1 Introduction
In his revision of the African snake genus Dasypeltis and further works on these egg-eating snakes, Gans [1–3] recognized six species, with two of them distributed in West Africa: D. scabra (Linnaeus, 1758), a savannah species with a wide distribution in most parts of Africa, and D. fasciata Smith, 1849, a species confined to the forest belt of West and Central Africa. Although this author recognized only two species in West Africa, he distinguished four colour patterns in this part of Africa, two among D. scabra and two among D. fasciata [1,3]. D. scabra was characterized by a dark dorsal rhombic pattern and comprised two groups of specimens, those with the lateral bars linked to the dorsal saddles (=“5L”), and those with these pattern elements alternating, thus “not linked” (=“5N”). D. fasciata was characterized by a series of square dorsal blotches with a row of rectangular lateral bars on each side, the pattern being produced by a darkening of the skin between the scales and the pigmentation of the lower edges of these, but this pattern was only typical of the specimens of the forest belt and a light coloured phase of D. fasciata was distributed in coastal areas of The Gambia and Senegal [1–3].
The taxonomy of the genus remained unchanged until Trape and Mané [4] described three new species and a new subspecies with a wide distribution in West Africa: D. confusa, which represented the “5L” form of Gans [1] and was distributed in wet savannah areas of West and Central Africa, D. gansi gansi, which corresponded to the light colored phase of D. fasciata and was distributed in dry savannah areas, D. gansi latericia, a previously undescribed reddish phase of the latter species that was found in several lateritic dry savannah areas, and D. sahelensis which included all specimens from the Sahel with a variant of the “5N” pattern where scales are only partially pigmented, a variant not mentioned by Gans [1] but clearly distinct from the very contrasted “5N” pattern observed in some specimens from wet savannah areas of West Africa. Since the latter pattern was similar to the one observed among typical D. scabra from Southern Africa, the status of the contrasted “5N” specimens from West Africa was considered unclear and required further investigations [4].
In this article, we investigate the phylogenetic relationship among the six color phases of the genus Dasypeltis in West Africa and we describe a new species in the D. scabra complex.
2 Material and methods
2.1 Specimens examined, colour patterns and morphological characters
The material examined consisted mainly of the collection accumulated by one of the authors (J.F.T.) between 1990 and 2010 (359 specimens from Senegal, 75 from Guinea, 68 from Niger, 48 from Mali, 28 from Benin, 14 from Togo, three from Cameroon, one from Mauritania) (Appendix 1). Most of this material is preserved at the Centre de Recherche IRD-UCAD de Hann in Dakar, Senegal (acronym: IRD), but a minority, in particular the types of the new species described in this article and in Trape and Mané [4], were deposited in the Muséum national d’Histoire naturelle de Paris (MNHN) or the Institut Royal des Sciences Naturelles de Bruxelles (IRSNB). Other specimens from West, Central or North Africa examined as part of this study are preserved at the Institut Fondamental d’Afrique Noire in Dakar (IFAN), the Centre National de la Recherche Scientifique et Technique in Ouagadougou (CNRST), the École Pratique des Hautes Études in Montpellier (BEV), and the MNHN in Paris (including formerly CAMHERP specimens).
Nomenclature of colour patterns (Fig. 1) and counts of pattern cycles follow Gans [1–3], Trape and Mané [4], Bates and Broadley [5] and Broadley and Bates [6]. Other characters used – number of ventrals, number of caudals, number of dorsal scale rows, type of head pitting, lateral serration, anal serration, depth of interprefontal sulcus, counts in head scales, number of labials entering the orbit, body and tail length – are those previously utilized by Gans [1,3].

Diagrams of eight colour patterns recognized by Gans [1]. 1: “linked scabra (5L)”; 2: “non-linked scabra (5N)”; 3: “intermediate scabra”; 4: “fasciata”; 5: “loveridgei”; 6: “palmarum”; 7: “medici”; 8: “lineolata”. Colour patterns of West African specimens studied by this author were classified either as “linked scabra (5L)”, “non-linked scabra (5N)”, “fasciata” or “light tan fasciata”.
2.2 Molecular study
We obtained tissues from specimens of each species, subspecies and colour pattern currently known in West Africa and/or Cameroon. We also obtained tissues from specimens of D. scabra from South Africa (Blouberg), where the type of this species was originated [7], and from Burundi. DNA from homogenized pieces of snake muscles conserved in ethanol (around 100 mg) was extracted using the BioRobot MDx Workstation (Quiagen, Courtaboeuf, France), with customized extraction protocol following the manufacturer's instructions and stored at 4 °C until use in Polymerase chain reactions (PCR) amplifications.
We amplified a portion of the 16S mt rDNA gene with the universal vertebrate primers 16SA-2290, CGCCTGTTTACCAAAAACAT and 16SB-2860, CCGGTCTGAACTCAGATCACGT [8]. Primers were manufactured by Eurogentec, Seraing, Belgium. PCR were performed in automated DNA control cyclers (GeneAmp 2400 and 9700; Applied Biosystems, Foster City, CA, USA). The PCR was performed using a HotStar Taq DNA Polymerase Kit (Qiagen) with 1.0 μL MgCl2, 0.2 μL HotStart Taq, 2.5 μL 10× PCR buffer, 2.5 μL dNTP (2 mM stock), 0.5 μL of a 10 μM solution of each primer, 12.8 μL sterile water and 5 μL of DNA. Cycling conditions were as follows: 94 °C 2 minutes followed by 40 cycles of 94 °C, 30 s; 53 °C, 30 s; 72 °C, 1 min.; the final elongation was at 72 °C; 3 min. We did not use positive controls. Sterile water was used as negative control. PCR products were visualized by electrophoresis on a 1.5% agarose gel, stained with ethidium bromide and examined using an ultraviolet transilluminator. The PCR products were purified using a QIAquick Spin Purification Kit (Quiagen) according to the manufacturer's instructions. Sequencing of amplicons was performed using the BigDye Terminator Cycle Sequencing Kit (Perkin Elmer Applied Biosystems) with ABI automated sequencer (Applied Biosystems). Obtained sequences were assembled (ChromasPro 1.49beta, Technelysium Pty Ltd, Tewantin, Australia), edited by BioEdit sequence alignment editor v.7.0.9.0 [9] and compared with those available in GenBank by NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences of mitochondrial 16S rDNA from studied snakes were concatenated and aligned with CLUSTAL W program, and then corrected manually to preserve conserved motifs. The evolutionary history was inferred using Bayesian phylogenetic analysis [10] by TOPALi 2.5 software (Biomathematics and Statistics Scotland) with integrated MrBayes application (http://mrbayes.csit.fsu.edu/) with GTR +Г substitution model for the whole alignments. The substitution model was chosen with FindModel online application (http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html) that uses Modeltest script. The sequence of Psammophis phillipsi (voucher IRD 355-T from Huiléhui, Togo) was used as outgroup. All sequenced amplicons were registered in Genbank (Table 1).
