1 Introduction
Iron is the second most abundant metal after aluminium in the Earth's crust. This partly explains why living organisms have selected iron to achieve a large number of essential biological processes. Among the significant roles of iron in biology, one can highlight the transport, storage and activation of molecular oxygen, the reduction of ribonucleotides and dinitrogen, the activation and decomposition of peroxides and the electron transport via disparate carriers spanning a redox potential of essentially 1 V [1]. Consequently, iron is required for the growth of almost all living cells. Because life appeared in an oxygen-free environment, large concentrations of soluble ferrous iron were thus available for the emerging biological macromolecules. When photosynthesis, converting water into oxygen, became efficient, a dramatic chemical change occurred at the Earth's surface: large increase of the redox potential and irreversible precipitation of ferric iron as oxides and hydroxides. Actually, ferrous iron is extremely reactive towards oxygen leading to highly water insoluble ferric compounds at physiological pH. For example at pH = 7, the solubility of [Fe(H2O)6]3+ is about 10−17 mol L−1. The reaction between ferrous iron and oxygen also results in the formation of harmful superoxide and hydroxyl radicals [2]. The advent of O2, at some time after the origin of life, was a dreadful event for most of the living organisms, which died. Survivors had to build up new machinery for solubilizing essential iron. The only way to make it bio-available was to synthesize soluble chelators (low-molecular weight compounds called siderophores) having a very high affinity for Fe(III) [3]. The chelation of Fe3+ then may efficiently compete with the formation of hydroxides and oxides.
1.1 What role for chemists?
Besides their interest for a better understanding of iron metabolism in microorganisms (chemical tools), synthetic siderophores are relevant for their potential application as clinical iron-sequestering agents. On the other hand, water-soluble iron complexes can be used to alleviate iron deficiency in plants, preventing and even reversing iron chlorosis.
The design of ligands needs a rational approach to understand the metal ion complexing abilities. The pFeIII values (calculated for [Fe(III)] = 1 μM, [L] = 10 μM at pH = 7.4) are representative of the affinity of the ligands for Fe3+. The octahedral arrangement of donor atoms is the most favourable geometry, allowing the maximum possible distance between their formal or partial negative charges. Tris-bidentate type ligands are thus suitable for the pre-organization of the coordination sphere in order to get high pFeIII values. Natural siderophores (and most of their synthetic models) containing three catecholates or hydroxamates groups give stable Fe(III) octahedral complexes. This follows, since ferric ion is a hard acid and is more strongly bound by hard bases. Nevertheless, efficient chelators have been developed, involving other chelating groups. Water solubility and the hydrophilic/lipophilic balance are important parameters associated with the “assimilation” of the chelator or its ferric complex. Furthermore, additional properties may be needed for specific purpose (probes for studying iron transport, diagnostic tools, agent suited for vectorisation, etc.). Several reviews have described one or the other of these fields (see [4]).
We present here an overview of our own works concerning 8-hydroxyquinoline-based iron chelators. We also describe amphiphilic chelators which mimic iron acquisition in marine bacteria.
2 O-TRENSOX, a promizing water soluble iron chelator
Taking into account the great interest in complexation of both ferrous and ferric iron for chelation therapy (the potentially dangerous iron is Fe(II) and redox recycling of iron may occur in cells), we have developed the water-soluble chelator O-TRENSOX [5] (Fig. 1). This ligand was synthesized by connecting the TREN spacer through a tris-amide moiety to the 7th position of 8-hydroxyquinoline-5-sulfonic acid, a bidentate ligand involving both hard phenolate oxygen and a softer pyridine nitrogen as donor groups. O-TRENSOX exhibits strong complexing ability towards ferric cation, of the same order of magnitude as the tris-catecholate (TRENCAMS) [6] and the tris-hydroxamate (TRENDROX) [7] analogs (Fig. 1). Moreover O-TRENSOX possesses strong complexing ability towards the ferrous cation: as expected, [O,Npyr] instead of [O,O] donor sets allow strong complexation of both Fe(III) and Fe(II).
