The chemistry of peroxide is of significance in many areas such as chemical and biochemical synthesis. Many oxidation reactions involve intermediates containing O–O bonds [1]. Epoxidation of fullerenes with peroxy acids is the most efficient method [2]. Singlet oxygen has been used for ring opening reactions of fullerenes in which a dioxetane intermediate is proposed [3]. Surprisingly the oxidation of a C60 diol derivative with Pb(OAc)4 only gave the dioxetane not the cage opened diketone [4]. Further derivatization of addends in some fullerene derivatives also form peroxide species with the peroxo group attached on the addends [5]. But stable and well-characterized fullerene peroxide is rare. We have found that t-butylperoxo radicals add to fullerenes to form stable fullerene mixed peroxides [6], which exhibits interesting reactions [7–9]. This report summarizes some of the results.
Fullerene is known to be excellent radical scavengers. This is well demonstrated in their reactions with t-butylperoxo radical tBuOO●. A number of fullerene mixed peroxides have been isolated with different number of t-butylperoxo groups. For C60 the reaction gave adducts with the general formula C60(O)m(OOtBu)n. Low concentration, long reaction time and irradiation of visible light favor the epoxides formation. High concentration of t-butylperoxo radical favors homo-peroxo adducts. Under optimized conditions C60(O)(OOtBu)4, C60(OOtBu)6, C60(O)(OOtBu)6 adducts can be prepared in 45%, 40% and 57%, respectively [10].

The addition to C70 is slightly different. Only the homo-peroxo adducts is formed with the general formula C70(OOtBu)n with n varies from 2 to 10. There is no epoxy moiety in the products. Due to its D5h-symmetric nature, C70 gave more isomers in particular for those with fewer addends. Thus four isomers were identified for the bis-adduct, two isomers for the tetrakis- and hexakis-adducts, and one isomer for the octakis- and decakis-adducts. The decakis-adduct is the major product (30%) under optimized conditions [8].

Various literature methods may be used to generate the t-butyl peroxo radical. Catalysis with Fe or Ru chlorides or coordination complexes requires a relatively large excess of TBHP and longer reaction time. The epoxy containing C60(O)(OOtBu)4 is best prepared under this condition. Stoichiometric oxidation with diacetoxyiodobenzene (DIB) or cerium ammonium nitrate (CAN) produces the t-butylperoxo radical efficiently in high concentration. The reaction is faster than the catalytic reaction and the number of addends on fullerene can be controlled by limiting the amount of DIB or CAN. t-Butyl peroxo radical is the best peroxo radical for the formation of fullerene mixed peroxides. Other peroxo radicals such as cumyl peroxo radical PhC(Me2)(OO●) reacts efficiently with C60, but complex mixtures were formed [7].
Formation of fullerene peroxides follows the stepwise radical addition mechanism [7,8]. The t-butylperoxo group already attached to the fullerene cage has a strong effect on the next t-butylperoxo group as a result of steric hindrance and/or the resulting radical stability. Para-addition on the hexagon is the dominant pattern. For C60 sequential para-addition occurs on the hexagons around a pentagon, i.e. the cyclopentadienyl addition mode [7]. For C70 two types of products are formed [8]: sequential para-addition along the belt and around a pentagon on the side of C70. The two pentagons on the poles of C70 do not facilitate the cyclopentadienyl mode in the present reactions. Perhaps the pole carbons are too much pyramidalized for effective double bond formation between them [11].
Given the well established peroxide chemistry, fullerene peroxides have much potential for further functionalization. Functional groups on fullerene derivatives usually exhibit unique chemical reactivity due to the influence of the fullerene cage. Trivial transformations readily achieved in classical organic synthesis may not take place or drastic conditions are needed in fullerene chemistry. Further investigation of the fullerene peroxides have revealed some interesting reactions and resulted in a number of other fullerene peroxide derivatives.
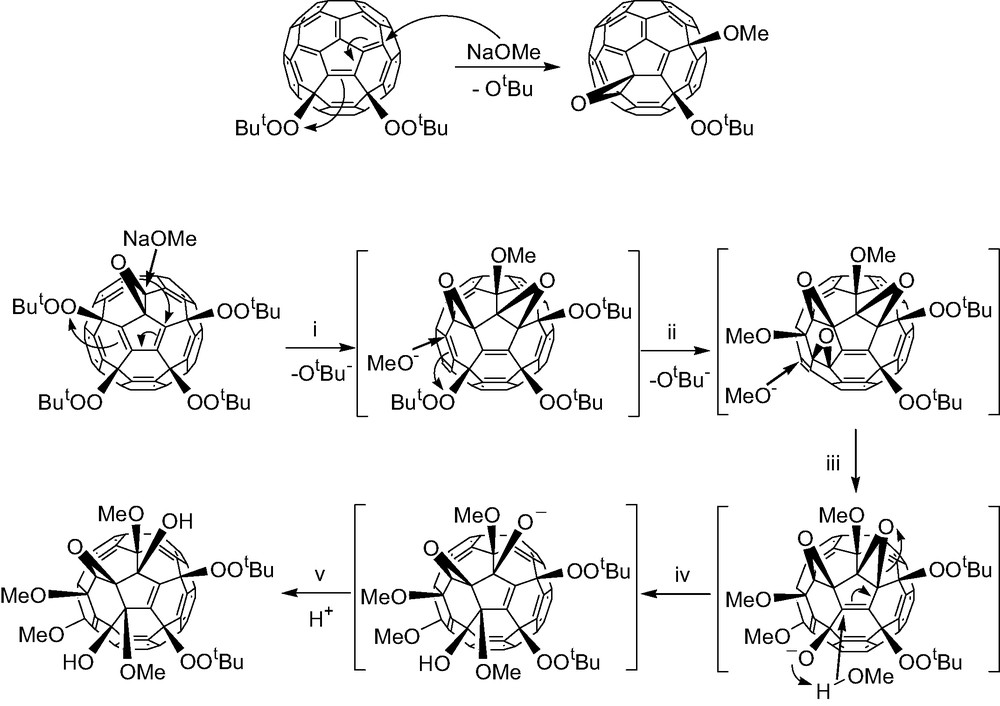
Nucleophiles react with the fullerene peroxides according to either SN1 or the extended SN2′ or SN2″ pathway. Thus addition of sodium methoxide results in the loss of a t-butoxide from the 1,4-bis-adduct C60(OOtBu)2 to give the epoxy containing product C60(O)(OMe)(OOtBu). The same reaction with C60(O)(OOtBu)4 is more complicated. Multi-addition of methoxy groups takes place resulting four isolable products, one of which is C60(O)(OH)2(OMe)4(OOtBu)2. Coordination of Na+ with the epoxy oxygen probably initiates its opening to leave a positive fullerene carbon for the first methoxide addition, a process similar to the classical SN1 reaction. The oxygen anion formed here through the epoxide opening then induces the loss of another t-butoxide through a SN2″ route forming the first intermediate C60(O)2(OMe)(OOtBu)3. Further reactions with sodium methoxide and methanol eventually gave the final product C60(O)(OH)2(OMe)4(OOtBu)2, the structure of which is confirmed by single crystal X-ray analysis [7].
