1 Introduction
2-(α-Hydroxyalkyl) 5-substituted 3-pyrrolidinols and their higher homologues 1a/1b are key structural features found in a number of polyhydroxylated bioactive alkaloids [1], and azasugars [2]. Castanospermine [3] (3) and bulgecinine [4] (4) are two typical examples among many others. For the asymmetric synthesis of such polyhydroxylated pyrrolidines, carbanion A represents a highly desirable synthon according to a conceptually attractive retrosynthetic analysis displayed in Scheme 1. However, although a huge number of methods have been developed for the carbanion-based C–C bond formation [5], and generation of chiral non-racemic N-α-carbanion of 4-hydroxy-2-pyrrolidinone A has been reported [6], the C–C bond formation based on synthon A, as well as generation and C–C bond formation of chiral non-racemic N-α-carbanion of 4-benzyloxy-2-pyrrolidinone B, remains as a challenging problem in carbanion chemistry [7,8].
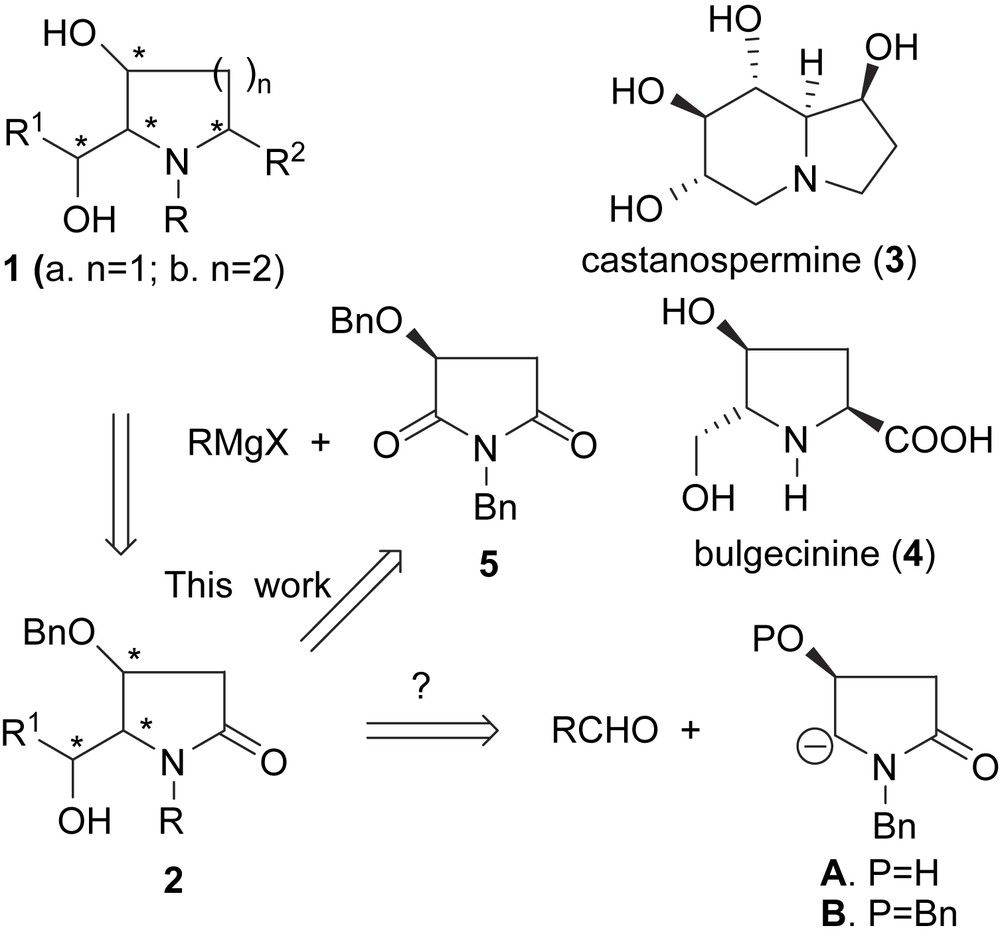
In recent years, we have been engaged [6] in the development of carbanion-based asymmetric approaches to 5-alkyl 4-hydroxy-2-pyrrolidinones (via tetramates) [9], 2-(α-hydroxyalkyl)-3-pyrrolidinols [7,10], 2,5-dialkyl-3-pyrrolidinols [9a], and 2-(α-hydroxyalkyl) 3-amino-pyrrolidines [11]. As a continuation of these studies and in connection with a related project [12], we have communicated recently a flexible approach to 5-(α-hydroxyalkyl) 4-benzyloxy-2-pyrrolidinones 2 [13], and we now report the full details of this method, and the results of further investigations on the key dehydration of diastereomeric N,O-acetals 6.
