1 Introduction
Coordination polymers are formed when divalent first-row transition metals are linked with the generally bidentate dicyanamide [dicyanamide is N(CN)2−, hereafter abbreviated dca] anion [1–3]. These structures are of current research interest because of their structural diversity and potential for magnetic ordering. The neutral, binary systems, M(dca)2, form rutile-like structures that exhibit ferromagnetic (Co and Ni), antiferromagnetic (V, Cr, Mn and Fe), or paramagnetic (Cu) ground states [4].
Anionic dicyanamide complexes of divalent manganese, iron, cobalt and nickel have been increasingly studied in recent years. Various structural topologies have been reported including one-dimensional chains [5] or ladders [6], two-dimensional sheets of squares [5,7], hexagonal honeycombs [8] or triangles [9], and three-dimensional triple rutile [7], perovskite [10–12] and lithium antimonite type [13] frameworks. These various topologies are a result of the templating effect of the cation. For example, the tetraphenylphosphonium and bis(ethylenedithio)tetrathiafulvalenium cations have strong propensities to form layered structures due to intermolecular aromatic or sulfur interactions, respectively. Thus, the structure of the anionic dicyanamide coordination polymer in these salts is also two-dimensional. However, in salts containing tetraalkylammonium cations, the interactions between cations are less important, and three-dimensional motifs result.
Although the spectroscopic properties of anionic, divalent copper dicyanamide complexes were first reported more than thirty years ago [14], their structural and magnetic properties have not been characterized. It was postulated that [As(C6H5)4]2[Cu(dca)4] contains tetrahedral copper(II) and a monomeric structure, while [N(CH3)4]2[Cu(dca)4] contains octahedral copper(II) in a polymeric structure. Copper(I) anionic dicyanamide complexes have been studied to only a slightly greater extent. The (PPh4)2Cu2(dca)3Br and PPh4Cu(dca)2 salts have been prepared, but not structurally characterized [15,16]. The crystal structure of [Rb(18-crown-6)]3Cu2(dca)5 contains Cu(I) with both tetrahedral and triangular coordinations while that of [Tl(18-crown-6)]Cu(dca)2 exhibits only tetrahedral copper sites [17].
The molecular superconductors with the highest superconducting transition temperatures (Tc), κ-(BEDT-TTF)2[Cu(dca)X] [BEDT-TTF is bis(ethylenedithio)tetrathiafulvalene; X = Cl [18], Br [19], Tc = 12.8 K at 0.3 kbar pressure and Tc = 11.6 K at ambient pressure] contain chains of diamagnetic Cu(I) ions linked by μ1,5-dca− anions, with the halide atoms completing the triangular coordination around the copper centers [20]. The alloyed charge transfer salts with mixed halides, κ-(BEDT-TTF)2[Cu(dca)XnY1−n] (X, Y = Cl, Br, I), are also known [21,22]. The isostructural κ-(BEDT-TTF)2[Cu(dca)I] salt is not a superconductor because of disorder in the ethylene group of the BEDT-TTF molecules [23]. At room temperature, the Cu–N distances in these structures are in the range 1.946–1.985 Å. The non-superconducting κ-(BEDT-TSF)2[Cu(dca)Br] [BEDT-TSF is bis(ethylenedithio)tetraselenafulvalene] salt also contains a similar Cu(dca)Br− anion [24]. In all of these salts, one-dimensional chains of Cu(dca)X− are arranged in two-dimensional layers interspersed between sheets of BEDT-TTF+0.5 or BEDT-TSF+0.5 cations which are arranged in a κ-type packing motif [25]. It has been suggested that small amounts of copper(II) impurities can alter the resistivity and physical properties of these systems [26]. A novel (BEDT-TTF)Cu(dca)2 salt has also been reported, which contains a three-dimensional BEDT-TTF network. In this salt the polymeric Cu(dca)2− anion contains chains of distorted tetrahedrally coordinated copper(I) atoms connected by double μ1,5-dca− bridges [16].
Hybrid materials that possess both conducting and magnetic components are currently being intensely studied [27]. We are interested in developing structure/property correlations in model compounds which contain anionic metal dicyanamide coordination polymers. It is anticipated that such magnetic, anionic lattices can be incorporated into charge transfer salts. We previously reported the synthesis and characterization of the (BEDT-TTF)2[Mn(dca)3] salt that contains a novel triangular, spin frustrated, anion lattice separated by conducting layers of ET radical cations [9]. We have now been able to extend this work to include an isostructural salt of cobalt(II), (BEDT-TTF)2[Co(dca)3]. In this article, we describe the crystallization and crystal structure of (PPh4)3Cu4(dca)11, which contains a unique anionic topology. It is anticipated that this salt may serve as an electrolyte necessary for the preparation of the (BEDT-TTF)2[Cu(dca)3] analog.