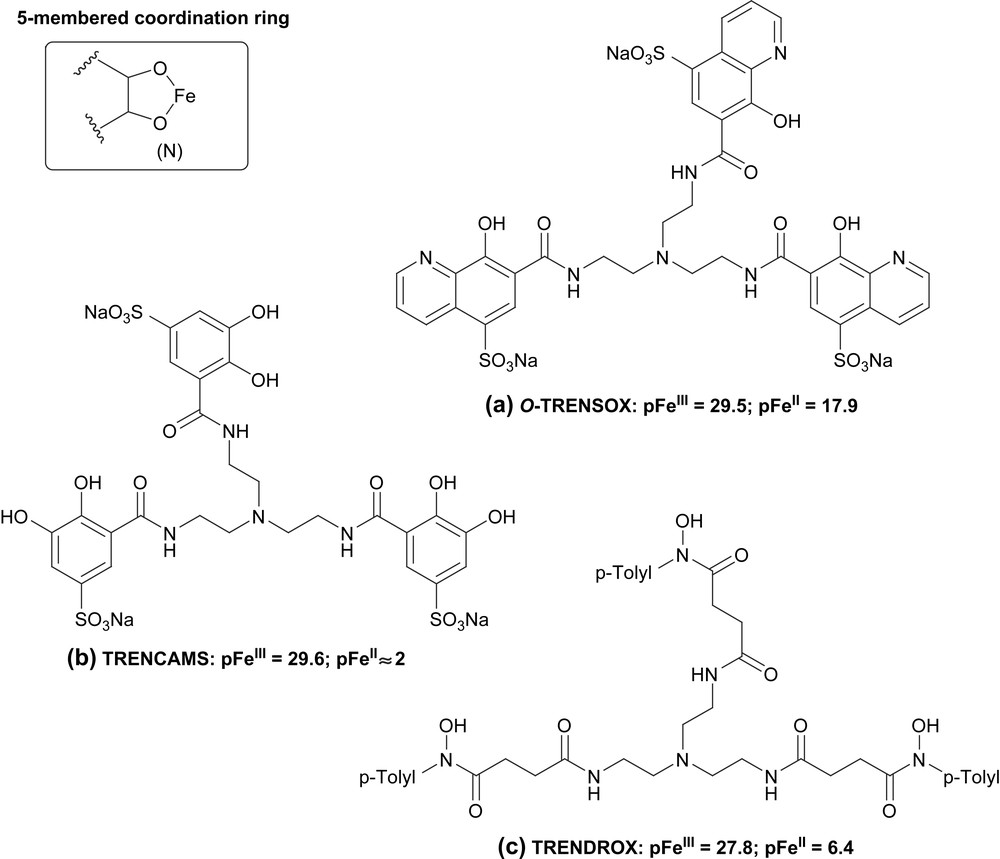
Formulae of O-TRENSOX (a) and its catechol (b) and hydroxamic acid (c) analogues.
2.1 Physicochemical properties of the complexes
2.1.1 Thermodynamic stability
The thermodynamic stability of Fe(III) complexes has been investigated by UV–vis spectrophotometry and potentiometric methods. At pH < 1, O-TRENSOX forms a protonated [FeLH5]2+ complex (orange colour, λmax = 435 nm, ɛ = 8200 M−1 cm−1) which deprotonates, over the pH range 1–2, in a green complex [FeLH]2− (λmax = 443 nm, ɛ = 5200 M−1 cm−1 and λmax = 495 nm, ɛ = 5200 M−1 cm−1) through a four-proton process. A pKa value of 5.60 for [FeLH]2− and an overall stability constant log β110 = 30.9 have been determined. Owing to the fact that pH can vary in a relatively large range between the different biological compartments, the knowledge of the pH dependence of the pFe value may be a decisive criteria for understanding the in vivo behaviour of a given iron chelator. The pFeIII = f(pH) plot [5] clearly shows that O-TRENSOX is the strongest synthetic iron chelator at pH < 7 and TRENCAMS is the strongest synthetic iron chelator at pH > 7. Cyclic voltammetry experiments indicate that the FeIII–O-TRENSOX/FeII–O-TRENSOX system is quasi-reversible, with a redox potential of 0.087 V vs NHE. This value is related to the high complexing ability of O-TRENSOX for both the ferric and ferrous iron, making it relevant for biological uses. The values log β110 = 19.3 and pFeII = 17.9 are calculated for the FeII–O-TRENSOX complex.
