genotypes were grown in the greenhouse in pots filled with a mixture of 2/3 perlite with 1/3 vermiculite. Tetraploid rootstocks were identified by flow cytometry among diploid seedlings. The genetic constitution was analysed with 5 simple sequence repeat (SSR) markers (Ci01H05, mCrCIR01E02, mCrCIR07E05, mCrCIR08A03 for Poncirus trifoliata; Ci01H05, mCrCIR01E02, mCrCIR06A8, mCrCIR07E05, mCrCIR08A03 for Carrizo citrange; mCrCIR01F04a, mCrCIR06A8, mCrCIR07E05, mCrCIR08A03, mCrCIR06B05 for Cleopatra mandarin) according to Froelicher et al. [25]. Twenty diploid and tetraploid plants of each genotype were then selected and transferred in a growth chamber. The temperature ranged between 16–18 °C at night and 25–27 °C during the day; relative humidity was maintained at 80% and the light/dark period was 16/8. Plants were irrigated twice a week with half diluted Hoagland solution [26]: 0.40 g/L KNo3; 0.82 g/L Ca (No3)2 4H2O; 0.40 g/L MgSO4. 7H2O; 0.07 g/L H3PO4; 2.86 mg/L H3BO3; 2.20 ZnSO4⋅ 7H2O; 0.09 mg/L MoO3; 0.11 mg/L CuSO4⋅4H2O; 9.15 mg/L MnSO4 and 67.0 mg/L Fe-Sequestrene. For each genotype, five plants of the same height were selected as control plants and five others were subjected to salt stress. Salt stress was applied by soaking pots twice a week with half diluted Hoagland solution complemented with 50 mM of NaCl for 47 days. Control plants were soaked in half diluted Hoagland solution.
2.2 Plants sampling and mineral analysis
Plant height and number of leaves were measured and counted every 10 days. The experiment was stopped when symptoms (necroses, burn and leaves fall-down) appeared for Poncirus trifoliata that is known to be one of the most salt stress sensitive rootstock. Plants samplings were fractionated into roots, stems and leaves. Those fractions were oven-dried at 60 °C for several days, weighed, crushed in a hammer-mill and stored at room temperature. Mineral analyses were carried out on dried leaves, stems and roots. Samplings were ground and 0.5 g of powder was burnt at 400 °C for 4 hours. The resulting ashes were dissolved in 100 mL of 0.5N concentrated nitric acid. Cations analysis was carried out by Inductively Coupled Plasma-Mass Spectometer (ICP) at the “Office de l'équipement hydraulique de la Corse”. The sodium content was expressed in mg/g of dry material, and the chloride concentration was determined using a specific chloride electrode (Orion, 9417BN). Chloride content was expressed in mg/g of dry material.
2.3 Statistical analysis
Data analysis (linear regressions, slopes values and
3 Results
3.1 Tetraploid frequency and genetic homogeneity analysis
Using flow cytometry, tetraploids were screened among plantlets from mother trees of the San Guiliano germplasm. The percentage of tetraploid seedlings from diploid genotypes is presented in Table 1. On polyacrylamide gels, all diploid and tetraploid seedlings presented the same SSRs profile as their parental tree, attesting to their nucellar origin (data not shown). Plants were then grown for 6 months and 10 diploid and tetraploid homogeneous plants were selected for the salt stress experiment.
Percentage of tetraploid citrus plants in diploid seedlings from the INRA-CIRAD germplasm of San Giuliano, Corsica
| Variety | Accession | Number of seedlings | % of tetraploid |
| Poncirus trifoliata | SRA 1074 | 1630 | 5.4 |
| Carrizo citrange | SRA 796 | 908 | 7 |
| Cleopatra mandarin | SRA 948 | 771 | 4 |
3.2 Growth rate and leaf number
At the beginning of the experiment, tetraploid plants were significantly smaller when compared to their corresponding diploid (Fig. 1). In the growth chamber, tetraploid control plants of Poncirus trifoliata, Carrizo citrange and Cleopatra mandarin presented a faster growth than diploid. Indeed, at the end of the experiment, tetraploid were as tall as the diploid, or were even taller (Fig. 1).