2 Experimental
2.1 Synthesis of (PPh4)(dca)
Tetraphenylphosphonium bromide (22.674 g, 54.07 mmol, Aldrich) was dissolved in 400 ml water. Sodium dicyanamide (4.813 g, 54.07 mmol, Lonza) was dissolved in 200 ml water. These two solutions were combined and stirred thoroughly. The heavy white precipitate of (PPh4)(dca) was collected by suction filtration, rinsed with deionized water and dried with diethyl ether. This product was recrystallized from hot ethanol: (PPh4)(dca) (14.3 g, 35.27 mmol) was dissolved in 50 ml hot ethanol, filtered, and slowly cooled to yield colorless crystals. Anal. Calcd (%) for C26H20N3P: C, 77.02; H, 4.97; N, 10.37. Found: C, 77.04; H, 5.00; N, 10.40. Mp: 215–217 °C. IR (cm−1): 2228m, 2189m, 2129vs, 2100w, 1585w, 1482m, 1438s, 1307s, 1189w, 1163w, 1106vs, 1074w, 1028w, 996m, 932w, 901w, 862w, 760m, 753m, 722vs, 690s.
2.2 Synthesis of (PPh4)3[Cu4(dca)11]
(PPh4)(dca) (1 g, 2.47 mmol) was dissolved in 25 ml ethanol (Aaper, 200 proof). Copper(II) nitrate hydrate (199 mg, 0.86 mmol, Aldrich) was dissolved in 5 ml ethanol. These two clear solutions were combined and warmed slightly to get rid of cloudiness. After two days, a green solid, containing some green rods, was collected by filtration. One green, transparent rod-like single crystal was chosen for the single crystal X-ray study described below. The green solid was rather inhomogeneous, assumed to contain some Cu(dca)2 along with other possible byproducts, and was not further characterized. Additional attempts to grow pure phase material through the use of aqueous solutions and other copper(II) precursors were unsuccessful. Mp: 94–96 °C. IR (cm−1): 2360s, 2339s, 2311s, 2253m, 2238m, 2175vs, 2169vs, 1483w, 1457w, 1438m, 1375m, 1363m, 1336m, 1188w, 1164w, 1110s, 1028w, 996m, 753m, 724s, 689s, 668m.
2.3 Synthesis of Cu(dca)2
Cu(dca)2 was prepared according to the literature procedure [4]. Aqueous solutions of copper(II) nitrate hydrate (1396 mg, 6 mmol, Aldrich) and sodium dicyanamide (1068 mg, 12 mmol, Lonza) were combined and the precipitate collected by filtration and dried in air.
2.4 X-ray crystallography
The crystal structure of (PPh4)3[Cu4(dca)11] was determined by X-ray diffraction using a Siemens SMART single crystal diffractometer equipped with a CCD-based area detector and a sealed-tube Mo Kα source (graphite monochromator). The detector frames were integrated by use of the program SAINT [28] and the intensities corrected for absorption by Gaussian integration based on the measured crystal shape using the program XPREP of SAINT. Other systematic variations were corrected by the analysis of replicate reflections using the program SADABS [29]. The structure was solved by use of direct methods, while full-matrix least-squares refinement on F2 (including all data) was performed, both using the program package SHELXTL [30]. The analysis of the intensity differences due to anomalous scattering indicated the presence of merohedral twinning by inversion symmetry, which was included in the refinement. A summary of the crystallographic data is given in Table 1, selected bond lengths are given in Table 2, and selected bond angles in Table 3. The atomic numbering scheme is illustrated in Fig. 1 and in the Supplementary data (Figs. S1–S3), and further details of the structure are available in the CIF file also deposited as electronic supplementary information.
X-ray crystallographic data for (PPh4)3[Cu4(dca)11]
| Formula | C94H72Cu4N33P3 | Fw | 1998.82 |
| a (Å) | 7.1911(4) | Space group | P21 (no. 4) |
| b (Å) | 55.050(3) | T (K) | 298 |
| c (Å) | 11.7964(7) | ρcalc (g cm−3) | 1.440 |
| β (°) | 99.166(3) | μ (mm−1) | 1.03 |
| V (Å3) | 4610.2(7) | R(Fo)a | 0.0558 |
| Z | 2 | Rw(Fo2)b | 0.1169 |
a R(Fo) = ∑‖Fo| − |Fc‖/∑|Fo| (I > 2σ).
b Rw(Fo2) = [∑w(|Fo2| − |Fc2|)2/∑wFo2]1/2 (all data).