2.1.2 Modes of coordination
The interesting feature of O-TRENSOX is to allow two coordination modes for each arm of the ligand depending on the protonation state of the complex: in the deprotonated species, Fe(III) is coordinated via the bidentate unit (nitrogen from pyridine and oxygen from hydroxyle) (cf Fig. 4, vide infra) while in the protonated form the so-called salicylate-like coordination is observed, in which all the pyridine nitrogens are protonated. A tris-salicylate coordination for Fe(III) stabilized by a network of intramolecular hydrogen bonds has been characterized by X-ray crystallography for the protonated complex prepared in acidic medium (Fig. 2a) [8]. Hydrogen bonding has been also revealed in the tris-8-hydroxyquinolinate complex between the amide hydrogens and the coordinated quinoline oxygens (Fig. 2b and c).
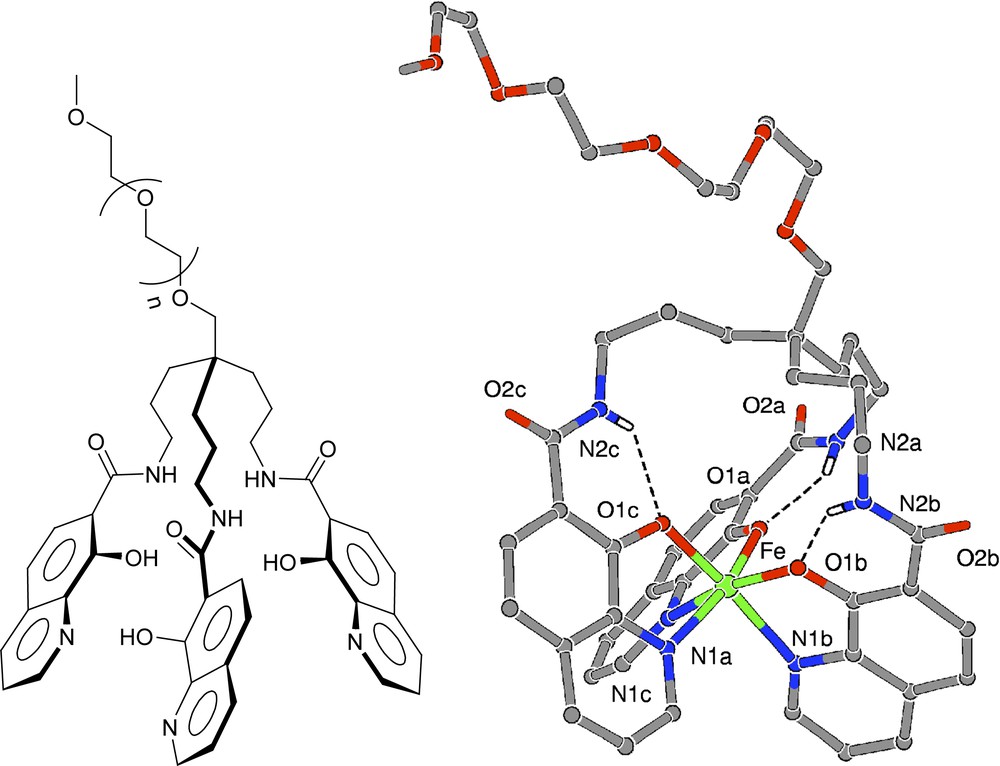
POE grafted iron chelators derived from O-TRENSOX (left) (n = 3: COX-200; n = 15: COX-750; n = 43: COX-2000). Structure of [Fe(COX-200)] (right).
