1 Introduction
Polyoxometalates have attracted increasing attention as polydentate ligands in order to obtain polynuclear complexes with interesting magnetic, catalytic and electrocatalytic properties [1–10]. Polyoxotungstates are generally used owing to their great stability over a wide range of pH. Among them, the open Wells–Dawson anion α-[Si2W18O66]16– is highly interesting because it is constituted of two A-α-{SiW9O34} subunits which delimit a large central cavity which can accommodate several metallic cations [11].
Usually, one potassium cation K1 is included in the cavity, slightly shifted to one side, and bridges the two {SiW9} subunits (Fig. 1a) [12] and two different coordination modes of transition metals have been observed. In the first one, the transition metal cation M1 is coordinated to only one half-anion (Fig. 1b), for example the cobalt cation in the derivative [{Co(H2O)}(μ-H2O)2K(Si2W18O66)]13– [12]. The presence of the potassium atom prevents the binding of a second transition metal cation with the same mode of coordination. In the second coordination mode, a transition metal M2 links the two subunits through one bond with each half-anion at the periphery of the cavity (Fig. 1c) as in the dicobalt complex [{Co(H2O)}(μ-H2O)2K{Co(H2O)4}(Si2W18O66)]11– [12].

Combined polyhedral/ball and stick representation of the anions [{K(H2O)3}2{K(H2O)2}(Si2W18O66)]13– (a), [{M1(H2O)}(μ-H2O)2K(Si2W18O66)]13– (b), [{M1(H2O)}(μ-H2O)2K{M2(H2O)4}(Si2W18O66)]11– (c), showing tungsten (polyhedra), silicon (white), potassium (light-gray ball), transition metal (black) and oxygen (small black) atoms.
Removal of the potassium cation K1 is necessary in order to include in the cavity more than one M1 type transition metal cation. A first example is the copper(II) complex [Cu5(OH)4(H2O)2(A-α-SiW9O33)2]10– synthesized by Kortz [13,14] in which four copper atoms are bound to the polyanion following the first coordination mode, the fifth cation capping the cationic copper cluster {Cu4(OH)4(H2O)2}4+.
The formation of the {Cu4(OH)4(H2O)2}4+ fragment in the cavity can be related to the ability of copper to form polycations. We report here the synthesis and structure of two complexes obtained with vanadium(V) which gives easily polyoxo-vanadium clusters and iron(III) for which polyhydroxo clusters are well-known.
2 Experimental section
2.1 Synthesis
2.1.1 K11[{KV2O3(H2O)2}(Si2W18O66)]·40 H2O (1)
K16α-[Si2W18O66]·25 H2O (10 g, 1.82 mmol) was added in 196 ml of water [11]. A solution of NaVO3 was prepared by dissolving NaVO3 (0.44 g, 3.65 mmol) in 7 ml of water at room temperature. The solution of NaVO3 was slowly added to the dimer in suspension and 1 M HCl (3.2 ml) is added (the pH was about 3.8). Thirty grams of solid KCl was added and an orange precipitate appeared. Orange crystals of 1 suitable for X-ray crystallography were obtained at room temperature within 1 day (yield: 20%). Elemental analysis (%) calcd for K11[{KV2O3(H2O)2}(Si2W18O66)]·40 H2O: V 1.76, K 8.09, W 57.03, Si 0.97; found: V 1.38, K 8.15, W 59.05, Si 1.30. IR (KBr pellet): ν = 1001(w), 959(m), 910(s), 877(s), 865(sh), 792(s), 734(s), 553(w), 524(w), 374(w), 351(w), 326(w) cm−1.
2.1.2 K2Na8[{Fe4(OH)6}(Si2W18O66)]·44 H2O (2)
K16α-[Si2W18O66]·25 H2O (10 g, 1.82 mmol) was added in 210 ml of water. Dissolution of the solid occurred during addition of 0.9 M FeCl3 aqueous solution (12 ml, 11 mmol), the pH was about 1.5. The pH is adjusted to 2.9 by 1 M KHCO3. 20 g of solid KCl was added and an ochre precipitate appeared. After gentle stirring for 30 min, the solid was collected by filtration through a fine frit. Yield: 7.3 g (73%). This crude product was dissolved in acetic acid/sodium acetate buffer, pH 4.7 (5 g in 120 ml) and ochre crystals of 2 suitable for X-ray crystallography were obtained at room temperature within 10 days (yield: 38%). Elemental analysis (%): calcd for K2Na8[{Fe4(OH)6}(Si2W18O66)]·44 H2O: Fe 3.85, Na 3.17, K 1.35, W 57.05, Si 0.97; found: Fe 3.95, Na 3.46, K 1.92, W 55.31, Si 1.60. IR (KBr pellet): ν = 1072(w), 1007(w), 962(m), 915(sh), 900(s), 856(sh), 773(s), 738(sh), 648(m), 547(w), 522(w), 481(w), 457(w), 413(w), 371(m), 327(m) cm−1.