Selected bond lengths for (PPh4)3[Cu4(dca)11]
| Cu(1)–N(13) | 1.972(8) | N(13)–C(9) | 1.127(10) |
| Cu(1)–N(10) | 1.976(7) | N(14)–C(10) | 1.316(11) |
| Cu(1)–N(12)a | 1.980(7) | N(14)–C(9) | 1.326(11) |
| Cu(1)–N(15)b | 2.001(8) | N(15)–C(10) | 1.134(10) |
| Cu(1)–N(1) | 2.147(7) | N(16)–C(11) | 1.129(11) |
| Cu(1)–N(33)c | 3.200(7) | N(17)–C(11) | 1.301(12) |
| Cu(2)–N(16) | 1.944(8) | N(17)–C(12) | 1.338(13) |
| Cu(2)–N(19) | 1.952(8) | N(18)–C(12) | 1.122(11) |
| Cu(2)–N(3) | 1.982(8) | N(19)–C(13) | 1.148(11) |
| Cu(2)–N(4) | 1.989(8) | N(20)–C(14) | 1.289(12) |
| Cu(2)–N(21)b | 2.518(8) | N(20)–C(13) | 1.315(12) |
| Cu(2)–N(18)a | 2.715(9) | N(21)–C(14) | 1.142(11) |
| Cu(3)–N(6) | 1.940(7) | N(22)–C(15) | 1.144(11) |
| Cu(3)–N(22) | 1.965(9) | N(23)–C(15) | 1.289(13) |
| Cu(3)–N(7) | 1.972(7) | N(23)–C(16) | 1.305(14) |
| Cu(3)–N(25) | 1.976(8) | N(24)–C(16) | 1.118(13) |
| Cu(3)–N(27)b | 2.615(9) | N(25)–C(17) | 1.122(11) |
| Cu(3)–N(24)a | 2.825(10) | N(26)–C(18) | 1.291(14) |
| Cu(4)–N(33)b | 1.981(7) | N(26)–C(17) | 1.292(12) |
| Cu(4)–N(30)a | 1.984(7) | N(27)–C(18) | 1.170(12) |
| Cu(4)–N(31) | 1.986(7) | N(28)–C(19) | 1.137(10) |
| Cu(4)–N(28) | 1.990(8) | N(29)–C(20) | 1.318(11) |
| Cu(4)–N(9) | 2.157(7) | N(29)–C(19) | 1.325(12) |
| Cu(4)–N(15)d | 3.207(8) | N(30)–C(20) | 1.142(10) |
| N(1)–C(1) | 1.158(10) | N(31)–C(21) | 1.156(10) |
| N(2)–C(1) | 1.293(11) | N(32)–C(21) | 1.296(10) |
| N(2)–C(2) | 1.312(11) | N(32)–C(22) | 1.306(12) |
| N(3)–C(2) | 1.108(10) | N(33)–C(22) | 1.164(10) |
| N(4)–C(3) | 1.110(10) | P(1)–C(23) | 1.778(8) |
| N(5)–C(4) | 1.247(12) | P(1)–C(29) | 1.789(7) |
| N(5)–C(3) | 1.277(13) | P(1)–C(41) | 1.790(8) |
| N(6)–C(4) | 1.169(10) | P(1)–C(35) | 1.792(7) |
| N(7)–C(5) | 1.141(10) | P(2)–C(55) | 1.797(8) |
| N(8)–C(5) | 1.280(12) | P(2)–C(67) | 1.799(9) |
| N(8)–C(6) | 1.317(11) | P(2)–C(47) | 1.810(8) |
| N(9)–C(6) | 1.129(10) | P(2)–C(61) | 1.820(8) |
| N(10)–C(7) | 1.161(10) | P(3)–C(87) | 1.786(8) |
| N(11)–C(7) | 1.302(11) | P(3)–C(93) | 1.786(8) |
| N(11)–C(8) | 1.320(11) | P(3)–C(73) | 1.795(8) |
| N(12)–C(8) | 1.156(10) | P(3)–C(79) | 1.799(8) |
a x + 1, y, z.
b x − 1, y, z.
c −x + 2, y + 1/2, −z + 1.
d −x + 2, y − 1/2, −z + 1.