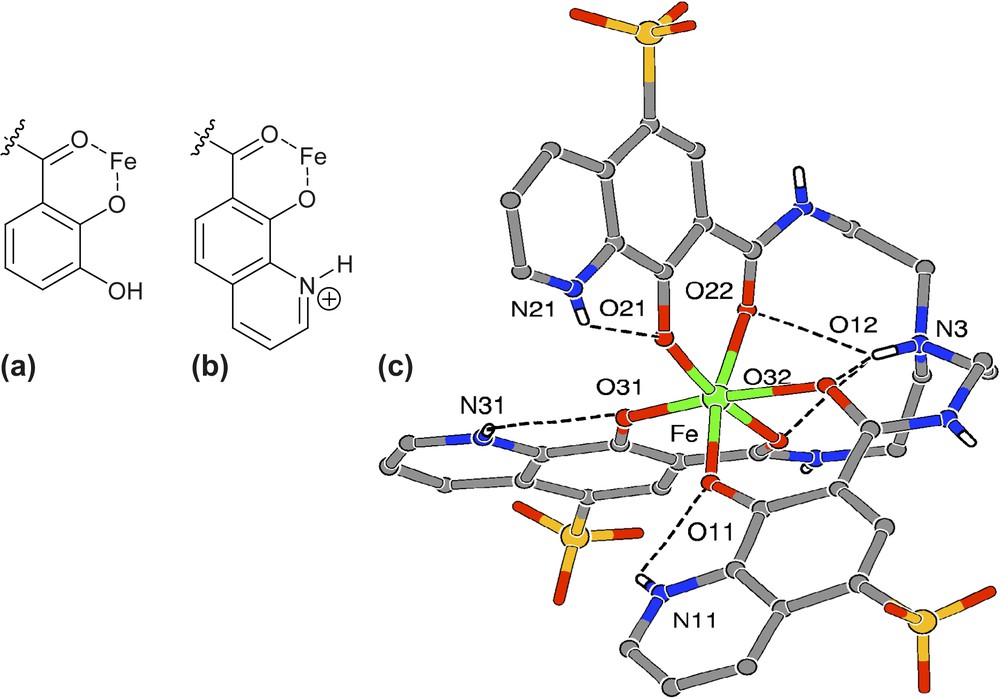
The tris-salicylate coordination modes in (a) catechol-amide, (b) hydroxyquinolinium-amide and (c) in the structure of Fe-O-TRENSOX.
It should be noted that N-TRENSOX, an analog of O-TRENSOX in which the 8-hydroxyquinoline subunits are connected to the tripodal spacer at the 2-position, was found to be significantly less efficient than O-TRENSOX with a pFe(III) value of 21.6 (which is eight orders of magnitude lower than for O-TRENSOX). This spectacular result has been assigned to a less efficient pre-organization of the octahedral coordination sphere with N-TRENSOX.
2.1.3 Fe(III) release mechanisms
Release kinetics of Fe(III) by O-TRENSOX has been studied through acid hydrolysis of the [FeL]3− complex. The kinetic data revealed a four-stage mechanism related to the successive protonation of the binding sites, their dissociations and their replacements by water ligands. Stage 1 has been found to occur very rapidly and has been attributed to the dissociation of a bidentate unit of the ligand. This reflects a high lability of one arm of the tripodal ligand, which is of interest from a ligand interchange process point of view. Stages 2 and 3 (643 M−1 s−1 and 0.26 s−1) correspond to a shift from a bis(8-hydroxyquinolinate) to a bis(salicylate) coordination involving two arms of the ligand. Stage 4 is related to the dissociation of the last coordinated arm of the ligand and is very slow (10 M−5 s−1). Taken together, these results demonstrate the ability of O-TRENSOX to retain iron even in low pH conditions (acidic media are encountered in gastrointestinal tract). This is a great advantage for potential applications in iron chelation therapy.