Growth rate of two salt-sensitive, Poncirus trifoliata and Carrizo citrange (A and B, respectively) and salt-tolerant, Cleopatra mandarin (C), diploid and tetraploid rootstocks, grown for 47 days in control and salt stress conditions. (•: diploid / control condition; ○: diploid / stress condition; ▾: tetraploid / control condition; ∇: tetraploid / stress condition). Vertical bars indicate the mean value ± SD.
Diploid and tetraploid plants were submitted to a 50 mM salt stress for 47 days. At the end of the experiment, the plant size of diploid and tetraploid Poncirus trifoliata grown in control and stress conditions were significantly different. The growth rate of diploid Poncirus trifoliata was much more affected by salt stress than for tetraploid Poncirus trifoliata since the ratio of the slopes (growth rate in control condition / growth rate in salt stress condition) obtained from linear regressions was greater for diploid (1.87) when compared to tetraploid (1.45) (Fig. 1A, Table 2). At the same time, no statistical difference of the plant height was observed for Carrizo citrange, whatever the ploidy level or the growth condition. Diploid of Poncirus trifoliata and Carrizo citrange plants presented chlorosis symptoms but no symptom was observed for their respective tetraploid genotypes. At the end of the experiment, it is worth noting that only the stressed plants of Poncirus trifoliata presented a lower number of leaves when compared to the control plants (
Growth rate analysis of diploid and tetraploid genotypes grown in control and salt stress conditions. Slope values were calculated from the linear regression obtained from data of Fig. 1
| Variety | Ploidy level | Growth condition |
Slope value /
|
Slope ratio of plants grown in control condition / stress condition |
| Poncirus trifoliata | 2X | Control | 0.15/0.88 | 1.87 |
| Stress condition | 0.08/0.87 | |||
| 4X | Control | 0.48/0.92 | 1.45 | |
| Stress condition | 0.33/0.91 | |||
| Carrizo citrange | 2X | Control | 0.07/0.92 | 1.40 |
| Stress condition | 0.05/0.97 | |||
| 4X | Control | 0.18/0.98 | 1.29 | |
| Stress condition | 0.14/0.97 | |||
| Cleopatra mandarin | 2X | Control | 0.03/0.86 | 1.00 |
| Stress condition | 0.03/0.85 | |||
| 4X | Control | 0.08/0.95 | 0.80 | |
| Stress condition | 0.10/0.99 |

Number of leaves of diploid and tetraploid plants after 47 days of growth in control and salt stress conditions. Vertical bars indicate the mean value ± SD, * indicate a statistical significant difference between the number of leaves of control and salt stressed plants for each couple of diploid and tetraploid genotype.
The salt stress did not induce any chlorosis symptom in diploid and tetraploid Cleopatra mandarin plants. Also, it did not limit the growth of both genotypes (Fig. 1C). Indeed, after 47 days of stress, tetraploid Cleopatra mandarin plants presented significant statistical differences of height when grown in control and salt stress conditions and the ratio of the slopes (control condition / salt stress condition) obtained from the linear regressions of tetraploid Cleopatra mandarin was less than 1 (Fig. 1C; Table 2). On Fig. 2 is presented a picture of the diploid and tetraploid Cleopatra mandarin plants grown in control and stress condition for 47 days. The right picture shows that the salt stress has promoted the growth of tetraploid Cleopatra mandarin plants. However, the number of leaves of tetraploid plants was smaller when compared to diploid whatever the growth conditions suggesting a faster development of the internodes of the stem in tetraploids (Fig. 3).

Pictures of diploid (left picture) and tetraploid (right picture) Cleopatra mandarin rootstocks grown in control and salt stress conditions for 47 days.