Selected bond angles for (PPh4)3[Cu4(dca)11]
| N(13)–Cu(1)–N(10) | 167.3(3) | C(4)–N(6)–Cu(3) | 178.4(8) |
| N(13)–Cu(1)–N(12)a | 86.2(3) | C(5)–N(7)–Cu(3) | 171.7(8) |
| N(10)–Cu(1)–N(12)a | 91.6(3) | C(5)–N(8)–C(6) | 124.0(8) |
| N(13)–Cu(1)–N(15)b | 92.5(3) | C(6)–N(9)–Cu(4) | 163.8(7) |
| N(10)–Cu(1)–N(15)b | 86.8(3) | C(7)–N(10)–Cu(1) | 161.9(6) |
| N(12)a–Cu(1)–N(15)b | 167.0(3) | C(7)–N(11)–C(8) | 119.3(7) |
| N(13)–Cu(1)–N(1) | 95.8(3) | C(8)–N(12)–Cu(1)b | 160.4(7) |
| N(10)–Cu(1)–N(1) | 96.9(3) | C(9)–N(13)–Cu(1) | 152.7(7) |
| N(12)a–Cu(1)–N(1) | 98.0(3) | C(10)–N(14)–C(9) | 119.5(7) |
| N(15)b–Cu(1)–N(1) | 95.0(3) | C(10)–N(15)–Cu(1)a | 154.1(8) |
| N(13)–Cu(1)–N(33)c | 88.8(2) | C(11)–N(16)–Cu(2) | 174.9(7) |
| N(10)–Cu(1)–N(33)c | 78.5(2) | C(11)–N(17)–C(12) | 120.5(10) |
| N(12)a–Cu(1)–N(33)c | 85.2(2) | C(13)–N(19)–Cu(2) | 178.1(7) |
| N(15)b–Cu(1)–N(33)c | 81.9(3) | C(14)–N(20)–C(13) | 122.3(9) |
| N(1)–Cu(1)–N(33)c | 174.5(2) | C(15)–N(22)–Cu(3) | 173.2(8) |
| N(16)–Cu(2)–N(19) | 176.1(3) | C(15)–N(23)–C(16) | 121.6(11) |
| N(16)–Cu(2)–N(3) | 88.6(3) | C(17)–N(25)–Cu(3) | 172.9(7) |
| N(19)–Cu(2)–N(3) | 91.7(3) | C(18)–N(26)–C(17) | 122.5(9) |
| N(16)–Cu(2)–N(4) | 88.8(3) | C(19)–N(28)–Cu(4) | 158.7(7) |
| N(19)–Cu(2)–N(4) | 90.8(3) | C(20)–N(29)–C(19) | 117.6(7) |
| N(3)–Cu(2)–N(4) | 177.1(3) | C(20)–N(30)–Cu(4)b | 160.8(7) |
| N(16)–Cu(2)–N(21)b | 90.5(3) | C(21)–N(31)–Cu(4) | 150.5(7) |
| N(19)–Cu(2)–N(21)b | 93.3(3) | C(21)–N(32)–C(22) | 119.9(7) |
| N(3)–Cu(2)–N(21)b | 85.6(3) | C(22)–N(33)–Cu(4)a | 154.7(7) |
| N(4)–Cu(2)–N(21)b | 95.7(3) | N(1)–C(1)–N(2) | 172.0(9) |
| N(16)–Cu(2)–N(18)a | 89.8(3) | N(3)–C(2)–N(2) | 170.4(10) |
| N(19)–Cu(2)–N(18)a | 86.4(3) | N(4)–C(3)–N(5) | 170.7(11) |
| N(3)–Cu(2)–N(18)a | 91.8(3) | N(6)–C(4)–N(5) | 171.0(10) |
| N(4)–Cu(2)–N(18)a | 86.8(3) | N(7)–C(5)–N(8) | 172.3(10) |
| N(21)b–Cu(2)–N(18)a | 177.4(3) | N(9)–C(6)–N(8) | 172.2(9) |
| N(6)–Cu(3)–N(22) | 91.2(3) | N(10)–C(7)–N(11) | 175.7(8) |
| N(6)–Cu(3)–N(7) | 176.6(3) | N(12)–C(8)–N(11) | 173.7(9) |
| N(22)–Cu(3)–N(7) | 87.5(3) | N(13)–C(9)–N(14) | 173.8(9) |
| N(6)–Cu(3)–N(25) | 89.9(3) | N(15)–C(10)–N(14) | 176.6(10) |
| N(22)–Cu(3)–N(25) | 177.5(4) | N(16)–C(11)–N(17) | 169.4(11) |
| N(7)–Cu(3)–N(25) | 91.3(3) | N(18)–C(12)–N(17) | 170.1(12) |