2.1.4 Selectivity of Fe(III) complexation towards other biologically relevant metal ions [9]
Toxicity of an iron chelator may reflect the chelation of other essential elements. A probe or a diagnostic tool should also be selective for a given metal ion. The complexation of several biologically relevant metal ions such as Cu(II), Zn(II) and Ca(II) has been studied. The case of Al(III) is also to be considered for the treatment of aluminium intoxication: Desferal®, the clinically used iron chelator, is also used in dialysis patients to remove Al(III). The pM values (calculated at pH 7.4) demonstrate a thermodynamic selectivity of O-TRENSOX for Fe3+ according to Fe3+ (29.5) >> Cu2+ (22.9) > Zn2+ (21.7) > Al3+ (20.0) > Fe2+ (17.9) > Ca2+ (13.6) and this selectivity is maintained over a pH range from 4 to 9.
3 Biological studies of O-TRENSOX and its iron complexes
3.1 Fenton Chemistry
In contrast to the Fe(III)–EDTA and Fe(III)–citrate complexes, Fe(III)–O-TRENSOX is not photoreducible. In the presence of ascorbate the corresponding ferrous complex does not induce radical damage when hydrogen peroxide is present as Fenton reagent. Tests were performed on supercoiled DNA as target molecules. The ligand may provide a kinetic barrier to the electron transfer to hydrogen peroxide [10].
3.2 Plant nutrition by FeIII–O-TRENSOX [10]
O-TRENSOX was found to be able to prevent and reverse iron chlorosis in several plant species grown in axenic conditions. It also allowed the iron nutrition and growth of Acer pseudoplatanus L. cell suspensions. The rate of iron uptake was monitored by 59Fe radio-iron; ferritins were shown to be the first iron-labeled protein during iron uptake and to further dispatch the metal. The rate of iron incorporation into ferritin was found to be notably higher with O-TRENSOX than with Fe–EDTA, which is the commercially available form of iron nutriment for plants.
3.3 Iron mobilization and cellular protection by O-TRENSOX [11]
Iron mobilization has been evaluated: (i) in vitro by using horse spleen ferritin and haemosiderin. DFO (desferrioxamine B) mobilized iron more rapidly at pH 7.4 than did O-TRENSOX, whereas at pH = 4, iron mobilization was similar with both chelators; (ii) in vitro by using cultured rat hepatocytes which had been loaded with 55Fe-ferritin, DFO was slightly more effective after 100 h than O-TRENSOX; (iii) in vivo i.p. administration to rats which had been iron loaded with iron dextran, O-TRENSOX mobilized 51.5% of hepatic iron over two weeks, compared to 48.8% for DFO. The effect of O-TRENSOX in decreasing the entry of 55Fe–citrate into hepatocyte cultures has been demonstrated. This protective effect of O-TRENSOX against iron toxicity induced in hepatocyte cultures by ferric citrate was shown by decreased release of the enzymes' lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase from the cultures and by decreased production of lipid radicals. O-TRENSOX was more effective than DFO in quenching hydroxyl radicals in an acellular system.
3.4 Antiproliferative and apoptotic effects of O-TRENSOX on differentiated human hepatoma cell lines [12]
The effect of O-TRENSOX has been investigated on proliferation and apoptosis in cultures of the human hepatoblastoma HepG2 and hepatocarcinoma HBG cell lines. O-TRENSOX was found to decrease DNA synthesis in a time- and dose-dependent manner with a higher efficiency than DFO. Mitotic index was also strongly decreased. O-TRENSOX induced accumulation of cells in the G1 and G2 phases of the cell cycle. The effect of O-TRENSOX on DNA synthesis and mitotic index were reversible after removing the chelator from the culture medium. O-TRENSOX also induced apoptosis in a dose- and time-dependent manner in proliferating HepG2 cells. In HBG cells O-TRENSOX induced apoptosis in proliferating and confluent cells arrested in the G1 phase of the cell cycle, demonstrating that inhibition of the proliferation and induction of apoptosis occurred independently. DFO induced DNA alteration only at concentration > 100 mM and without induction of caspase 3-like activity, indicating that DFO is not a strong inducer of apoptosis. O-TRENSOX, at concentrations of 20–50 pM, strongly inhibits cell proliferation and induces apoptosis in proliferating and non-proliferating HepG2 and HBG cells, respectively. Consequently, O-TRENSOX is a potential drug for chemotherapy.