3.3 Ion contents
Ion contents were investigated in the root, leaf and stem samplings. Stem ion contents are not presented since no significant change of ion accumulation occurred. Only results for sodium and chloride are presented on Fig. 4 since the other cations did not present significant changes. For Poncirus trifoliata, Carrizo citrange and Clepoatra mandarin, sodium content increased similarly in leaves for diploid and tetraploid plants (Fig. 4A). At the root level, only diploid and tetraploid of Poncirus trifoliata and tetraploid of Cleopatra mandarin presented a greater sodium accumulation when submitted to salt stress (Fig. 4C).
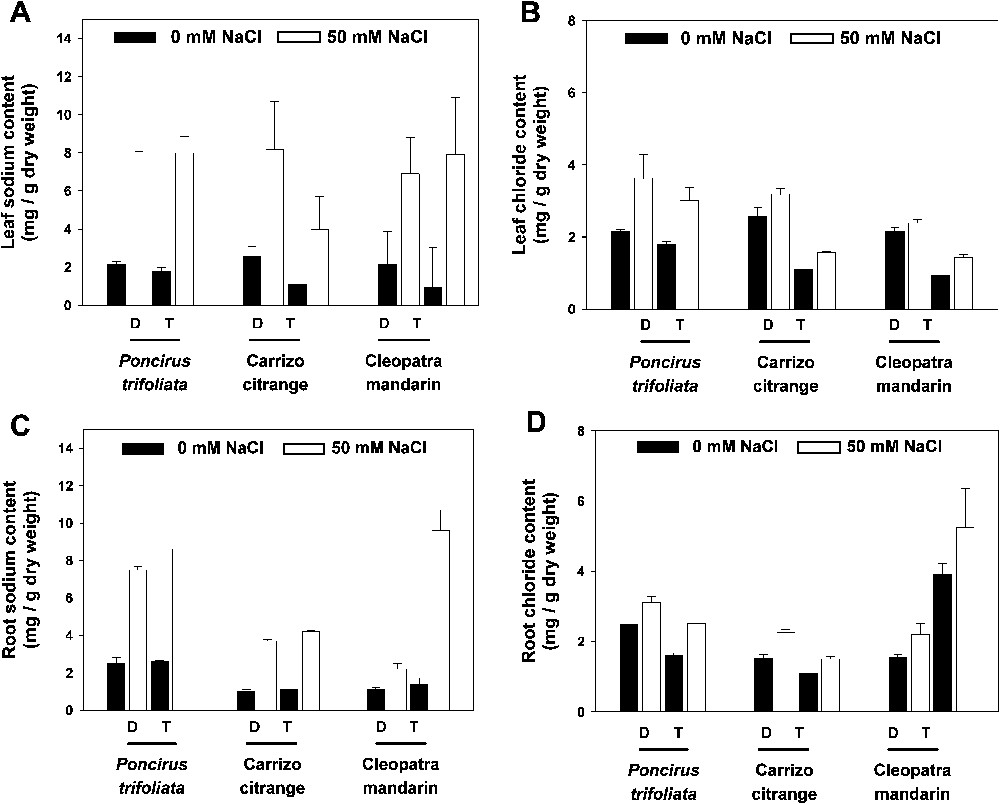
Sodium and chloride content (mg/g dry weight) of leaves (A and B) and roots (C and D) from diploid and tetraploid plants (D: Diploid; T: Tetraploid) after 47 days of growth in control and salt stress conditions. Vertical bars indicate the mean value ± SD.
