1 Introduction
The parent isoindole (I) was first described in 1973 [1]. Comprehensive reviews described the properties of isoindoles [2–4]. More recent monographs present annelated tricyclic isoindoles [5,6].
[4 + 2]-Cycloaddition is a common reaction for an isoindole aromatic system [7,8], and the identification of unstable isoindoles is often achieved by their Diels–Alder adducts with maleimides. This reaction for original isoindole system is well studied, endo- or exo-adducts are formed depending on kinetic or thermodynamic control conditions and there were the unique criteria for the adduct configuration determination [9–11]. A Michael conjugate addition is a possible side-reaction in such cases [12–17].
Isoindoles can be modified by annelation at border a with other aromatic and heteroaromatic rings. It becomes thus a part of a larger aromatic system with a nodal nitrogen in position 2. Nevertheless in some cases it keeps a part of autonomy by reacting at the first stage like a classical isoindole.
A theory of cycloaddition reactions for a annelated isoindoles was recently developed [18,19]. Several reactions of pyrido[2,1-a]isoindole system with different dienophiles are reported, which demonstrated that the reaction pathway depends on the dienophile and radical system [20–32]. Reactions of maleimides and other dienophiles with 6-methyl-6H-isoindolo[2,1-a]quinazolin-5-one [33,34] and maleimides with 1-methyl-2-R-1H-[1,2,3]triazolo[5,1-a]isoindoles as well as 1-methyl-1H-tetrazolo[5,1-a]isoindole [35] are also reported. The formation of adducts with a 7-azabenzonorbornene structure is common for the reactions of simple isoindoles with dienophiles, but new rearrangements of intermediate moieties were found.
Nevertheless, in the pyrimido[2,1-a]isoindole family, though the preparation of 2,4-dimethylpyrimido[2,1-a]isoindole and some homologous pyrimido[2,1-a]isoindole is described [36], and despite the fact that a preferred Diels–Alder reactions on the pyrrole fragment was predicted from quantum chemical calculations (PPP, CNDO/2) [13,14], the aromatic system, formally three cis-dienic moieties, is not studied in reactions with dienophiles.
Thus a research in this area is the goal of the presented work.
2 Results and discussion
First, we tested the reaction between 2,4-dimethylpyrimido[2,1-a]isoindole (I) and N-p-tolylmaleimide (II, R = p-tolyl) (Scheme 1). We found that I reacts with II in 1:2 molar ratios, even if different ratios were tested. This observation was also previously reported for its structural analog pyrido[2,1-a]isoindole (Ia) [25].

[R = H (a), benzyl (b), o-CH3–C6H4 (c), p-CH3–C6H4 (d), 2,5-CH3–C6H3 (e), p-CH3O–C6H4 (f), α-naphtyl (g)].
The reaction of I yielded a series of different products. In example, in the case of 2,4-dimethylpyrimido[2,1-a]isoindole and p-anisylmaleimide, more than 10 reaction products in noticeable concentration were observed by TLC. Further heating of the reaction mixture lead to the accumulation of a yellow fluorescent product. It was isolated pure as yellow needles, in low yield, by flash chromatography.
Other maleimides reacted in the same manner either with 2,4-dimethylpyrimido[2,1-a]isoindole or with its 6H-salt in presence of triethylamine, giving similar products which were isolated by column chromatography. In the case of N–H or N-benzylmaleimide, the corresponding products precipitate spontaneously from the reaction mixtures.
The obtained compounds are yellow solids with low solubility in organic solvents and strong green fluorescence in solutions. The spectral data are in accordance with the structure VI. A probable reaction path is presented in Scheme 1. The Michael adduct III contains an isoindole aromatic system. Therefore, it is able to accept one maleimide molecule more, giving the 7-azabenzonorbornene adduct IV, a combined Michael and Diels–Alder system. The exo-isomer of IV extrudes the bridged nitrogen thus yielding the naphthalene V (Scheme 2), which then hydrolyzes to end product VI.

Elimination in 7-azabenzonorbornene skeleton of exo-adducts IV.