List of vouchers for molecular studies and GenBank accession numbers.
| Species | Collection number | Country | Localitya | GenBank |
| D. parascabra | IRD 1555-G | Guinea | Dalakan | JQ801307 |
| D. parascabra | IRD 1613-G | Guinea | Dalakan | JQ801308 |
| D. confusa | IRD 254-T | Togo | Mont Agou | JQ801313 |
| D. confusa | IRD 116-T | Togo | Fazao | JQ801322 |
| D. sahelensis | BEV 9448 | Morocco | Tan-Tan | JQ801329 |
| D. sahelensis | BEV 9449 | Morocco | Mirleft | JQ801328 |
| D. sahelensis | IRD 309-N | Niger | Piliki | JQ801315 |
| D. sahelensis | IRD 315-N | Niger | Piliki | JQ801316 |
| D. sahelensis | IRD 1703-N | Niger | Tarka | JQ801309 |
| D. sahelensis | IRD 1704-N | Niger | Tarka | JQ801310 |
| D. gansi | IRD S-6811 | Senegal | Kabrousse | JQ801319 |
| D. gansi | IRD S-7819 | Senegal | Kountanto | JQ801320 |
| D. gansi | IRD S-7826 | Senegal | Kountanto | JQ801321 |
| D. gansi | IRD 331-N | Niger | Piliki | JQ801317 |
| D. fasciata | IRD TR-3692 | Cameroon | Sissor | JQ801332 |
| D. fasciata | IRD TR-3793 | Cameroon | Sissor | JQ801333 |
| D. fasciata | IRD TR-3794 | Cameroon | Kendem | JQ801334 |
| D. fasciata | IRD 28-T | Togo | Yo | JQ801314 |
| D. fasciata | IRD 357-T | Togo | Diguengué | JQ801318 |
| D. fasciata | IRD 2107-G | Guinea | Nzébéla | JQ801311 |
| D. fasciata | IRD 2174-G | Guinea | Topapa | JQ801312 |
| D. scabra | BB T3SA | South Africa | Blouberg | JQ801323 |
| D. scabra | BB TASA | South Africa | Blouberg | JQ801324 |
| D. latericia | IRD TR-550 | Guinea | Kifaya | JQ801326 |
| D. latericia | IRD TR-2952 | Senegal | Sabodala | JQ801325 |
| D. latericia | IRD S-8611 | Senegal | Sabodala | JQ801319 |
| D. cf lineolata | BEV 109891 | Burundi | – | JQ801330 |
| D. cf lineolata | BEV 109892 | Burundi | – | JQ801331 |
| P. phillipsi | IRD 355-T | Togo | Diguengué | JQ801306 |
a See Appendix 1 for geographic coordinates.
3 Results
3.1 Phylogeography
A total of 29 specimens were included in the phylogenetic analyses. Obtained sequences were 498–505 base pairs long. Phylogenetic tree showed distinct topology with eight haplotype clades (Fig. 2). The haplotypes of D. scabra from South Africa appear clearly distinct from all West African specimens, in particular those that were until recently attributed to this species since they presented a “scabra pattern”, either the “5L” pattern typical of D. confusa, the “light colored 5N” pattern of D. sahelensis, or the “well contrasted 5N” pattern of the Guinean specimens which belong to an undescribed species. D. confusa and D. sahelensis belong to two very distinct and well supported clades, and there is more similarity between D. scabra from South Africa and the three species of the D. fasciata complex than between D. scabra and the undescribed species from Guinea which shares with D. scabra a “well contrasted 5N” pattern.

Evolutionary relationships of the Dasypeltis species. The evolutionary history was inferred using Bayesian phylogenetic analysis with GIR+T substitution model for the whole alignments based on 16S rDNA sequences (498 base pairs long). The numbers to the left of nodes are Bayesian posterior probabilities multiplied by 100. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
Within the D. fasciata complex, the molecular analysis confirms the validity of D. gansi, a savannah species with a “light tan fasciata” pattern. The subspecies D. gansi latericia, with a “reddish fasciata” pattern, appears closer to D. scabra than D. gansi and D. fasciata and thus, must be raised to a specific rank.
3.2 Dasypeltis parascabra, sp. nov.
3.2.1 Holotype
MNHN 2010.0438, previously IRD 1555-G, collected between June and October 2005 at Dalakan, Guinea (9°58′N, 9°32′W), by a villager who gave it to the last author (Fig. 3).

Dasypeltis parascabra (sp. nov.). General view of the holotype.
3.2.2 Paratypes
Five specimens, all from Dalakan, collected between June and October 2005 by villagers: IRSNB 2660, previously IRD 1557-G; IRSNB 2661, previously IRD 1613-G; MNHN 2010.0439, previously IRD 1618-G; IRD 1556-G; IRD 1619-G.
3.2.3 Diagnosis
The genetic results and the following characters: nasal entire, dark rhombic pattern with the pigment uniformly distributed on scales and the lateral bars alternating with the dorsal saddles (“not-linked scabra” pattern of Gans [1]). Head with two transversal black bars between the nasals and the eyes, and two or three V shaped black bars on the back of the head and the nape.
3.2.4 Etymology
The name of the new species refers to its close resemblance to D. scabra.
3.2.5 Description of holotype
The holotype is a gravid female of the following dimensions: total length 605 mm, snout-vent length 509 mm, tail length 96 mm, ratio total length: tail length 6.3.