3.5 Mixed ligands derived from O-TRENSOX [13]
Two mixed ligands involving one hydroxyquinolinate and two catecholate moieties (TRENSOXCAMS2, Fig. 3a) or two 8-hydroxyquinolinate and one catecholate moieties (TRENSOX2CAMS, Fig. 3b) have been studied. These two molecules constitute models to study cooperative effects in iron complexation in homologous series from O-TRENSOX to TRENCAMS. The pFe = 32.2 of TRENSOXCAMS2 constituted to our knowledge the greatest pFe for an abiotic iron chelator up to 2005.

Mixed ligand chelators and their corresponding pFe: (a) TRENSOXCAMS2 and (b) TRENCAMSSOX2.
4 From hydrophilic to lipophilic iron chelators [14]
Octanol–water partition coefficient, referred to as log P or log KOW, is a measure of a molecule's lipophilicity, giving an indication of its ability to cross cell membranes by a passive diffusion process. The octanol/water partition coefficients of O-TRENSOX (log P = 0.02) and its iron complex (log P < 0.005) are typical values for hydrophilic compounds (log P < 1) and therefore they cannot diffuse passively through the membrane. Moreover, transport by membrane aquaporin channels seems also forbidden since O-TRENSOX has a too high molecular mass to be transported out by those permeases. Accordingly, O-TRENSOX and its iron complex would not be expected to exhibit a biological activity requiring crossing the cell membranes. Nevertheless, O-TRENSOX exhibits cellular protection by iron mobilization from rat hepatocytes in vitro or in vivo, and the ferric complex of O-TRENSOX allows the metabolism of iron by plant cells. These questioning results led us to study the role of lipophilicity on the biological activity. We have synthesized a series of iron chelators having the same coordination sphere as O-TRENSOX (expecting the same pFe) with different hydrophilic/lipophilic balance modulated by a polyoxyethylenic chain (POE or PEG) of variable length, grafted on the molecular framework (Fig. 4).
The ferric complex of COX-200 was studied by X-ray crystallography and was the first example of a tripodal tris-hydroxyquinoline structure with an 8-hydroxyquinolinate coordinating mode (compared to the O-TRENSOX ferric complex which was crystallized in acidic medium and showed salicylate coordinating mode). So grafting a polyoxyethylenic chain does not change the structure of the coordination sphere (Fig. 4) and has negligible effect on the pFe. Indeed, the COX-2000 derivative exhibits a pFe value of 29.1, which is very close to that of O-TRENSOX.
The three COX ligands and their corresponding iron complexes constitute three interesting pairs with increasing hydrophilicity. The COX-200 pair is only octanol soluble (log P = 15.67 and 9, respectively, for the ligand and the ferric complex). The COX-750 pair is partitioned between the two phases octanol and water (log P = 0.78 and 0.9) and finally the COX-2000 pair, with the longer polyoxyethylenic chain, is only soluble in water (log P = 0.03 and 0.01). We have undertaken biological studies with the characterized complexes to determine their ability to supply iron to plants and to determine the ability of different ligands to mobilize iron.
4.1 Plant cell nutrition
The first results have been obtained with axenic plant cell cultures of Arabidopsis thaliana. The ferric complexes of O-TRENSOX, COX-750 and COX-2000 lead to the same kinetic results concerning the growth of the cells. These results reveal that the lipophilic/hydrophilic balance of the ferric complex is not, here, a criteria of the ability of the complex to allow incorporation of iron into the cells!
4.2 Iron mobilization from ferritin [15]
Mobilization of iron by C-SOX (a sulphonated hydrophilic analog of COX with an H atom instead of the POE chain) and COX-750 was similar and furthermore more efficient than DFO.
4.3 Iron mobilization from iron overloaded hepatocytes [15]
Despite its lack of lipophilicity, O-TRENSOX was found to be able to mobilize iron from hepatocytes. Rat hepatocyte cultures have been incubated with 55Fe-dextran and then treated by O-TRENSOX, COX-2000 or COX-750, respectively. Similar results are observed with the three chelators, which mobilize 30% of the cellular iron after six days. Again, the lipophilic/hydrophilic balance of the free chelators does not seem to be a criterion of the ability to mobilize intracellular iron! Our results question whether the use of partition coefficients is pertinent in the prediction of the activity of drugs, particularly for iron chelators designed for chelation therapy.