Concerning the chloride ion distribution in leaves, no significant difference was observed between diploid and tetraploid Poncirus trifoliata with quite the same profile of accumulation in roots. For Carrizo citrange and Cleopatra mandarin, the accumulation in leaves was significantly lower (
4 Discussion
In order to compare salt stress tolerance of tetraploid rootstocks to their corresponding diploid, we looked for polyploid plantlets among the seedlings. Depending on the genotype, the percentage of tetraploids ranged from 4 to 7 percent, which is quite high (Table 1). If the germination of tetraploids when compared to diploid plants was not delayed, the growth rate was usually smaller, leading to smaller plants for all the investigated genotypes (Fig. 1). Analysis of the genetic conformity of the plants using SSR molecular markers did not reveal any zygotic plants. Our results suggest that the nonconformity of the citrus rootstocks which may be encountered in the field is mainly due to the presence of tetraploid plants. Plants of the same height of each genotype were then investigated for salt stress tolerance. In control growth conditions, tetraploid of Poncitrus trifoliata, Carrizo citrange and Cleopatra mandarin presented a faster growth than diploid genotypes, suggesting that the high humidity associated to the high temperature and artificial light of the growth chamber promoted the growth of tetraploid plants.
Salt stress for 47 days affected the growth of diploid and tetraploid plants of Poncirus trifoliata and Carrizo citrange when compared to control conditions (Fig. 1; Table 2). Contrary to most plant species, salt-induced damage in citrus leaves are not associated to excess of sodium accumulation but to chloride ions [27–30]. Indeed, sodium accumulations were not very different between the diploid and tetraploid genotypes we studied. It is interesting to note that leaf fall was observed at the end of the experiment only for Poncirus trifoliata diploid but for tetraploid showing clearly the better tolerance of tetraploid plants (Fig. 3). Also, for Poncirus trifoliata and Carrizo citrange, symptoms of chlorosis were observed only for diploid plants. The leaf chloride accumulation of Poncirus trifoliata tetraploid was not significantly lower than for diploid. However, because of leaf fall, and the use of leaves still attached to the tree for ion content analysis, we cannot exclude that the chloride content was underestimated in diploid Poncirus trifoliata. For Cleopatra mandarin, during the whole duration of the experiment, salt stress promoted the growth of the tetraploid plants when compared to control conditions (Figs. 1 and 2, and Table 2). Indeed, for Carrizo citrange and, above all, for Cleopatra mandarin plants, chloride accumulation was significantly lower for tetraploid when compared to diploid. Cleopatra mandarin is one of the most salt tolerant rootstock known to be a chloride excluder [31]. Moya et al. [30] have proposed that chloride ions are translocated in the leaves throughout the transpiration stream. So, we may suppose that the greater growth rate of Cleopatra mandarin tetraploid, when compared to diploids, is in relation to the lower accumulation of this ion in the leaves. Curiously, a greater accumulation of chloride and sodium was observed in root samplings of tetraploid Cleopatra mandarin. Hence, it would be necessary to monitor ionic concentrations at the cellular level in order to check if specific ions compartmenting at the root level is involved and may explain why chloride ions are note translocated to the leaves in tetraploid Cleopatra mandarin plants.
Moreover, since tetraploids have specific behaviour such as thicker leaves, a difference in the number and size of the stomata when compared to their respective diploid plants [11], one may expect that those anatomical traits could lead to better adaptation to abiotic stresses as it has been observed in paper beach [11]. In plume, Pustovoitova et al. [10], observed that tetraploids are more tolerant to water deficiency, and this tolerance is associated to a greater ABA synthesis. From the seed to the plant, if polyploids synthesise more ABA, it could explain the reduced growth we observed in tetraploid when grown in the greenhouse or in the field. Better regulation of the transpiration associated to a better osmotic adjustment in water stress conditions may explain the greater tolerance observed in prune and wheat polyploid [10,32].
Finally, it is required to study the comportment of those tetraploid genotypes when grafted in order to verify if the greater tolerance to water stress is also observed for the association. If this hypothesis is verified, tetraploids could be a new source of rootstocks for the citrus industry.
Acknowledgements
We thank F. Luro and S. Jaffuel for helpful discussions and C. Jacquemond, F. Curk and Jean-Marc Gandoin (Unité GEQA, INRA, San Giuliano, France) for providing diploid seedlings and technical assistance. François Santoni from Office de l'équipement hydraulique de la Corse (OEHC) is also thanked for ICP assays.



Vous devez vous connecter pour continuer.
S'authentifier