2.2 X-ray crystallography
Single crystals of compounds 1 and 2 were mounted on a glass fiber for indexing and intensity data collection at 293 K on a Bruker X8-APEX2 CCD area-detector diffractometer using Mo Kα radiation (λ = 0.71073 Å). Six sets for 1 and four sets for 2 of narrow data frames (45 s per frame for 1 and 20 s for 2) were collected at different values of θ (for 2 and 4 initial values of ϕ and ω, respectively, for 1; for 3 and 1 initial values of ϕ and ω, respectively, for 2) using 0.5° increments of ϕ or ω.
Crystal data and structure refinements for 1: orange crystal, dimensions 0.20 × 0.10 × 0.06 mm3, triclinic, space group P
Crystal data and structure refinements for 2: ochre crystal, dimensions 0.40 × 0.20 × 0.06 mm3, triclinic, space group P
Data reduction was accomplished using SAINT V7.03 [15]. The substantial redundancy in data allowed a semi-empirical absorption correction (SADABS V2.10) [15] to be applied, on the basis of multiple measurements of equivalent reflections. The structure was solved by direct methods, developed by successive difference Fourier syntheses, and refined by full-matrix least-squares on all F2 data using SHELXTL V6.14 [16].
3 Results and discussion
The complex 1 K11[{KV2O3(H2O)2}(Si2W18O66)]·40 H2O has been obtained by the reaction of metavanadate with [KSi2W18O66]15–. Three cations are included in the cavity, a potassium atom K5 and two vanadium atoms V1 and V2. The two vanadium atoms are bound to only one {SiW9} subunit following the coordination mode M1 (Fig. 2) and the potassium atom is bound to the other subunit. V1 and V2 are linked by an oxygen atom (V1–O68–V2, angle 165°) and their coordination sphere is completed by two terminal oxygen atoms. The corresponding bond lengths (V1–O67 = 1.62 Å, V1–O69 = 2.10 Å, V2–O70 = 1.59 Å, V2–O71 = 2.13 Å) show that one terminal oxygen on two is protonated. Bond-valence sum calculations show that bridging O68 and the terminal atoms O67, O70 are not protonated (Table 1). On the contrary, the other terminal positions O69, O71 are occupied by a water molecule. The vanadium group bound to the upper {SiW9} subunit in Fig. 2 can be then written [VO(H2O)–O–VO(H2O)]4+. The potassium atom K5 is linked to the divanadium group through to the four terminal oxygen atoms O67, O69, O70, O71 and O68 so that it is localized in the pseudo-symmetry plane containing the Si atoms. Consequently, the symmetry of the idealized anion is Cs. Values of the angles V1–O69–K5 (79.6°), V2–O71–K5 (79.3°) on the one hand and V1–O67–K5 (98.9°), V2–O70–K5 (99.5°) on the other hand are in agreement with H2O and O bridging ligands between vanadium and potassium, respectively. The polyoxometallic cluster included in the cavity of [Si2W18O66]16– can be written [KV2O3(H2O)2]5+.
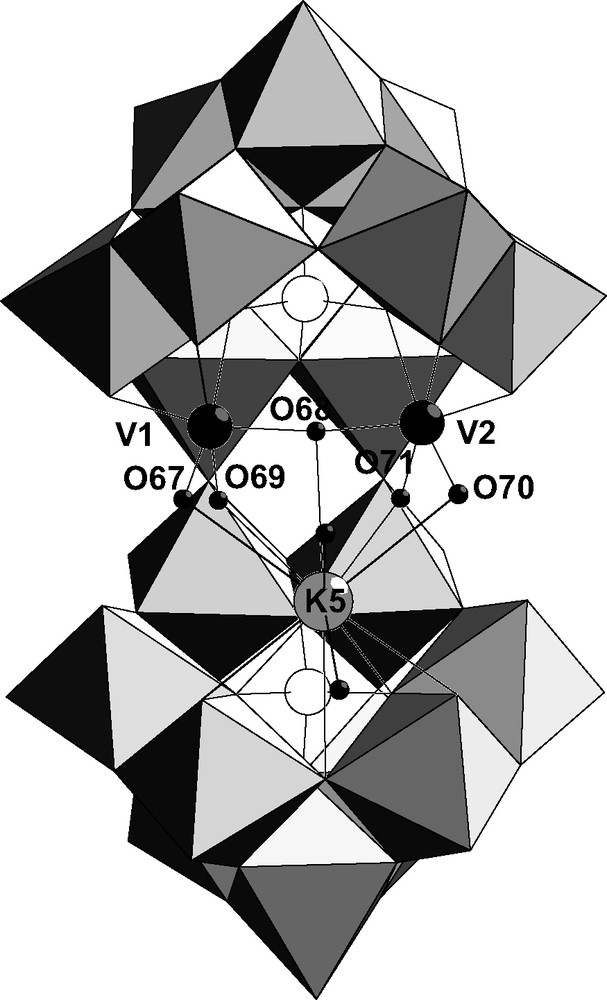
Combined polyhedral/ball and stick representation of α-[{KV2O3(H2O)2}(Si2W18O66)]11– 1 showing tungsten (light-gray polyhedra), potassium (light-gray ball), vanadium (black), silicon (white) and oxygen (small black) atoms.