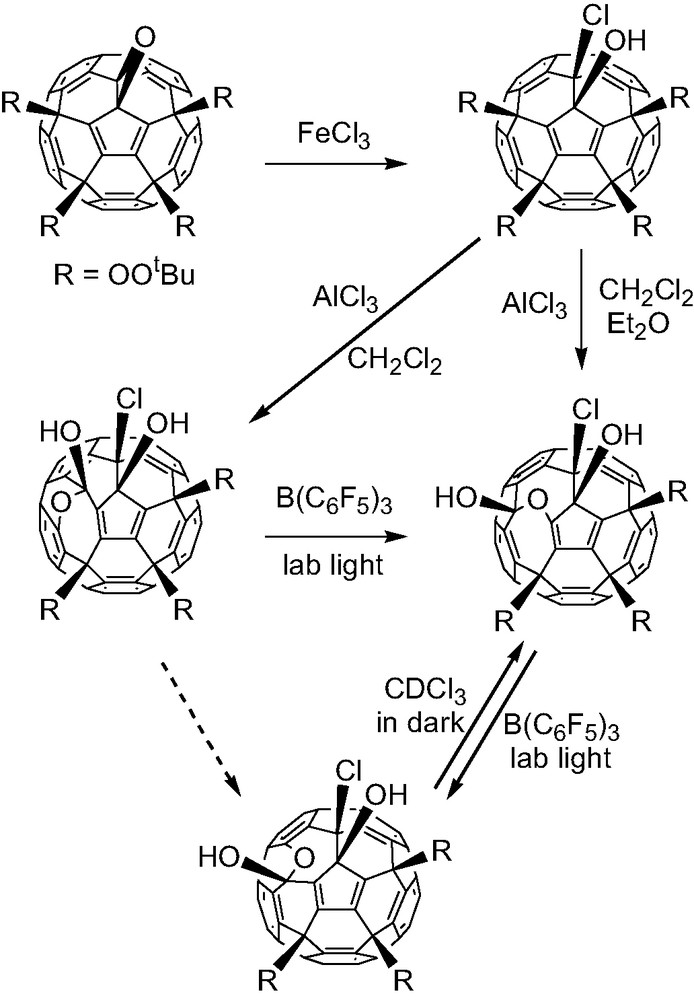
Various Lewis and Bronsted acids can also react with the fullerene peroxides. Ferric chloride opens the epoxide effectively to give the vicinal hydroxyl chloride. In the presence of boron trifluoride addition of methanol, t-butylhydroperoxide and hydrogen peroxide yielded the corresponding epoxide opened derivatives. Aluminum chloride results in heterolysis of the peroxo O–O bond and formation of hemiketals after rearrangement. The hemiketals have similar stability and can be interconverted under milder conditions [9].
Most of the fullerene peroxides were characterized by their spectroscopic data. In a few cases structures were confirmed by single crystal X-ray analysis [9]. The fullerene peroxides are readily soluble in both polar and non-polar solvents due to presence of the t-butylperoxo groups. The relatively high solubility greatly facilitates the separation by normal flash chromatography with more choice of solvents, and in particular reduces the time of obtaining 13C NMR spectra. The compounds are stable under atmospheric conditions. Most samples can be stored at r.t. for months without noticeable decomposition. We have carried out the reaction with up to 1 g C60 and never had any problem. But considering the high local concentration of the peroxo groups, one must take great care in dealing with such fullerene peroxides to avoid possible explosion.
We have shown that fullerene peroxides can be readily prepared. Such compounds react with various acids and sodium methoxide to give characterizable products. Preliminary work with other nucleophiles, such as primary and secondary amines and carbon anions, indicates that atoms other than oxygen can be added to these fullerene peroxides. Many of the compounds prepared here are racemic mixtures. Chirality is another aspect to be addressed in the future [12]. Cleavage of the C–C and C=C bonds by peroxides are classical reactions in organic synthesis. Work is in progress to open up the fullerene cage starting from fullerene peroxides.
Acknowledgements
Many thanks to all my co-workers and students for their hard work, whose names appear in the references. I am also grateful to the NSFC and the Ministry of Education of China for financial support.