| N(6)–Cu(3)–N(27)b | 94.9(3) | N(19)–C(13)–N(20) | 172.1(10) |
| N(22)–Cu(3)–N(27)b | 87.6(3) | N(21)–C(14)–N(20) | 174.9(11) |
| N(7)–Cu(3)–N(27)b | 88.2(3) | N(22)–C(15)–N(23) | 171.6(12) |
| N(25)–Cu(3)–N(27)b | 94.6(3) | N(24)–C(16)–N(23) | 169.6(14) |
| N(6)–Cu(3)–N(24)a | 85.0(3) | N(25)–C(17)–N(26) | 173.9(10) |
| N(22)–Cu(3)–N(24)a | 91.1(3) | N(27)–C(18)–N(26) | 175.1(11) |
| N(7)–Cu(3)–N(24)a | 92.0(3) | N(28)–C(19)–N(29) | 171.8(9) |
| N(25)–Cu(3)–N(24)a | 86.8(3) | N(30)–C(20)–N(29) | 174.2(9) |
| N(27)b–Cu(3)–N(24)a | 178.6(3) | N(31)–C(21)–N(32) | 173.7(9) |
| N(33)b–Cu(4)–N(30)a | 165.4(3) | N(33)–C(22)–N(32) | 174.9(10) |
| N(33)b–Cu(4)–N(31) | 91.6(3) | C(23)–P(1)–C(29) | 107.8(4) |
| N(30)a–Cu(4)–N(31) | 86.5(3) | C(23)–P(1)–C(41) | 109.7(4) |
| N(33)b–Cu(4)–N(28) | 87.0(3) | C(29)–P(1)–C(41) | 109.9(4) |
| N(30)a–Cu(4)–N(28) | 92.1(3) | C(23)–P(1)–C(35) | 110.5(4) |
| N(31)–Cu(4)–N(28) | 169.0(3) | C(29)–P(1)–C(35) | 111.9(4) |
| N(33)b–Cu(4)–N(9) | 95.2(3) | C(41)–P(1)–C(35) | 107.0(4) |
| N(30)a–Cu(4)–N(9) | 99.3(3) | C(55)–P(2)–C(67) | 106.6(4) |
| N(31)–Cu(4)–N(9) | 95.6(3) | C(55)–P(2)–C(47) | 109.6(4) |
| N(28)–Cu(4)–N(9) | 95.4(3) | C(67)–P(2)–C(47) | 112.1(4) |
| N(33)b–Cu(4)–N(15)d | 81.9(2) | C(55)–P(2)–C(61) | 109.4(4) |
| N(30)a–Cu(4)–N(15)d | 83.6(2) | C(67)–P(2)–C(61) | 112.2(4) |
| N(31)–Cu(4)–N(15)d | 89.3(3) | C(47)–P(2)–C(61) | 106.8(4) |
| N(28)–Cu(4)–N(15)d | 79.7(2) | C(87)–P(3)–C(93) | 110.6(4) |
| N(9)–Cu(4)–N(15)d | 174.4(2) | C(87)–P(3)–C(73) | 114.2(4) |
| C(1)–N(1)–Cu(1) | 168.8(8) | C(93)–P(3)–C(73) | 105.9(4) |
| C(1)–N(2)–C(2) | 123.3(8) | C(87)–P(3)–C(79) | 107.9(4) |
| C(2)–N(3)–Cu(2) | 169.1(8) | C(93)–P(3)–C(79) | 108.9(4) |
| C(3)–N(4)–Cu(2) | 163.2(8) | C(73)–P(3)–C(79) | 109.2(4) |
| C(4)–N(5)–C(3) | 129.8(9) |
a x + 1, y, z.
b x − 1, y, z.
c −x + 2, y + 1/2, −z + 1.
d −x + 2, y − 1/2, −z + 1.
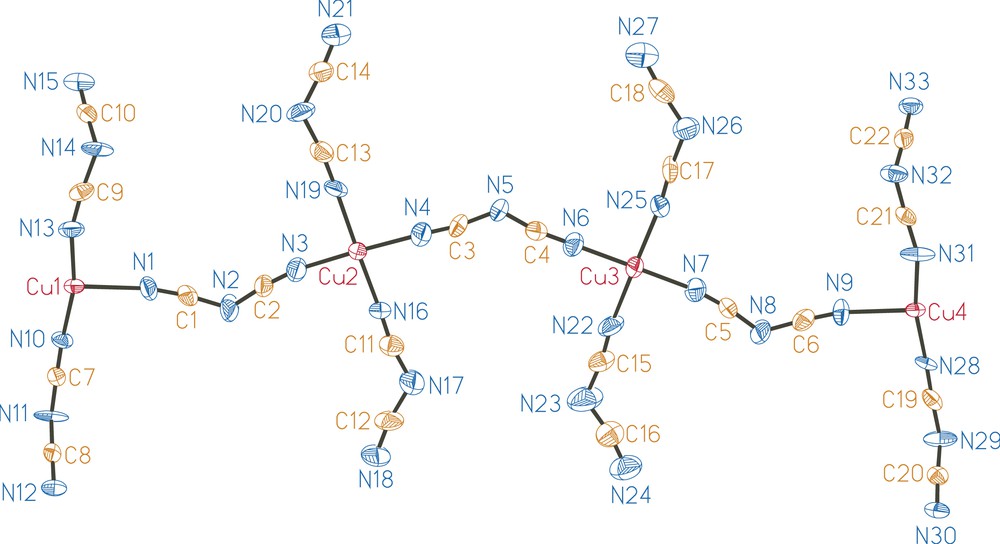
Atomic numbering scheme of the [Cu4(dca)11]3− anion in the (PPh4)3[Cu4(dca)11] salt. Thermal ellipsoids are drawn at the 50% probability level.
2.5 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were collected using a Bruker Vertex 70 spectrometer equipped with a mercury cadmium telluride (MCT) detector and a Pike ATR accessory.