For the benzo[f]isoindole, in 1H-NMR (DMSO-d6), the signals for the protons system are observed in the aromatic region. The 1H spectra of VI present typically two doublets at 8.4–8.6 ppm and two triplets at 7.7–7.9 ppm. Protons of the succinimide ring give doublets of doublets (7.6, 17.5 Hz) at 2.69–3.27 ppm (3.5 ppm in C5D5N) for one CH2 group, doublets of doublets (10, 17.5 Hz) at 3.06–3.58 ppm (3.74 ppm in C5D5N) for the other CH2 protons, the Hγ-proton signal is sometimes double (5.13–5.71 and 6.07–6.38 ppm), most likely because of hindered rotation in molecule, since all obtained compounds gave only one peak in HPLC. The NH2 protons appear like a broad singlet (7.4–8.2 ppm) and interchange with D2O. The COSY H–H spectrum of VIf in C5D5N presents three groups of cross-peaks for o-quinoid, succinimide and peripheral p-anisyl radical protons, respectively.
Similar fragmentations of 7-azabenzonorbornenes with extrusion of the bridged nitrogen and formation of a naphtalene system were described elsewhere [37]. Some reactions of 1-aminoisoindole derivatives were followed by the rearrangement of the 7-azabenzonorbornene adducts. The first example described is the reaction of the 2-aryl-3-dimethylaminoisoindole with maleimides followed by the subsequent cleavage of the 7-azabenzonorbornene intermediate and formation of 3,4-dihydronaphtalene derivatives [37]. Nevertheless no further elimination and full aromatization of the naphtalene system is observed due to the relative configuration of the starting hydrogen and nitrogen atoms in VIII. The final structure of IX points to an endo-configuration of the intermediate VIII (Scheme 3).
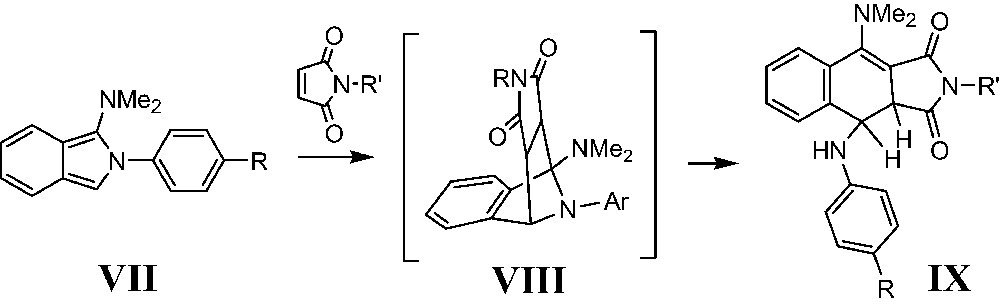
One more similar aromatization reaction was also described for isoindoles X, which turn into naphtalenes XI (Scheme 4) [38].

The obtained benzo[f]isoindole derivatives present interesting photo physical properties and high fluorescence quantum yields (Table 1).
UV–visible and fluorescence spectra of compounds VI in acetonitrile
| λ absorption (nm) | λ emission (nm), λ excitation (nm) | Quantum yield | |
| a | 338; 402.9 | 449, ex = 380 | 0.53 |
| b | 342; 402.9 | 456, ex = 380 | 0.19 |
| c | 344.3; 399.9 | 455, ex = 400 | 0.49 |
| d | 346; 407.8 | 456, ex = 400 | 0.21 |
| e | 345.5; 403.8 | 458, ex = 400 | 0.22 |
| g | 338.3; 402.9 | 460, ex = 380 | 0.30 |
| x | 266; 436 | 478, ex = 425 | 0.77 |
| x reference compound coumarin 314 |
The UV spectra present two main bands near 340 and 400 nm with quite the same intensity, the latter (π–π*) only being sensitive to excitation. The fluorescence spectra were recorded at 10–6 or 10–7 M to reach the concentration range requirement (absorbance UV # 0.05) and the relative quantum yields were determined by comparison with Coumarin 314 (from Aldrich). Finally, the functional groups (i.e. NH2) may be used for further modifications like grafting moieties in their perspective application as fluorescent probes.
In conclusion, the reaction between the 2,4-dimethylpyrimido[2,1-a]isoindole and maleimides is rather complex, passing through a Michael/Diels–Alder intermediate, the 7-azabenzonorbornene adduct IV which after an original rearrangement leads to a new series of benzo[f]isoindole derivatives VI. Finally, the theoretical suggestion of a diene behavior of pyrimido[2,1-a]isoindole (6,10b positions) got experimental support.
3 Experimental part
3.1 General methods
The 1H-NMR spectra (400.396 MHz in DMSO-d6 and C5D5N) were recorded with Varian Mercury 400 with TMS as internal standard.