Head short, weakly distinct from the neck (Fig. 4). Snout rounded. Eye medium, pupil vertical. Rostral just visible above. Two prefrontals, two internasals, the prefontals larger than the internasals. Interprefrontal sulcus faint. Frontal large, slightly longer than wide, only marginally pitted. Nasal large, longer than wide, entire on both sides of the head, with nostril opening in the middle of its anterior part. Loreal absent. A single preocular, twice as wide than long, between eye and nasal. Two postoculars. Seven supralabials, the third and the fourth in contact with the eye. 2 + 2 temporals on left, 2 + 4 on right. Six infralabials on right and seven on left, the first three contact the anterior chin shields. Ventral plates commence immediately after posterior chin shields.
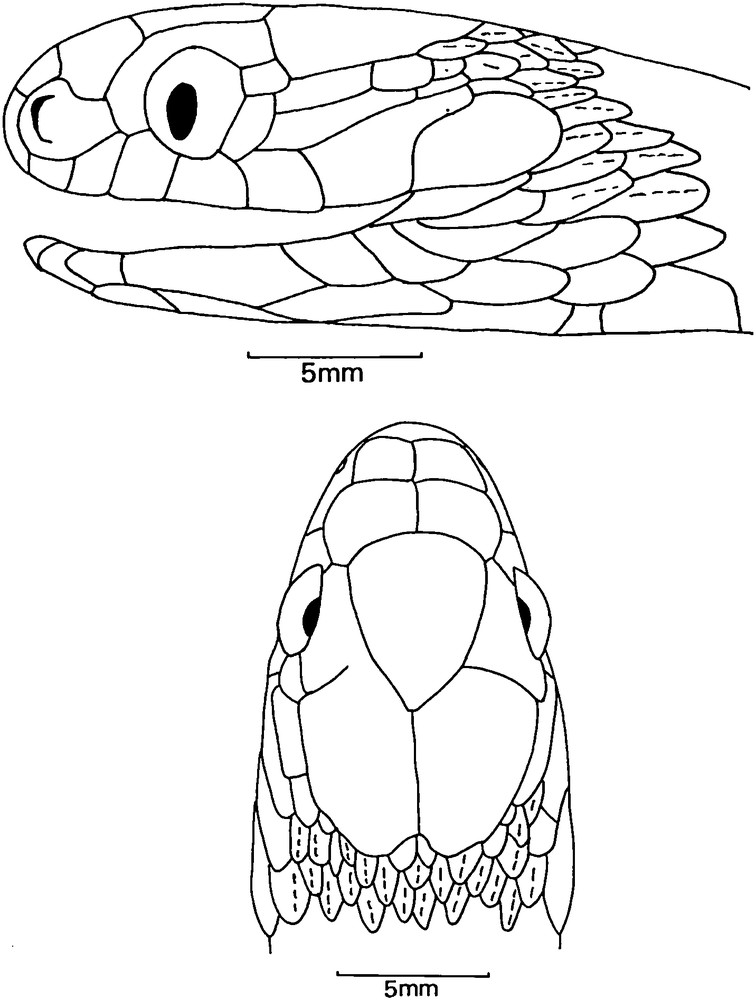
Dasypeltis parascabra (sp. nov.). Lateral and dorsal scalations of the head of the holotype.
Dorsal scales strongly keeled, in 23 rows around midbody, third, fourth, and fifth rows, counting from the ventrals on each side, reduced in size, serrated and inclined, vertebral row not enlarged. Dorsal scales in anal region faintly serrated. Ventrals 224, anal entire, subcaudals 64, all divided.
Colour pattern very contrasted (Fig. 5), corresponding to the “5N” pattern of Gans [1], with 66 black or dark brown dorsal blotches between the neck and the base of the tail, each dorsal blotch alternating with black or dark brown fine and irregular lateral bars. The pattern is produced by a darkening of the whole surface of a dozen scales for the dorsal blotches, and half or the whole surface of about ten scales for the lateral bars. The same pattern continues on the tail but become progressively indistinct toward the tip. Between the black blotches and bars, the dorsal scales are brown. On the head, in dorsal view, there are two black cross bars (one on the anterior edge of the prefrontals, and one on the anterior edge of the frontal and the supraoculars, respectively), and three V shaped black bars (starting from the middle of the frontal, the posterior edge of the frontal and the posterior edge of the parietals, respectively). The belly is cream with each ventral presenting dark markings, at least one on both side at approximately one quarter of the width of the ventral, and laterally every two or three ventrals, either as an extension of the lateral bars or more often lightly separated from the bars.

Dasypeltis parascabra (sp. nov.). Views of the head (A: lateral; B: dorsal) and the body (C: dorsal; D: ventral) of the holotype.
3.2.6 Description of paratypes
The five paratypes include:
- • one complete adult male (IRSNB 2660) of the following dimensions: total length 591 mm, snout-vent length 497 mm, tail length 94 mm, ratio total length: tail length 6.3;
- • one mutilated adult male (IRD 1556-G) comprising the posterior part of the trunk (268 mm from the trunk section to the vent) and an intact tail (77 mm);
- • three mutilated specimens comprising only the head and the anterior part of the trunk: IRSNB 2661 (193+ mm), MNHN 2010.0439 (128+ mm), IRD 1619-G (111+ mm).
All paratypes present the “5N” pattern of the holotype. The four paratypes with an intact head have the nasal entire on both sides of the head and share the following characters: pupil vertical, two prefrontals, two internasals, interprefrontal sulcus faint, frontal only marginally pitted, loreal absent, a single preocular, two postoculars, seven supralabials with the third and the fourth in contact with the eye (but six on one side, with the second and the third in contact with the eye in IRSNB 2660), 2 + 3 temporals, seven infralabials with the first three in contact with the anterior chin shields.
All paratypes have dorsal scales strongly keeled, in 22 rows around midbody for the two males and in 21–23 rows at the trunk section or the nearest of estimated midbody for the other paratypes. The third, fourth, and fifth rows, counting from the ventrals on each side, are always reduced in size, serrated and inclined. The intact male has 216 ventrals and 67 subcaudals. The other male with an intact tail has 69 subcaudals. In both specimens the dorsal scales in anal region are faintly serrated.