3 Results and discussion
As illustrated in Fig. 1, the crystal structure of (PPh4)3[Cu4(dca)11] contains a polymeric anion with μ1,5-dca− bridges between copper(II) sites. As further illustrated in Fig. 2, the [Cu4(dca)11]−3 anions form two-dimensional layers in the ab plane. This anionic layer is constructed of linear trans μ1,5-dca− bi-bridged chains parallel to the a axis, which are in turn linked by three single μ1,5-dca− bridges parallel to the b axis. This forms a triple ladder (with single μ1,5-dca− rungs and double μ1,5-dca− legs) which runs along the a axis. Adjacent ladders are in close contact along the b axis, even though they are not covalently bonded to each other, thus completing the layer. As illustrated in Fig. 3, this structure thus represents a new member of the family of chain-like structures previously observed in the EPh4+ (E = P, As) salts of metal dicyanamides: a single bi-bridged chain is observed for (PPh4)2[Co(dca)4] [5], a double bi-bridged chain (ladder) for (AsPh4)2[M2(dca)6(H2O)(ethanol)x] (M = Co, Ni) [6], and the infinite bi-bridged chain (square lattice) for (EPh4)[M(dca)3] (E = P, As; M = Mn, Co, Ni) [5–7].
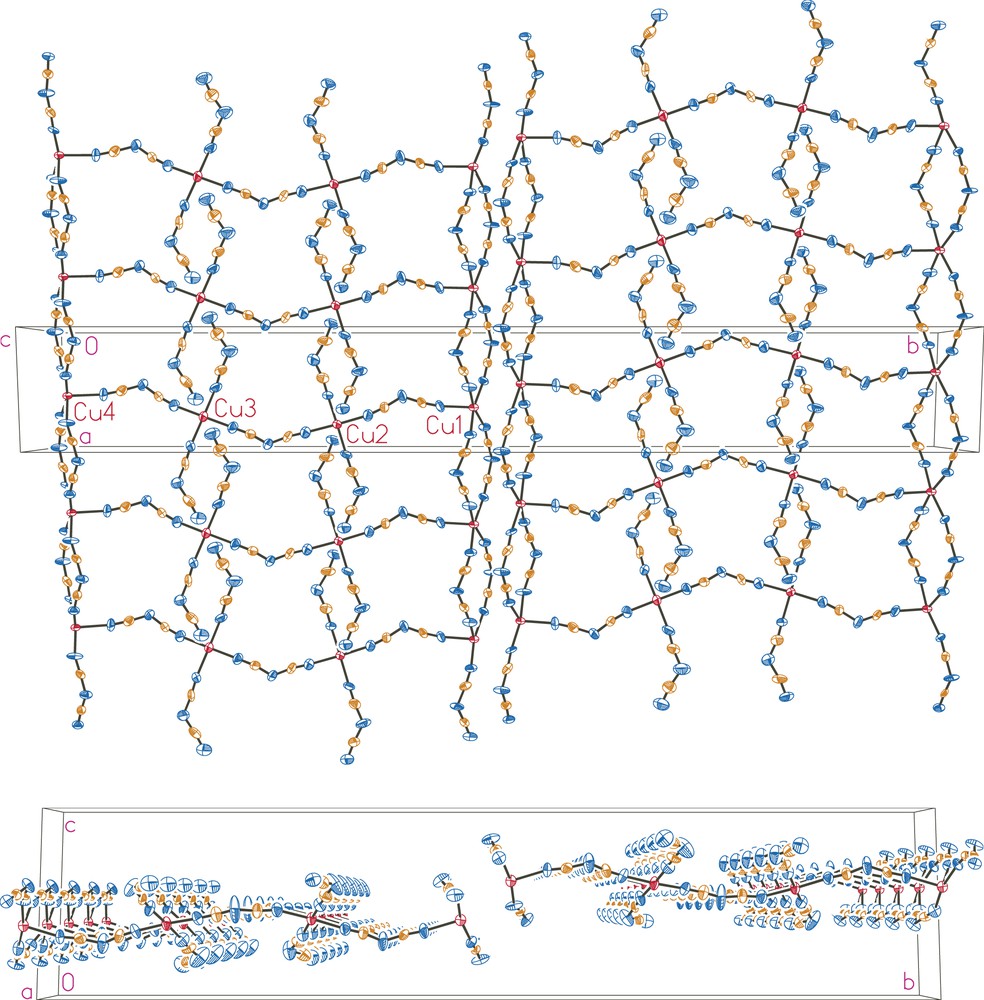
Packing diagrams of the [Cu4(dca)11]3− anion layer. Thermal ellipsoids are drawn at the 50% probability level. Top: view along the c axis; bottom: view along the a axis.
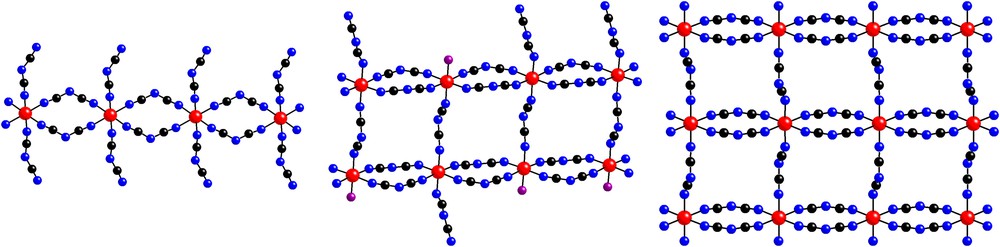
Schematic diagrams of the chain structure observed in (PPh4)2[Co(dca)4] [5] (left), the double bi-bridged chain (ladder) observed in (AsPh4)2[M2(dca)6(H2O)(ethanol)x] (M = Co, Ni) [6] (center, note that the terminal dca, water and ethanol are disordered; water and ethanol are illustrated as a single oxygen atom), and the infinite bi-bridged chain (square lattice) in (EPh4)[M(dca)3] (E = P, As; M = Mn, Co, Ni) [5–7] (right). The metal atoms are illustrated by circles with cross marks (online: red), the carbon atoms by a black circle (online: black), the nitrogen atoms by an open circle (online: blue) and the oxygen atoms as circles with a single stripe (online: violet). In all cases, the double μ1,5-dca− bridges lie in the horizontal direction and the single μ1,5-dca− bridges in the vertical direction.