5 Self-assembling of an amphiphilic iron(III) chelator: mimicking iron acquisition in marine bacteria [16]
Among the mechanisms proposed for iron solubilization and transport into the cells [17], the self-assembling of amphiphilic siderophores from marine bacteria described by Butler and coworkers [18] exemplifies a noteworthy process. These siderophores (marinobactins and aquachelins) involving hydrophilic polar peptidic head-groups and hydrophobic fatty acid tails are surface-active amphiphiles that form self-assembled structures. We thought that the bioinspired use of this noteworthy strategy might resolve the crucial problem of the hydrophilic/lipophilic balance encountered with abiotic siderophores designed for iron nutrition. Amphiphilic iron chelators could also offer a new approach in iron chelation therapy.
To this end, three synthetic amphiphilic catechol-based chelators have been synthesized (Fig. 5): La and Lb are bidentate ligands with different chain length (C12 and C16, respectively) and LT is a tris-bidentate ligand with a C16 length chain; the physicochemical properties of their ferric complexes have also been characterized.

Chemical formulae of the synthetic amphiphilic ligands: La, Lb and LT.
The equilibria of the metal complexes of the tripodal chelator LT were studied by means of spectrophotometric and potentiometric titrations and showed that [FeLTH]2− and [FeLTH2]− are the major species at pH 7.4 (the full deprotonated ligand is noted as L6−). The spectral parameters (λmax = 540 nm, ɛ = 4000 M−1 cm−1) are consistent with a mixed salicylate/8-hydroxyquinolinate coordination. It should be noted that at pH = 9.2, the spectral parameters (λmax = 490 nm, ɛ = 4300 M−1 cm−1) indicate a tris-catecholate coordination. The spectrophotometric titration of a mixture of Fe(III) and La or Lb in a 1:3 molar ratio was carried out over the pH range 5–10. At pH 7.4, the spectrum (λmax = 540 nm, ɛ = 3000 M−1 cm−1) is characteristic of a bis-salicylate coordination, corresponding to the [Fe(La/bH)2]+ species.
The free ligands as well as their iron complexes exhibit tensioactive properties with CMC < 10−5 M in water/methanol 95/5 (v/v) solution at pH = 7.4 (MOPS buffer). The low CMC of the iron-free ligands are in the same range as for marinobactin. Dynamic light-scattering studies with 10−4 M solution of the Fe(III) complexes of La, Lb and LT in the same medium revealed the presence of spherical particles of 130 nm, 100–110 nm and 200–250 nm diameter, respectively. The free ligands, which do not scatter light, are limited to micellar assembly. It should be noted that similar observations have been made for the siderophores from marine bacteria.
Cryo-transmission electron microscopy (cryo-TEM) images of 1.4 × 10−3 M solution of the Fe(III) complexes showed polydisperse spherical particles ranging from 100 to 250 nm. An example is given in Fig. 6. The objects observed in the images of Fig. 6 indicate a structuring of the molecules of complexes in agreement with an external hydrophilic surface as schematically depicted by Butler and coworkers [18]. The actual structure of these particles is upon investigation.