The UV–visible spectra were recorded on a PerkinElmer Lambda-19 spectrophotometer equipped with a 60 mm integration sphere for solid measurements.
The fluorescence spectra were realized at room temperature with a PerkinElmer LS 50-B luminescence spectrophotometer.
The chemical ionization mass-spectra were recorded on API-365 PerkinElmer Sciex at the ‘Service commun de spectrométrie de masse’ of the Paul Sabatier University, Toulouse.
Elemental analysis was realized with a Carlo Erba Instrumentation analyzer.
3.2 Common procedure for reaction of 2,4-dimethylpyrimido[2,1-a]isoindole with maleimides
The addition of 0.2 mmol of isoindole (or of the mixture of its 6-H perchlorate salt with 1 ml of triethylamine) to a solution of 0.4 mmol of maleimide in 20 ml of butanol leads to a deep red reaction mixture. Followed by TLC, the maleimide disappeared within 4 hours in the solvent at reflux but further reaction proceeds slowly. After 30 h heating, the TLC reveals the presence of a fluorescent spot together with spots of seven other compounds and tarry residue in the reaction mixture. Their concentration did not change during the last 5 h. After evaporation of the butanol, the remaining dark red viscous oil was dissolved in 10 ml of CHCl3 and purified by flash chromatography with CHCl3 through silica gel. A red-colored phase with a green fluorescence was collected. After standing, yellow crystalline needles of the product were formed and then collected on filter. Yields are comprised between 20% and 80% and listed in the adduct properties.
3.3 HPLC-analysis of the reaction mixtures and the separated adducts
The analytical separations were realized with a HPLC system from HPLC 3D SysteMS with a UV DAD 200/4 detector. At 25 °C, 5 μl of sample dissolved in acetonitrile were injected in a Hypersil ODS-C18 (2, 1 × 200 mm) column. It was eluted with a mobile phase 10/90 CH3CN/H2O and a gradient to 10–90% of CH3CN in 35 min (flow rate: 0.25 ml/min).
3.4 Adducts properties
We give as example the nomenclature for VIa: 4-amino-9-(2,5-dioxo-pyrrolidin-3-yl)-benzo[f]isoindole-1,3-dione.
VIa. Yield 65%. M.p. 309 °C (subl.); HPLC retention time (min) 11.23; MW (m/z) 309.28 (309). Elemental analysis, calculated, (found), % C: 62.14 (62.27); H: 3.58 (3.62); N: 13.59 (13.35).
1H-NMR (in DMSO-d6): 11.63, 11.22, 11.19, 11.09 (four singlets, 2H), 8.48 (d, 1H), 8.40 (d, 1H), 7.78 (t, 1H), 7.71 (t, 1H), 6.07 and 5.13 (dd, 1H), 3.06 (dd, 1H), 2.69 (dd, 1H).
13C-NMR (in DMSO-d6): 37.35 (CH2), 38.47 (CH), 103.12 (C aromatic), 122.11, 125.04, 125.74, 125.90, 126.04, 126.16, 126.45, 127.79, 130.26, 134.65, 136.68 (C, CH aromatic), 145.36 (C–NH2), 169.62, 171.31, 178.09, 179.82 (C=O).
VIb. Yield 80%. M.p. 304 °C; HPLC retention time (min) 24.33; MW (m/z) 489.52 (489). Elemental analysis, calculated, (found), % C: 73.61 (73.82); H: 4.74 (4.76); N: 8.58 (8.51).
1H-NMR (in DMSO-d6): 8.52 (d, 1H), 8.45 (d, 1H), 7.81 (t, 1H), 7.74 (t, 1H), 7.25–7.50 (m, 12H), 6.13 and 5.34 (dd, 1H), 4.71 (m, 4H), 3.24 (dd, 1H), 2.83 (dd, 1H).
13C-NMR (in DMSO-d6): 35.95, 37.13, 42.63 (CH2), 38.12 (CH), 101.5 (C aromatic), 121.71–137.39 (C, CH aromatic), 145.63 (C–NH2), 167.91, 169.34, 176.61, 178.39 (C = O).
VIc. Yield 45%. M.p. 193 °C; HPLC retention time (min) 25.32; MW (m/z) 489.52 (489). Elemental analysis, calculated, (found), % C: 73.61 (73.77); H: 4.74 (4.85); N: 8.58 (8.53).