The colour pattern is always very contrasted, corresponding to the “5N” pattern of Gans [1] and similar to the holotype, with black or dark brown dorsal blotches between the neck and the base of the tail, each dorsal blotch alternating with black or dark brown fine and irregular lateral bars. The number of dorsal blotches is 62 in the intact male. For each specimen, the pattern is produced by a darkening of the whole surface of a dozen of scales for the dorsal blotches, and half or the whole surface of about ten scales for the lateral bars. Between the black blotches and bars, the dorsal scales are brown. On the head, in dorsal view, there are always two black cross bars, one on the anterior edge of the prefrontals, and one on the anterior edge of the frontal and the supraoculars, respectively), and two or three V shaped black bars (starting from the middle of the frontal, the posterior edge of the frontal and the posterior edge of the parietals, respectively, with the latter not V shaped in two specimens). The pattern of the belly of the paratypes is similar to the holotype, with each ventral presenting dark markings, at least one on both side at approximately one quarter of the width of the ventral, and laterally every two or three ventrals.
3.2.7 Comparison with other species
Two other species in West Africa present a “scabra” pattern: D. sahelensis Trape and Mané, 2006 and D. confusa Trape and Mané 2006. D. sahelensis and the new species are unique in the genus Dasypeltis in possessing an entire nasal. The colour pattern of D. sahelensis is “5N”, but much less contrasted than the new species since the pigment in D. sahelensis is mainly distributed on the skin between the scales and on the lateral and posterior edges of the scales. In addition, the lateral bars are smaller, the markings on the head are different (Y shaped in D. sahelensis versus V shaped in D. parascabra) and D. sahelensis is the only species with a median black stripe on the frontal (Fig. 6). Furthermore, the number of subcaudals is lower in D. sahelensis – average 51.6, maximum 58 in females; average 59.7, maximum 67 in males (Table 2) – than in the new species (female: 64; males: 67 and 69). D. confusa is sympatric with D. parascabra in Dalakan. It differs from the new species by its pattern “5L” instead of “5N” and in having a semi-divided nasal (constant on both sides among 83 D. confusa specimens examined by us, including eight specimens from Dalakan) instead of an entire nasal.

Views of the head patterns of the six West African species of the genus Dasypeltis. A–E. Preserved holotypes of D. confusa, D. latericia, D. parascabra, D. gansi and D. sahelensis. F. In life specimen of D. fasciata.
Main characteristics of West African species of Dasypeltis.
| Species (n° of specimens) |
Dorsals | Ventrals | Subcaudals | Nasal | Pattern | |||
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | |||
| D. parascabra (2 ♂, 1 ♀) |
22 | 23 | 216 | 224 | 67–68.0–69 | 64 | Entire | Non-linked scabra |
| D. sahelensis (22 ♂, 43 ♀) |
21–21.5–23 | 21–22.3–23 | 207–213.8–221 | 212–223.7–237 | 55–59.7–67 | 45–51.6–58 | Entire | Non-linked scabra |
| D. confusa (18 ♂, 24 ♀) |
23–24.1–25 | 23–24.8–26 | 213–220.2–227 | 224–232.9–242 | 65–68.9–73 | 53–59.8–67 | Semi-divided | Linked scabra |
| D. gansi (42 ♂, 48 ♀) |
21–22.2–25 | 21–22.9–25 | 221–231.1–240 | 235–245.6–255 | 68–76.5–83 | 59–64.2–73 | Semi-divided | Light tan fasciata |
| D. latericia (65 ♂, 75 ♀) |
21–21.8–25 | 21–23.1–25 | 219–231.6–243 | 234–245.1–262 | 66–74.9–86 | 59–64.9–72 | Semi-divided | Latericia |
| D. fasciata (12 ♂, 7 ♀) |
20–22.3–23 | 21–22.8–25 | 227–236.3–245 | 244–247.8–254 | 71–79.9–88 | 70–75.4–84 | Semi-divided | Fasciata |
From D. scabra and the other species of Dasypeltis from other parts of Africa, D. parascabra differs in possessing an entire nasal. D. abyssina Duméril, Bibron and Duméril, 1854, from Ethiopia and Eritrea, that we recently resurrected from the synonymy of D. scabra [4], also present much higher ventral counts (260 for the holotype, 273 for a female from Gheleb, Eritrea [1] than D. parascabra. In addition, our molecular analysis shows clear differences between the new species and D. scabra from South Africa, where the type of Linnæus is presumed to originate, and; the specimens from Burundi with a “5N” pattern classically attributed to D. scabra.
From other African species, D. parascabra differs as follows: from D. fasciata Smith, 1949, D. gansi Trape and Mané, 2006, D. latericia Trape and Mané, 2006, D. inornata Smith, 1849, D. medici medici Bianconi, 1859, D. medici lamuensis Gans, 1957, D. atra Sternfeld, 1912, and D. palmarum Leach, 1818, the new species differs by the aspect of the nasal and a very different pattern. In addition, the number of ventrals is lower in the new species than in D. fasciata (227–245 in males, 244–254 in females in West Africa), D. gansi (221–240 in males, 235–255 in females), D. latericia (219–243 in males, 234–262 in females) and D. palmarum (221–241 in males, 227–232 in females) [1,4,5,11]. The number of subcaudals is much higher in D. inornata (81–109 in males, 69–84 in females) than in the new species. Both D. medici medici and D. medici lamuensis, have diagnostic pigmented apical pits on the dorsal scales [1,5]. D. atra is a mountain species from East Africa that may present several colour forms but never the “scabra” pattern [3,6,12].
3.2.8 Geographic distribution
In Guinea, D. parascabra was also collected at Mt. Nimba (ZMB 75496, see a colour picture of this specimen in Böhme et al. [13]) and at Kouroussa (MNHN 1902.19). This species is also distributed in Liberia (MNHN 1986.1847, Mt Nimba) and in Ivory Coast where five out of 33 specimens from Lamto, attributed to D. scabra by Roux-Estève [14], belong to D. parascabra (MNHN 1977.535, 1977.542, 1977.549, 1977.555, 1977.558). The “5N” pattern – typical of D. parascabra in West Africa – was also mentioned by Gans [1] for six specimens from Ghana (Somanya), Togo (Adele) and Nigeria (Lagos).