There are four crystallographically independent copper atoms in the structure. These atoms show considerable Jahn–Teller distortion, with the axial nitrile nitrogen atoms considerably further from the copper. Cu2 and Cu3 are both octahedrally coordinated to six nitrile nitrogen atoms. Within the basal plane, the Cu–N distances range from 1.944(8) to 1.989(8) Å and 1.940(7) to 1.976(8) Å, respectively. The axial Cu–N distances are more than 0.5 Å longer: 2.518(8) and 2.715(9) Å for Cu2 and 2.615(9) and 2.825(10) Å for Cu3. This coordination geometry is similar to that observed in Cu(dca)2, where the average equatorial distance is 1.975(1) Å and the average axial distance is 2.478(2) Å [31]. Cu1 and Cu4 are both coordinated in a square pyramidal fashion to five nitrile nitrogen atoms. Within the basal plane, the Cu–N distances range from 1.972(8) to 2.001(8) Å and 1.981(7) to 1.990(8) Å, respectively, while the apical Cu–N distances are slightly longer: 2.147(7) and 2.157(7) Å, respectively.
The Cu⋯Cu separations along the singly bridged chains are about 1.1 Å longer than along the bi-bridged chains. The Cu1⋯Cu1, Cu2⋯Cu2, Cu3⋯Cu3, and Cu4⋯Cu4 distances through the double μ1,5-dca bridges, are defined by the a axis length, 7.1911(4) Å. The Cu⋯Cu separations through single μ1,5-dca bridges, which lie along the b axis are 8.352(1) Å for Cu1⋯Cu2, 8.159(1) Å for Cu2⋯Cu3 and 8.344(1) Å for Cu3⋯Cu4. The shortest Cu⋯Cu interactions are between the legs of adjacent triple ladders: the Cu1⋯Cu4 distances are 4.003(1) and 6.497(1) Å.
The cyano stretching region of the infrared spectrum for (PPh4)3[Cu4(dca)11] is compared to that of PPh4(dca) and Cu(dca)2 in Fig. 4. As compared to the non-coordinated dca− anion in the PPh4(dca) salt, the absorption bands are shifted to higher wavenumbers in the metal complexes. The bands below 2250 cm−1 have been assigned to (νas)CN and (νs)CN modes, while those at higher wavenumbers have been assigned to (νas + νs)CN combination modes [32]. The spectrum of the binary Cu(dca)2 complex is similar to that previously reported for the M(dca)2 (M = Cr, Mn, Co and Ni) analogs [32], while the (PPh4)3[Cu4(dca)11] spectrum is similar, but more complex, than that of the (NR4)[M(dca)3] (R = C3H7, C4H9 and C5H11; M = Mn and Ni) salts [13]. The additional complexity is due to the greater diversity of dca− coordination in (PPh4)3[Cu4(dca)11].