2 Results and discussion
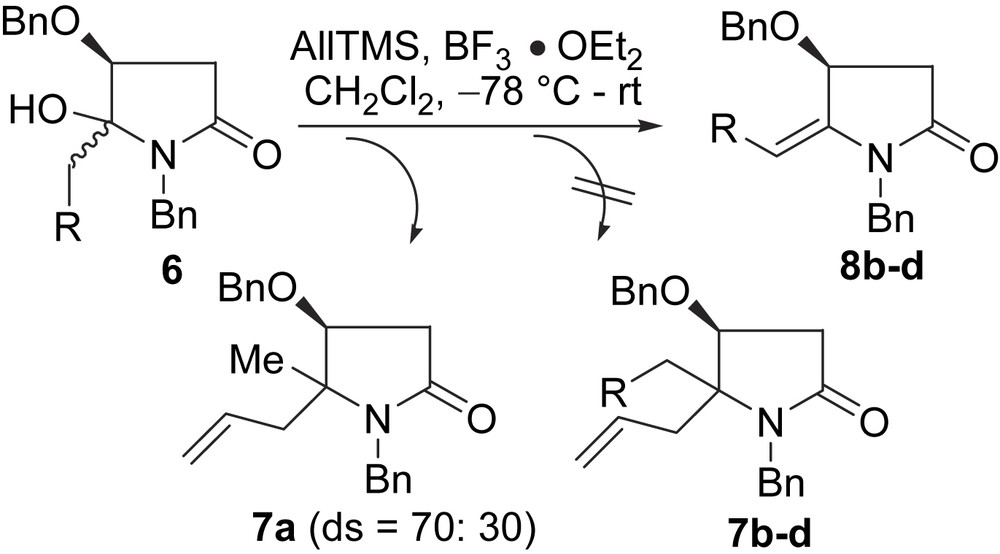
Our approach to 2 stemmed from some unexpected results obtained in a related project [12]. When we attempted the α-amidoallylation (AllTMS, BF3·OEt2, CH2Cl2, −78 °C to rt, 15 h) of N,O-acetals 6a–d, only 6a led to the desired α-amidoallylation product 7a, the reaction of 6b–d gave the dehydrated products 8b–d, respectively, in 75–82% yields (Scheme 2 and Table 1). In view of the recent advances in the enamide chemistries [14,15], it was realized that these findings would found a basis for a versatile approach to 5-(α-hydroxyalkyl)-4-benzyloxy-2-pyrrolidinones 2, and thus provide an alternative solution to the challenging problem of generating and reaction of synthon B.
Results of the attempted α-amidoallylations of 6a–da
| Entry | Starting material (alkyl group) | Product (yield, %) | |
| 1 | 6a (R = H) | 7a (86) | |
| 2 | 6b (R = n-Pr) | 8b (78) | |
| 3 | 6c (R = i-Pr) | 8c (75) | |
| 4 | 6d (R = n-C6H13) | 8d (82) |
a Reaction conditions: AllTMS, BF3·OEt2, CH2Cl2, −78 °C to rt, 15 h.
To this end, further investigations [13] on the acid catalyzed dehydrations [14] of N,O-acetals 6 [12] have been undertaken and TsOH·H2O turned out to be an effective and simple catalyst, which was used for further investigations.
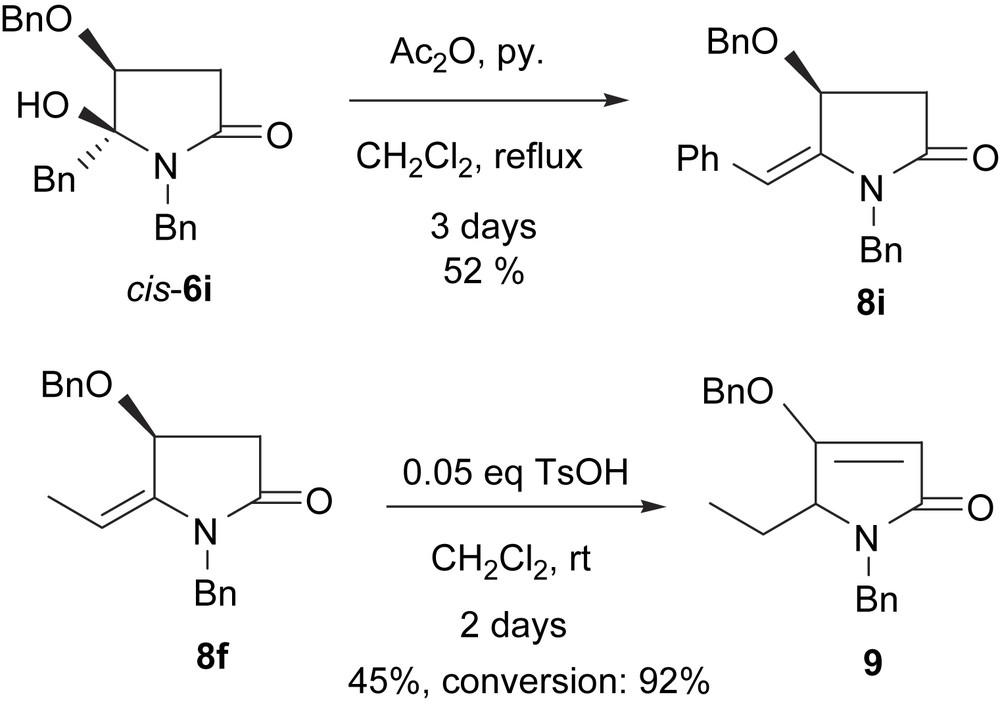
The requisite N,O-acetals 6 were obtained by Grignard reaction of (S)-N,O-dibenzylmalimide (5) as described previously [12] (Scheme 3). Most of the N,O-acetals (6a–h) were obtained with excellent C-2 regioselectivities (only one regioisomer was obtained in each case) and with diastereoselectivities ranging from 6:1 to 8:1. Recent single-crystal X-ray crystallographic analysis and NOESY experiments showed that the major diastereomer is trans-6 [16]. One exception is the reaction with benzyl magnesium bromide, which led to 6i as a 1:1 diastereomeric mixture. The results of the TsOH (5% mol. equiv)-promoted dehydration reactions of the major diastereomers of 6 are displayed in Table 2.