4 Discussion
Both morphological and molecular data show that six species can be recognized in the genus Dasypeltis in West Africa: D. fasciata, D. gansi, D. latericia, D. confusa, D. sahelensis and D. parascabra. Until the recent work of Trape and Mané [4,11] and this study, only two species were known in West Africa [1,15]: D. fasciata, which is distributed in rainforest areas of West and Central Africa, and D. scabra, a species that now appears to be absent from West Africa and to have a much more restricted distribution than previously thought, possibly being limited to Southern Africa (Broadley and Bates, unpublished data).
All West African species correspond to specific colour patterns (Fig. 7), most of them long recognized, in particular by Gans [1]. The relatively low number of specimens from West Africa in museums, the small differences in scale counts between species, and probably reluctance of many herpetologists to give too much importance to colour pattern, may explain why they remained confounded. Many snake species present high variability in coloration, and differences may be puzzling, e.g. in the case of the boomslang Dispholidus typus. It appears probable that the genus Dasypeltis comprises even more species than those currently recognised, particularly in the Eastern part of Africa, as suggested by our study which shows molecular divergences and morphological differences between Southern and Eastern African specimens of the D. scabra complex. In particular, specimens from Burundi clearly differ molecularly from the South African vouchers, suggesting that they belong to a different species for which the name of D. lineolata Peters, 1878, which was based on a specimen from Kenya, could perhaps apply (but D. lineolata may be a colour phase of D. atra [6,and D.G. Broadley, pers. comm.], see also Hughes [16]).

Views of the dorsal patterns of the six West African species of the genus Dasypeltis. A, C, E. Preserved specimens of D. confusa, D. parascabra and D. sahelensis. B, D, F. In life specimens of D. latericia, D. gansi and D. fasciata.
Interestingly, our study also confirms the synonymy between D. fasciata (type from Sierra Leone, Guinean rainforest block) and D. macrops Boulenger, 1907 (type from Efulen, Southern Cameroon, Congolese rainforest block). Colour patterns and scale counts of these two taxa are similar, and molecular differences between West African and Central African populations appear insufficient to justify a subspecies rank for D. macrops. Similarly, populations of D. sahelensis from Morocco and from West Africa have similar colour pattern and are very close molecularly, suggesting that they were separated recently, probably less than 6000 BP when the Sahara desert extended at the end of the Holocene.
We examined the synonymy in the genus Dasypeltis in order to check if a name was available for the new species described in this paper. According to Gans [1], the synonymy of D. scabra comprised Rachiodon abyssinus Duméril, Bibron and Duméril 1854, from Ethiopia, Dasypeltis scaber var. capensis Peters, 1864, from Cape of Good Hope (South Africa), Dasypeltis scaber var. mossambicus Peters, 1864, from Tete and Boror (Mozambique), Dasypeltis scaber var. breviceps Peters, 1864, from Kafferland (inland from Port Elisabeth, South Africa), Dasypeltis scaber var. lineolata Peters, 1878, from Kitui, Ukamba (Kenya), and Dasypeltis scabra loveridgei Mertens, 1954, from Windhoek (Namibia). As indicated before, R. abyssinus is a valid species with much higher scale counts than D. parascabra. All types of Peters and Mertens are from Southern or Eastern Africa where all specimens with a « non-linked scabra » pattern have a semi-divided nasal and thus differ from D. parascabra.
The six West African Dasypeltis species have broadly distinct ranges but some of them are sympatric on part of their ranges (Fig. 8). The only exception appears to be D. parascabra, which is sympatric with D. confusa in almost all areas where it is currently known. Both species are typical of Guinean savannah areas of West Africa, but so far, D. parascabra is only known from West Africa although D. confusa is also known from Cameroon, Central African Republic, South Sudan, Uganda, Gabon, Congo Brazzaville, Democratic Republic of Congo, Angola and western Zambia [5,17,18, Broadley pers. comm., Trape unpublished]. D. fasciata is the only forest species, and we never observed true sympatry with D. confusa even in the deforested areas of southeastern Guinea and the minute relict forests of Togo. D. gansi presents a curious distribution. It is mainly a Sudan savannah species, but it is absent from southeastern Senegal and Western Mali where it is replaced by D. latericia. On the coast of Senegal, D. gansi reaches the Senegal river delta and the northern bank of the river in southern Mauritania. In southwestern Senegal, several areas of Guinea and in the Dahomey gap, D. gansi is in sympatry with D. confusa. D. sahelensis is widely distributed in the Sahel and relict populations exist in southern Morocco. To the East, this species does not appear to reach Egypt (Fayum) or Sudan (Sennar Province), as specimens in the BMNH classically attributed to D. scabra have semi-divided nasals (Broadley pers. comm.). In the most sudano-sahelian part of this range, D. sahelensis is sympatric with D. gansi in several areas of coastal Senegal north of the Gambia and in southern Niger.

Geographic distribution of West African species of the genus Dasypeltis. Data from this study and from Gans [1], Chirio and LeBreton [18], Geniez and Guillod [19], Trape (unpublished data) and Chirio (unpublished data).
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
We thank J.-D. Durand for advice and assistance in Dakar during preliminary molecular studies of Dasypeltis, and C. Balde and Y. Mané for invaluable assistance during snake collections. We are grateful to C. Kelly and B. Branch for providing tissue samples of D. scabra from South Africa, and to P. Geniez and P.-A. Crochet for providing tissue samples of D. cf lineolata from Burundi and D. sahelensis from Morocco. We thank D.G. Broadley, A. Barlow and M.F. Bates for helpful comments on a first draft of this article.