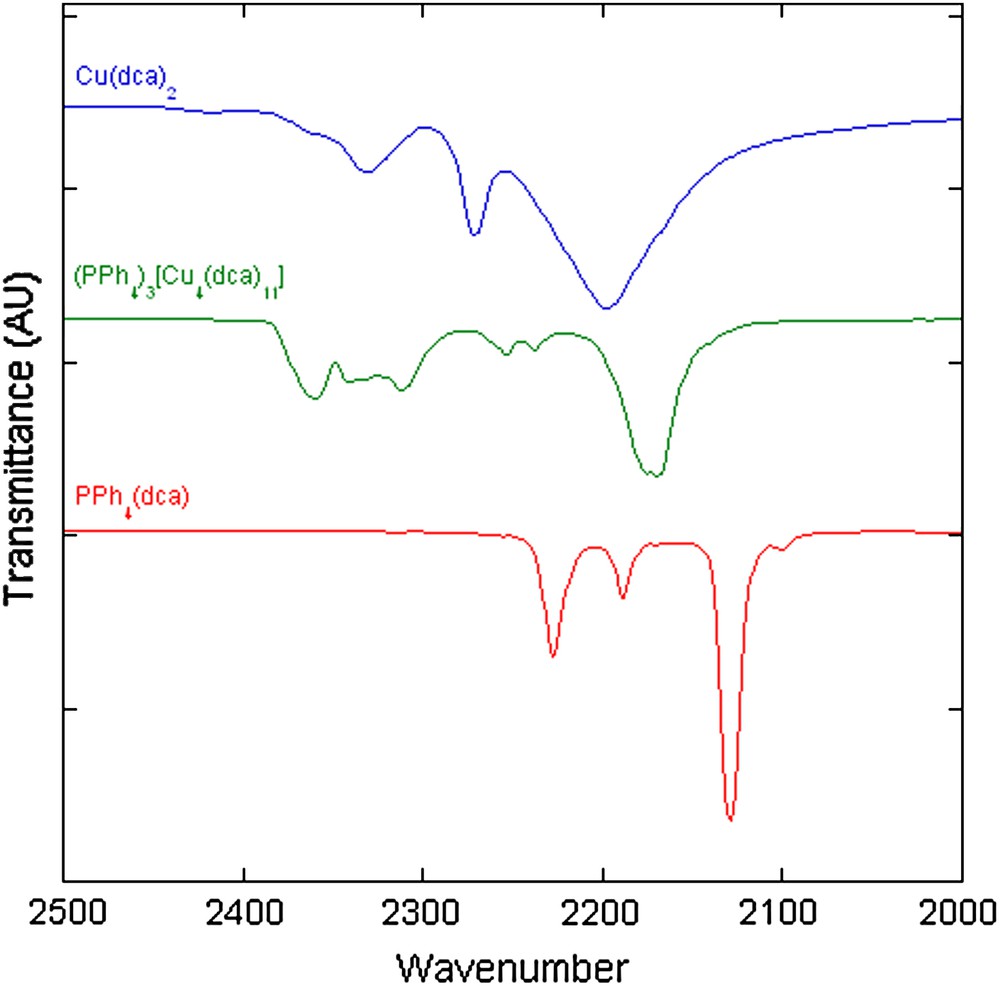
Comparison of the cyano stretching region of the infrared spectra of PPh4(dca), (PPh4)3[Cu4(dca)11], and Cu(dca)2. Spectra are offset for clarity.
Small single crystals of (PPh4)3[Cu4(dca)11], mechanically separated from a significant amount of polycrystalline material that formed simultaneously, were used for the X-ray and infrared analyses. It has been determined by elemental analysis that this polycrystalline byproduct is not of the same composition as the single crystals. We speculate that a large fraction of this powder may be neutral Cu(dca)2. It has not yet been possible to isolate a suitable quantity of phase pure (PPh4)3[Cu4(dca)11] for magnetic measurements. We are currently investigating various crystallization conditions in order to selectively crystallize (PPh4)3[Cu4(dca)11].
Because of the strong propensity of electron donor molecules, such as BEDT-TTF, to crystallize in two-dimensional motifs, we anticipate that similar anionic layers such as Cu4(dca)11− can be utilized as the charge compensating component in charge transfer salts. Through the use of the PPh4Mn(dca)3 electrolyte, we have previously shown that it is possible to electrocrystallize the (BEDT-TTF)2[Mn(dca)3] salt [9]. We report that we have recently electrocrystallized the isostructural (BEDT-TTF)2[Co(dca)3] salt through the use of the PPh4Co(dca)3 electrolyte. However, until now, there has been no suitable electrolyte to form the analogous (BEDT-TTF)2[Cu(dca)3] salt. We hope that the (PPh4)3[Cu4(dca)11] salt described in this paper will provide a suitable electrolyte for the formation of copper(II) dicyanamide-based charge transfer salts. We note that the Jahn–Teller distortion forces a structural change in the PPh4+ series of salts and that the (PPh4)3[Cu4(dca)11] salt is therefore not isostructural to the PPh4M(dca)3 (M = Mn, Co). It is therefore expected that if an BEDT-TTF-based charge transfer salt can becrystallized with a copper(II) dicyanamide anion, it may not be isostructural to (BEDT-TTF)2[M(dca)3] (M = Mn, Co).
4 Conclusions
We are interested in the crystallization of hybrid charge transfer salts that possess both electrically conducting and magnetic sublattices. Through the appropriate choice of templating cations, dicyanamide-based two-dimensional anionic coordination polymers can be constructed with divalent first-row transition metals. For this purpose, we have synthesized and structurally characterized the first anionic dicyanamide coordination polymer containing divalent copper. In this (PPh4)3[Cu4(dca)11] salt, the anion possesses a novel triple ladder motif and contains three distinct sets of Cu⋯Cu separations: long [8.352(1) to 8.519(1) Å], through single μ1,5-dca− bridges; intermediate [7.1911(4) Å], through double μ1,5-dca− bridges; and short [4.003(1) and 6.497(1) Å], through space interactions.
Acknowledgement
Work at Argonne National Laboratory was supported by the Office of Basic Energy Sciences, Division of Materials Science, U.S. Department of Energy under contract no. W-31-109-ENG-38.