Bond-valence sum calculations of oxygen atoms bound to vanadium and potassium in the complex 1 [{KV2O3(H2O)2}(Si2W18O66)]11– and to iron, potassium or sodium in the complex 2 [{Fe4(OH)6}(Si2W18O66)]10–
| Polyanion | Atoms | Bond-valence sum |
| 1 | O67 | 1.91 |
| O68 | 2.07 | |
| O69 | 0.49 | |
| O70 | 2.01 | |
| O71 | 0.47 | |
| 2 | O67 | 1.23 |
| O68 | 1.08 | |
| O69 | 1.20 | |
| O70 | 0.97 | |
| O71 | 1.14 | |
| O72 | 1.20 |
In the crystal, the anions are associated by their open faces through three potassium cations K5, K6 and K8. These two anions are related by an inversion center K1 and these inversion pairs are linked in linear chains by potassium cations K4, K10 and K12 (Fig. 3).
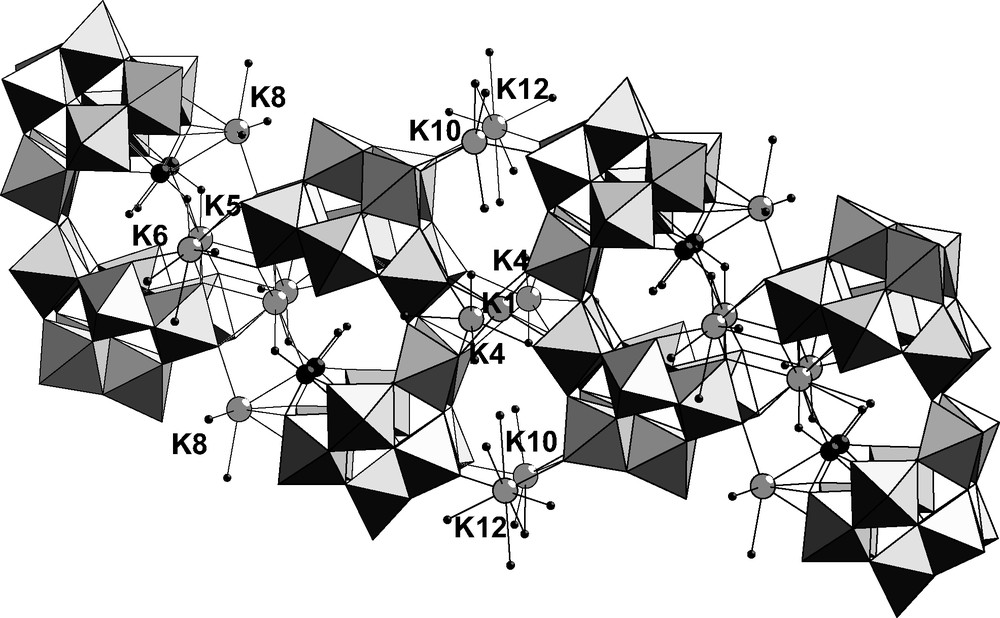
Combined polyhedral/ball and stick representation of K11α-[{KV2O3(H2O)2}(Si2W18O66)] 1 chains in the lattice side view showing tungsten (light-gray polyhedra), potassium (light-gray ball), vanadium (black) and oxygen (small black) atoms.