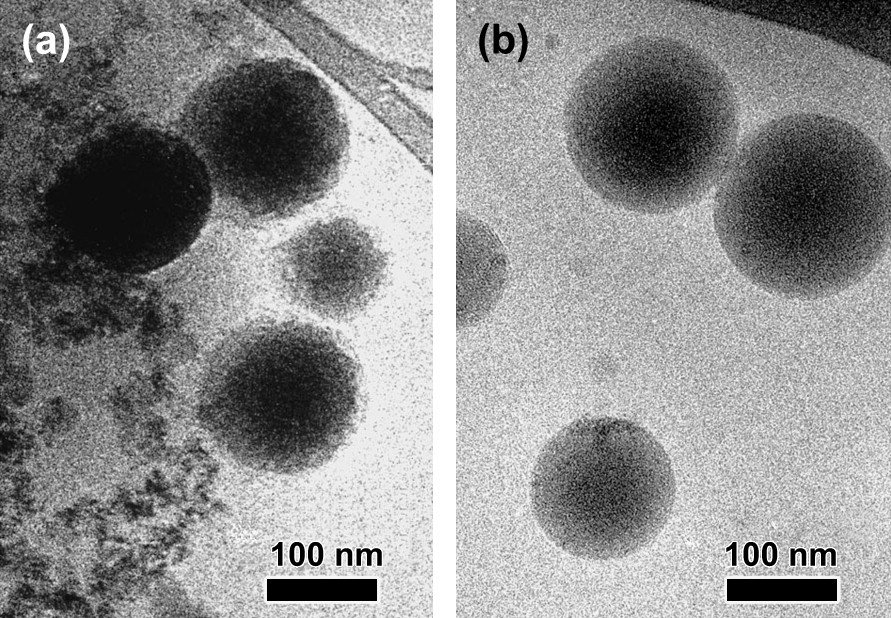
Cryo-TEM images of Fe(III)–Ln complexes in water/methanol 95/5 (v/v) solution at pH = 7.4 (MOPS buffer) embedded in vitreous ice for La 5 min after addition of 1% octanol (a); 3 h after addition of 1% octanol (b).
For a better understanding of the biological relevance of the self-assembling process (in correlation to the very interesting discussion of Butler and coworkersin Ref. [18a]), studies of bacterial iron nutrition have been performed with the gram-negative Erwinia chrysanthemi which produces two siderophores upon iron deficiency: chrysobactin (a monocatecholate) and achromobactin, which is produced only when chrysobactin synthesis is repressed [19]. Preliminary results show that: (i) the iron aggregates allow nutrition; (ii) the best results are obtained with the iron complex of La; (iii) when it is preserved, the natural siderophore way is used; (iv) nutrition is allowed in its absence. Moreover, we have shown that 70–75% of iron transport involves TonB dependent receptors Fct and/or 88Da with Cbu for the inner membrane. For the remaining 25–30%, it can be suggested that the aggregate merges with the outer membrane, releasing iron in the periplasm and then using Yfe to internalize iron (Yfe is not TonB dependent). Further experiments are needed to reach more definite conclusions concerning the precise role of the self-assembling process in iron acquisition. This process demonstrated by Butler and coworkers in marine bacteria opens fascinating perspectives for new applications in iron nutrition and for iron chelation therapy.
6 Conclusion
We are currently developing chemical tools (probes) with the aim of a better understanding of the biological properties: spin-labeled derivatives of O-TRENSOX to allow a localisation of the chelator in membranes, radioactive O-TRENSOX, fluorescent probes and a selective iron(III) electrode based on O-TRENSOX. On the other hand, we are currently studying amphiphilic fluorescent chelators.
The future age of the research on abiotic siderophores, suited for iron chelation therapy and/or iron nutrition involves a better knowledge of the penetration of the free ligand or of its iron complex through the biological membranes. The permeability and biological activity of siderophores are dictated by their three-dimensional structure and chirality. How their shapes fits specific membrane proteins is the main question which needs to be answered. Chemists have to turn their attention to this question. A better knowledge of the membrane proteins, whose structures are largely unknown, is needed for this purpose. The chemistry of the iron assimilation process is an open and fascinating field of new research.
Acknowledgments
The authors thank the following for fruitful collaboration or discussions: Drs. A.M. Albrecht (Strasbourg), A. Butler (Santa Barbara), D. Expert (Paris), M.-J. Stébé (Nancy), R. Crichton (Louvain la Neuve), A.L. Crumbliss (Durham), G. Lescoat (Rennes), A. Shanzer (Rehovot), F. Taran (Saclay) and J. Desbrières, M. Fontecave, J.-P. Laulhère, J.-L. Putaux, E. Saint-Aman (Grenoble).