1H-NMR (in DMSO-d6): 8.53 (d, 1H), 8.47 (d, 1H), 7.83 (t, 1H), 7.71 (t, 1H), 7.52 (s, 2H) 7.30 (m, 8H), 5.45 (dd, 1H), 3.45 (dd, 1H), 2.83 (dd, 1H), 2.28 (s, 3H), 2.25 (s, 3H).
13C-NMR (in DMSO-d6): 17.65, 17.87 (Me), 37.65 (CH2), 38.8 (CH), 101.7 (C aromatic), 122.82–137.05 (C, CH aromatic) 145.84 (C–NH2), 167.4, 168.75, 175.7, 177.6 (C = O).
VId. Yield 45%. M.p. 268 °C; HPLC retention time (min) 26.77; MW (m/z) 489.52 (489). Elemental analysis, calculated, (found), % C: 73.61 (73.80); H: 4.74 (4.81); N: 8.58 (8.46).
1H-NMR (in DMSO-d6): 8.59 (d, 1H), 8.50 (d, 1H), 7.88 (t, 1H), 7.78 (t, 1H), 7.52 (s, 2H) 7.30 (m, 7H), 7.20 (m, 1H), 5.41 and 6.38 (dd, 1H), 3.37 (dd, 1H), 2.99 (dd, 1H), 2.39 (s, 3H), 2.35 (s, 3H).
VIe. Yield 40%. M.p. 161 °C; HPLC retention time (min) 27.75; MW (m/z) 517.57 (517). Elemental analysis, calculated, (found), % C: 74.26 (74.32); H: 5.26 (5.28); N: 8.12 (8.05).
1H-NMR (in DMSO-d6): 8.53 (d, 1H), 8.48 (d, 1H), 7.79 (t, 1H), 7.72 (t, 1H), 7.45 (s, 2H), 7.2–7.3 (m, 6H), 5.58 (dd, 1H), 3.41 (dd, 1H), 2.85 (dd, 1H), 2.2–2.3 (m, 12H).
13C-NMR (in DMSO-d6) 17.25, 17.38, 20.72, 20.81 (Me), 37.31 (CH2), 38.91 (CH), 101.75 (C aromatic), 123.02–136.62 (C, C-H aromatic), 145.75 (C–NH2), 167.43, 168.75, 175.75, 178.02 (C = O).
VIf. Yield 45%. M.p. 293 °C; HPLC retention time (min) 23.95; MW (m/z) 521.52 (521). Elemental analysis, calculated, (found), % C: 69.09 (69.20); H: 4.45 (4.52); N: 8.06 (7.95).
1H-NMR (in DMSO-d6) 8.58 (d, 1H), 8.47 (d, 1H), 7.86 (t, 1H), 7.78 (t, 1H), 7.54 (bs, 2H), 7.36 (d, 2H), 7.20 (d, 2H), 7.05 (m, 4H), 5.41 (dd, 1H), 3.91 (s, 3H), 3.89 (s, 3H), 3.17 (dd, 1H), 3.02 (dd, 1H).
1H-NMR (in C5D5N) 8.82 (d, 1H), 8.63 (d, 1H), 7.83 (t, 1H), 7.68 (t, 1H), 8.2 (bs, 2H), 7.73 (d, 2H), 7.55 (d, 2H), 7.04 (d, 2H), 6.98 (d, 2H), 5.71 (t, 1H), 3.63 (s, 3H), 3.58 (s, 3H), 3.74 (dd, 1H), 3.50 (dd, 1H).
VIg. Yield 30%. M.p. 167 °C; HPLC retention time (min) 27.42; MW (m/z) 561.59 (561). Elemental analysis, calculated, (found), % C: 76.99 (77.10); H: 4.13 (4.19); N: 7.48 (7.40).
1H-NMR (in DMSO-d6): 8.45 (d, 1H), 8.38 (d, 1H), 7.67 (t, 1H), 7.60 (t, 1H), 7.3–7.6 (m, 16H), 5.24 (m, 1H), 3.58 (dd, 1H), 3.27 (dd, 1H).
Acknowledgements
The authors wish to acknowledge the Paul Sabatier University (Toulouse, France) for financial support in the frame of the Toulouse–Kiev co-operation agreement and the Ukrainian Minister of Science and Education for the grant No. F7/442-2001 to the 03.07/00019 project in fundamental research.