Polyanion 2 [{Fe4(OH)6}(Si2W18O66)]10– has been synthesized by addition of FeCl3 to K16α-[Si2W18O66]·25 H2O. No potassium cation but four iron atoms are included in the pocket as a {Fe4(OH)6}6+ fragment in which the iron atoms are coordinated to only one half-anion as V atoms in 1 (Fig. 4). Bond-valence sum calculations (Table 1) reveal that Fe atoms are linked by either single hydroxo (Fe1–OH–Fe2, Fe3–OH–Fe4) or double hydroxo bridges (Fe1–(OH)2–Fe4, Fe2–(OH)2–Fe3). The Fe–OH bond lengths range from 1.93 to 2.04 Å and the Fe–OH–Fe angles from 134.9 to 140.1° for the single bridges and from 96.4 to 98.8° for the double bridges. Moreover, the cationic assembly is achieved by two sodium ions Na3 and Na5 which bridged the two {SiW9} half-units. However, the two Na are not equivalent since Na3 is bound to {Fe4(OH)6}6+ by an OH ligand in the interior of the pocket but Na5 to an OH ligand in the exterior of the pocket. Without Na cations, the symmetry of the complex would be C2v, but the non-equivalence of the Na atoms leads to a lack of all symmetry elements in the crystal.

Combined polyhedral/ball and stick representation of [{Fe4(OH)6}(Si2W18O66)]10– 2 showing tungsten (light-gray polyhedra), sodium (medium-gray ball), iron (black), silicon (white) and oxygen (small black) atoms.
In the crystal, two [{Fe4(OH)6}(Si2W18O66)]10– anions are associated by their open faces through one potassium cation K1 bound to terminal oxygen atoms of the pocket. These two anions are related by an inversion center and these inversion pairs are linked in linear chains by a potassium cation K2 and one sodium cation Na4.
Anion 2 can be characterized in solution by its polarogram (Fig. 5). In acetic acid/sodium acetate buffer, pH 4.8, it shows four successive waves of 4, 2, 4 and 2 electrons, at –0.50, –0.70, –0.82 and –0.93 V, vs. the saturated AgCl/Ag reference electrode. The polarogram of the ligand α-[Si2W18O66]16– in the same medium shows three waves of 2, 4, 2 electrons, at –0.68, –0.81 and –0.93 V. So, the three last waves are attributed to the reduction of tungsten atoms of the polyanion and the first one to the reduction of the iron cluster [Fe4(OH)6]6+ in one step from FeIII to FeII. This simultaneous reduction of iron atoms is irreversible since the {Fe4(OH)6} fragment is not stable with FeII as observed in polyoxotungstic complexes when, as in 2, all the iron are equivalent. Aqueous solution of 2 is stable during at least 24 h since there is no evolution of the polarogram during this time.
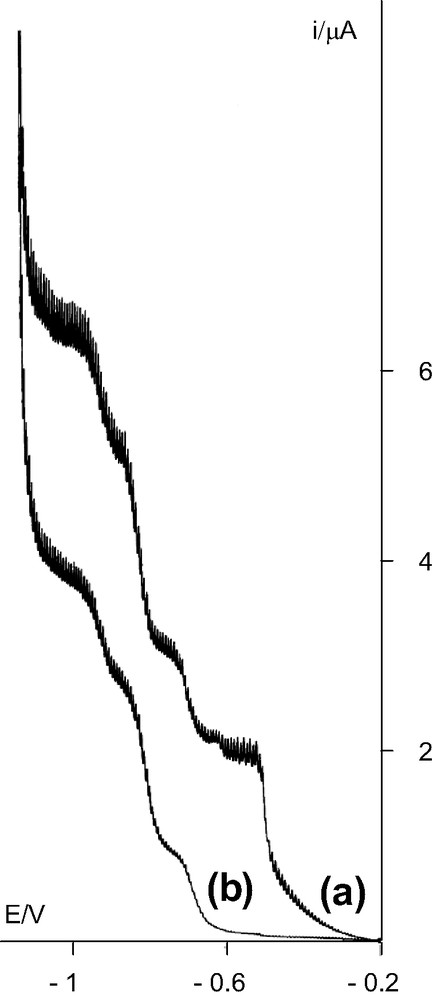
Polarograms of the anion 2 (5.6 × 10−4 mol l−1) (a) and of α-[{K(H2O)2}(Si2W18O66)]15– (5.7 × 10−4 mol l−1) (b) in acetic acid/sodium acetate buffer, pH 4.8, vs. the saturated AgCl/Ag reference electrode.
4 Supplementary material
Two X-ray crystallographic files in CIF format. This material can be obtained from the Fachinformationszentrum Karlsruhe, Abt. PROKA, 76344 Eggenstein-Leopoldshafen, Germany (fax: +49 7247 808 666; e-mail: crysdata@fiz-karlsruhe.de), on quoting the depository number CSD-416411, 416412, respectively.