Appendix 1 List of material examined
| Dasypeltis confusa |
| Senegal. MNHN 2006.0303 (Holotype): Ibel (12°31′N, 12°23′W); MNHN 2006.0304: Mpak (12°28′N, 16°14′W); MNHN 2006.0305, MNHN 2006.0306, MNHN 2006.0307, IRSNB 2619: Bouroffaye (12°30′N, 16°16′W); IRSNB 2620, IRSNB 2621: Djibonker (12°32′N, 16°21′W); IRD S-83, S-2366: Mlomp (12°34′N, 16°35′W); IRD S-4683, S-4691: Koumbacara (12°42′N, 14°29′W); IRD S-6299: Niandouba (12°58′N, 13°58′W); IRD S-6945: Fafakourou (13°04′N, 14°33′W); IRD S-4716, S-4729, S-4735: Guénoto (13°33′N, 13°50′W). |
| Guinea. IRD 363-G: Kilissi (09°57′N, 12°51′W); IRD 437-G: Kouroumaya (09°56′N, 12°49′W); IRD 530-G, 531-G, 532-G, 533-G, 534-G, 536-G: Kalekouré (09°55′N, 12°48′W); IRD 100-G, 101-G, 102-G, 103-G, 106-G: Foulaya (10°00′N, 12°55′W); IRD 77-G, 83-G: Friguiagbé (09°57′N, 12°57′W); IRD 228-G, 235-G: Camaraboundji (09°54′N, 13°01′W); IRD 842-G: Seffan (09°54′N, 12°47′W); IRD 1067-G: Madina (10°15′N, 14°10′W); IRD 1104-G: Hamdalaye (11°01′N, 14°14′W); IRD 1199-G, 1211-G, 1230-G, 3266-G, 3287-G, 3303-G: Yatia (10°01′N, 10°58′W); IRD 1340-G, 1343-G, 3122-G, 3203-G, 3236-G: Sangolabadou (09°14′N, 10°02′W); IRD 1552-G, 1553-G, 1554-G, 1558-G, 1614-G, 1615-G, 1616-G, 1617-G, 3027-G: Dalakan and vicinity (09°58′N, 09°32′W; 10°00′N, 09°31′W); IPG 2605-I: Kindia (10°04′N, 12°51′W); IRD 3625-G: Konsankoro (09°02′N, 08°59′W); IRD 2439-G: Tabakourou (11°32′N, 09°08′W); IRD 1887-G, 1891-G, 1892-G, 1897-G, 1920-G: Yonkia (10°04′N, 13°38′W); IFAN 53.8.92: Keoulenta (07°40′N, 08°18′W). |
| Mali. IRD 3731-M: Mamarabougou (11°13′N, 05°28′W); IFAN 52.8.50:? Diafarabé (14°08′N, 05°01′W). |
| Benin. IRD 23-B, 75-B, 87-B, 94-B, 141-B; 177-B, 291-B, 336-B: Lanta (07°06′N, 01°52′E). |
| Togo. IRD 176-T, 181-T, 191-T, 193-T: Aledjo (09°15′N, 01°12′E); IRD 153-T: Diguengué (08°05′N, 00°38′E); IRD 116-T*, 165-T: Fazao (08°41′N, 00°48′E); IRD 218-T: Huiléhui (07°09′N, 01°18′E); IRD 254-T*: Mont Agou (06°51′N, 00°45′E). |
| Ivory Coast. MNHN 1977.524, 1977.525, 1977.526, 1977.527, 1977.528, 1977.529, 1977.530, 1977.531, 1977.532, 1977.533, 1977.534, 1977.536, 1977.537, 1977.538, 1977.539, 1977.540, 1977.541, 1977.543, 1977.544, 1977.545, 1977.546, 1977.547, 1977.548, 1977.550, 1977.551, 1977.552, 1977.553, 1977.554, 1977.556, 1977.557: Lamto (06°13′N, 05°01′W). |
| Dasypeltis gansi |
| Senegal. IRSNB 2613 (Holotype), IRD S-5943, S-5949, S-6521, S-6526, S-6543, S-6551, S-7330: Mahamouda Chérif (12°58′N, 16°30′W); IRSNB 2614, MNHN 2006.0308, MNHN 2006, MNHN 2006.0310, MNHN 2006.0311, MNHN 2006.0312, MNHN 2006.0313, IRD S-43, S-2160, S-2163, S-2249, S-2346, S-3400: Mlomp (12°34′N, 16°35′W); IRD S-6810, S-6875, S-6882: Boukote (12°25′N, 16°45′W); IRD S-7414: Kaguite (12°24′N, 16°23′N); IRD S-6811*: Kabrousse (12°21′N, 16°43′W); IRD S-4935, S-4936: Mpak (12°28′N, 16°14′W); IRD S-5112: Kamaracounda (12°30′N, 16°05′W); IRD S-5913, S-5917: Djibonker (12°32′N, 16°21′W); IRD S-4675: Koumbacara (12°42′N, 14°29′W); IRD S-4949: Tiarap (12°46′N, 14°31′W); IRD S-4003, S-4016, S-4019, S-4020, S-4025, S-4045, S-4050, S-4055, S-4069, S-4070, S-4091, S-4111: Némataba (12°48′N, 15°01′W); IRD S-4769, S-4776, S-7495: Takoudialla (12°50′N, 14°04′W); IRD S-3933, S-3961, S-3979: Goundaga (12°51′N, 14°05′W); IRD S-6327: Marewe (12°52′N, 14°08′W); IRD S-3878, S-3881, S-3884: Dabo (12°53′N, 14°29′W); IRD S-3913, S-3918: Fafakourou (13°04′N, 14°33′W), IRD S-618, S-619, S-760: Keur Bakar Mané (13°37′N, 16°17′W); IRD S-5880, S-6165: Médina Djikoye (13°37′N, 16°18′W); IRD S-504: Keur Ayip Kâ (13°39′N, 16°19′W); IRD S-6445, S-6450, S-7819*, S-7826*: Kountanto (13°39′N, 16°14′W); IRD S-1443, S-1445, S-1450, S-1462, S-1533, S-1534, S-1535, S-4884, S-5896: Dielmo (13°43′N, 16°25′W); IRD S-1260, S-1261, S-1579, S-1717, S-6311: Keur Lahine Fatim (13°44′N, 16°23′W); IRD S-389: Dakar (14°43′N, 17°28′W); IRD S-221, S-3408: Keur Moussa (14°47′N, 17°07′W); IRD S-7203: Touba Ndiaye (15°09′N, 16°52′W); IRD S-3308, S-3311, S-6352: Mbakhana (16°05′N, 16°22′W). |
| Mauritania. BEV.11172: Rkiz lake margin (16°48′N, 15°18′W). |
| Guinea. IRD 1346-G: Sangolabadou (09°14′N, 10°01′W); IRD 3269-G: Yatia (10°01′N, 10°18′W). |
| Mali. IRD 2355-M, 2356-M: Ballabougou (12°52′N, 06°52′W). |
| Niger. IRD 273-N: Téla (12°08′N, 03°28′E); IRD 331-N*: Piliki (13°08′N, 01°57′E); IRD 252-N: Cissia (13°52′N, 10°25′E). |
| Benin. IRD 15-B, 17-B, 36-B, 47-B, 79-B, 100-B, 104-B, 120-B, 204-B, 218-B, 219-B, 257-B, 266-B, 284-B, 286-B, 292-B, 295-B, 307-B, 312-B, 341-B: Lanta (07°06′N, 01°52′E). |
| Togo. IRD 408-T, 415-T, 431-T, 472-T, 473-T, 478-T: Huiléhui (07°09′N, 01°18′E); IRD 172-T, 179-T: Alédjo (09°15′N, 01°12′E); IRD 158-T: Fazao (08°41′N, 00°48′E). |
| Dasypeltis latericia |
| Senegal. IRSNB 2615 (Holotype), IRD S-2086, S-2087, S-2098, S-2490, S-2491, S-2495, S-5722: Boundoukondi (12°31′N, 12°20′W); MNHN 2006.0314: Mamakono (13°13′N, 12°03′W); IRD S-1814, S-1815, S-1821, S-1826, S-1853, S-3203, S-3228, S-5655: Ndébou (12°31′N, 12°27′W); IRSNB 2616, IRD S-1941, S-1943, S-1971, S-1983, S-1989, S-2548, S-2549, S-2574, S-2576, S-2603, S-2625, S-2632, S-2635, S-4302, S-4304, S-4344, S-4833: Bandafassi (12°32′N, 12°19′W); IRD S-5057, S-5063: Koté (12°33′N, 12°51′W); IRD S-1885, S-1887, S-1892, S-1893, S-1894, S-1895, S-1897, S-1899, S-1902, S-1906, S-1908, S-1918, S-2118, S-2131, S-2140, S-2383, S-2385, S-2386, S-2387, S-2388, S-2390, S-2392, S-2395, S-2400, S-2401: Landiéni (12°33′N, 12°22′W); IRD S-4954, S-4957, S-4981: Ebarakh (12°38′N, 12°52′W); IRD S-5104, S-5105: Oubadji (12°40′N, 13°03′W); IRD S-2520, S-2525, S-2529: Mako (12°51′N, 12°21′W); IRD S-4555: Massamassa (12°55′N, 11°55′W); IRD S-4391, S-5441: Sambarabougou (13°06′N, 11°51′W); IRD S-4461, S-4485, S-4495, S-4506, S-4516: Mamakono (13°13′N, 12°03′W); IRD S-5197, S-5198, S-5199: Guénoto (13°33′N, 13°50′W).; IRD S-1750, S-1753, S-1771, S-2901, S-2905, S-2919, S-2925, S-2941, S-2942, S-2943, S-2945, S-2954, S-2961, S-2966, S-2989, S-2990, S-2991, S-2992, S-3008, S-3031, S-3033, S-3057, S-3117, S-3128, S-3133, S-3141, S-3143, S-3175, S-4177, S-4186, S-4203, S-5599, S-5732: Ibel (12°31′N, 12°23′W); IRD S-2024, S-2028, S-2036, S-2038, S-2047, S-2049, S-2050, S-2645, S-2649, S-2653, S-2704, S-2707, S-2711, S-2738, S-2753, S-2778, S-2807, S-5716: Nathia (12°28′N, 12°22′W); IRD S- 5503, S-5542, S-5566: Wassangara (13°12′N, 11°33′W); IRD S-5299: Saïnsoutou (13°23′N, 11°38′W); S-8611*, S-8617, S-8618, TR-2952*: Sabodala (13°09′N, 12°07′W). |
| Guinea. IRD TR-550*: Kifaya near Koundâra (12°09′N, 13°05′W); IRD 2459-G (ex TR-1665), IRD 2520-G (ex TR-1666): Tabakourou (11°32′N, 09°08′W). |
| Mali. IRD 2371-M: Koundian (13°10′N, 10°40′W); IRD 1930-M: Titiéna (11°27′N, 06°33′W); IRD 153-M: Sébékourani (12°12′N, 08°42′W); IRD 161-M, 162-M, 163-M, 171-M, 172-M, 2619-M, 4063-M: Zamoko (13°09′N, 07°57′W); IRD 1351-M, 1713-M, 1714-M: Séoulasso (13°14′N, 04°42′W); IRD 27-M, 276-M, 285-M, 288-M: Bangaya (13°14′N, 10°43′W); IRD 138-M, 139-M, 2357-M, 2358-M, 2359-M: Niamou (14°01′N, 08°03′W). |
| Dasypeltis sahelensis |
| Senegal. MNHN 2006.0315 (Holotype): Tialé (15°14′N, 16°49′W); IRD S-2291: Keur Bakar Mané (13°37′N, 16°17′W); IRD S-1716: Keur Lahine Fatim (13°44′N, 16°23′W); MNHN 2006.0316, IRSNB 2622, IRD S-5012, MNHN 2006.0317, IRSNB 2623, IRD S-5029: Delbi (14°14′N, 15°18′W); MNHN 2006.0318, IRD S-6696, S-6753: Bellé (14°25′N, 12°19′W); IRD S-5072: Lougué Tiékodié (14°39′N, 13°59′W); IRD S-4897: Doli (14°45′N, 15°09′W); IRD S-4945, S-4946: Gassane (14°49′N, 15°18′W); IRSNB 0329: Mbawane (14°53′N, 17°08′W); IRD S-3453: Vélingara Ferlo (15°01′N, 14°41′W); IRD S-3499: Kéllol (15°17′N, 13°08′W); IRD S-7234, MNHN 2006.0320: Loumbol (15°19′N, 13°44′W); MNHN 2006.0321: Calbansall (15°21′N, 16°23′W); IRD S-7249: Tiguéré Yéné (15°42′N, 13°16′W); MNHN 2006.0322, S-3337: Mbakhana (16°05′N, 16°22′W); IRD S-3281, S-3301, S-3302: Nder (16°15′N, 15°53′W); IRD S-7265: Thily (16°28′N, 14°09′W); IRD S-7300: Tivaoune II (16°28′N, 15°02′W); IRD S-6378: Walaldé (16°30′N, 14°12′W); IRD S-237, S-238: Fété-Olé (16°15′N, 15°08′W); IRD S-7297: Pathé Galo (16°37′N, 14°27′W); IRD S-4875: Ngandiouf (15°16′N, 16°24′W). |
| Mali. IRD 1395-M, 1505-M: Séoulasso (13°14′N, 4°42′W); IRD 1230-M, 1231-M, 2360-M: Toumboula (14°20′N, 07°48′W); IRD 130-M, 412-M, 2361-M, 2362-M, 2363-M: Bouyanga (14°30′N, 9°39′W); IRD 1535-M, 3561-M, 3567-M, 3573-M, 3583-M: Gaoudel (15°59′N, 04°05′W); IRD 2335-M, 2340-M, 3503-M, 3509-M, 3510-M, 3512-M, 3523-M, 3524-M: Koyretao (16°04′N, 03°58′W). |
| Mauritanie. IRD 110-MT: Lahouvitch (16°38′N, 15°59′W). |
| Niger. IRD 305-N, 309-N*, 315-N*, 1405-N, 1418-N, 1434-N, 1437-N, 1445-N, 1452-N, 1466-N, 1467-N, 1468-N, 1470-N, 1473-N, 1491-N: Piliki (13°08′N, 01°57′E); IRD 189-N: Saboulayi (13°30′N, 07°50′E); IRD 587-N, 1022-N: Aholé (13°33′N, 04°01′E); IRD 820-N, 831-N, 899-N, 908-N: Karosofoua (13°37′N, 06°37′E); IRD 59-N, 394-N: Baboul (13°42′N, 8°35′E); IRD 10-N, 106-N, 115-N, 120-N, 130-N, 133-N, 400-N, 401-N, 402-N, 423-N, 431-N, 432-N, 433-N, 435-N, 444-N, 1106-N, 1112-N, 1149-N, 1195-N, 1206-N, 1240-N, 1262-N, 1269-N, 1273-N, 1703-N*, 1704-N*, 1705-N, 1706-N, 1707-N: Tarka Dakouara (14°12′N, 8°49′E); IRD TR-1545: Korri Solomi (17°35′N, 07°42′E); IRD 1051-N, 1071-N, 1083-N: Cissia (13°52′N, 10°25′E); IRD 1363-N: Tékhé (14°01′N, 06°01′E); IRD 1543-N, 1552-N, 1561-N, 1569-N, 1579-N: Téla (12°08′N, 03°28′E); IRD 1662-N, 1686-N: Tounga Yacouba (13°54′N, 05°26′E). |
| Burkina Faso. CNRST HV-2369: Zogoré (13°24′N, 02°34′W); CNRST HV-5827: Dori (14°01′W, 00°01′W). |
| Morocco. BEV.9448*: 43 km NE of Tan-Tan (28°37′N, 10°46′W); BEV.9449*: 10 km S of Mirleft (29°30′N, 10°03′W) BEV.9450: 9 km W of Tiznit (29°40′N, 09°50′W). |
| Dasypeltis fasciata |
| Guinea. IRD 4163-G, 4170-G, 4197-G: Ballassou (08°23′N, 09°18′W); IRD 2653-G, 2769-G, 2793-G, 2936-G, 3828-G, 3899-G: Konipara (07°51′N, 09°02′W); IRD 2107-G*: Nzébéla (08°05′N, 09°05′W), IRD 3131-G, 4132-G: Sangolabadou (09°14′N, 10°01′W); IRD 2174-G*: Topapa (07°22′N, 08°55′W). |
| Togo. IRD 28-T*: Yo (06°56′N, 00°35′E); IRD 356-T, 357-T*, 361-T, 363-T, 365-T,: Diguengué (08°05′N, 00°38′E). |
| Liberia. MNHN 1973.1233, 1973.1224: Monrovia (06°19′N, 10°46′W). |
| Cameroon. IRD TR-3692*, TR-3793*: Sissor (03°08′N, 13°39′E); IRD TR-3794*: Kendem (05°44′N, 09°43′E). |
* Specimens used for molecular studies.
Appendix 2 Key to the West African species of Dasypeltis
| 1a. Nasal entire.……………………………………….………….……………………….............2 |
| 1b. Nasal semi-divided.…………………………………..………………………………..............3 |
| 2a. Pattern consisting of dark brown dorsal blotches alternating with dark brown lateral bars, the pigment mainly distributed on the lateral and posterior edges of the scales and on the underlying skin. A median black stripe on the frontal in addition to pairs of symmetric markings. Sahel.…………………………………………………………….…..........................D. sahelensis |
| 2b. Pattern consisting of black dorsal blotches alternating with black lateral bars, the pigment distributed both across the individual scales and on the underlying skin. No median black stripe on the frontal, only pairs of symmetric markings. Guinean savannah….………..……………………………………………………………….D. parascabra |
| 3a. When present, pattern consisting of brown dorsal blotches alternating with brown lateral bars or blotches, the pattern produced by a darkening of the skin between the scales and by pigmentation of the lower edge of these.….………………………………………...............................................................................4 |
| 3b. Pattern consisting of black or dark brown dorsal blotches linked to black or dark brown lateral bars, the pigment distributed both across the individual scales and on the underlying skin. Guinean and Sudan and Guinean savannah……….………………..……...........................................................................D. confusa |
| 4a. Lateral bars narrow or indistinct, their width less than half the width of the spaces between bars. Sudan and Guinean savannah…..…………………………….……..……............................5 |
| 4b. Lateral blotches large, their width at least twice the width of the spaces between blotches. Rain forest …………….………..….…………………………….…................................................D. fasciata |
| 5a. Ground colour in life pale grey or beige. Lateral bars often indistinct both in juveniles and adults. Dorsal blotches often indistinct in adults. One or two postoculars……………………………………………………………………..….............D. gansi |
| 5b. Ground colour in life light reddish-brown. Dark dorsal blotches and lateral bars always well marked both in juveniles and adults. Two postoculars……………………......................................................................................D. latericia |