Grignard reactions with 5 and the subsequent TsOH-mediated dehydration reactions of 6
| Entry | RCH2MgX | Product 6 (yield %) | Product 8 (yield %) |
| 1 | CH3MgI | 6a (95)a | 8a (78c, 88d)e |
| 2 | n-PrCH2MgBr | 6b (95)a | 8b (74c, 97d) |
| 3 | i-PrCH2MgBr | 6c (86)a | 8c (83c, 95d) |
| 4 | n-C6H13CH2MgBr | 6d (90)a | 8d (63c, 92d) |
| 5 | n-BuCH2MgBr | 6e (81)a | 8e (69c, 91d) |
| 6 | MeCH2MgBr | 6f (83)a | 8f (67c, 95d) |
| 7 | EtCH2MgBr | 6g (99)a | 8g (79c, 93d) |
| 8 | BnCH2MgBr | 6h (95)a | 8h (55c, 89d) |
| 9 | PhCH2MgBr | 6i (92)b | 8i (77c, 93d) |
a Diastereomeric ratios: 6:1–8:1, only the major diastereomers were used for the dehydration.
b Diastereomeric ratio: ca. 1:1.
c Isolated yields.
d Yield based on the recovered starting material (cis-diastereomer).
e Conditions used: Ac2O/py/DMAP (cat), CH2Cl2, reflux, 2 days.
It is worth noting that the dehydration of 6a was unsuccessful under several acidic conditions (TsOH; TFA; CSA; HCl; H2SO4) [14c,14d]. To our delight, the desired dehydration product 8a (yield: 67%) was obtained by refluxing a mixture of 6a and Ac2O/py in CH2Cl2 for 2 days [14e]. If a catalytic amount of DMAP was added, higher yield (78%) of 8a was obtained (Table 2, entry 1).
More importantly, all the dehydration reactions were incomplete, and partial epimerization of trans-diastereomer (trans-6) to cis-diastereomer (cis-6) was observed according to TLC monitoring. To gain an insight into the reactivity difference between two pairs of diastereomers (cis-6/trans-6), the dehydration of either pure diastereomer or diastereomeric mixtures of cis-6e/trans-6e were performed separately. Pure diastereomer trans-6e provided the dehydration product 8e in 69% yield, alongside with 29% of cis-6e (Table 3, entry 1). The result implicates clearly partial epimerization of trans-6e to cis-6e under the reaction conditions. Starting from a 25:75 mixture of cis-6e/trans-6e, 8e and cis-6e were obtained in 62% and 34% yield, respectively (entry 2). A 1:1 mixture of cis-6e and trans-6e gave lower yield (48%) of 8e and higher portion of cis-6e (50%, entry 3). When started from pure diastereomer cis-6e (entry 4), little desired dehydration product 8e was observed. These results suggested that the yields of 8e are depended on the content of the trans-diastereomer in the starting diastereomeric mixture (cis-6e/trans-6e), namely, only the trans-diastereomer (trans-6e) can be readily converted into 8e.
The reactivity difference between diastereomers (cis-6e and trans-6e) in the dehydration reactiona
| Entry | Ratio of starting 6e (cis/trans) | Reaction time | Yield (%) of 8e/(recovered cis-6e) |
| 1 | 0:100 | 1 h | 69 (29)b |
| 2 | 25:75 | 1 h | 62 (34)b |
| 3 | 50:50 | 1 h | 48 (50)b |
| 4 | 100:0 | 1–12 h | c |
| 5 | 100:0 | 20 mind | 67 (33)e |
a Reaction conditions: 0.05 mol. equiv. TsOH, CH2Cl2, rt.
b Isolated yield.
c Detected by TLC monitoring, little product formed, most of the starting material remained unchanged.
d Reaction run in CDCl3 in a NMR tube and monitored by 1H NMR.
e Ratio of the two diastereomers determined by 1H NMR.
When performing the reaction under modified conditions (Ac2O, py) and at higher temperature with longer reaction time, the dehydration of the cis-diastereomer (cis-6i) was also observed (Scheme 4). However, prolonged reaction time led to the migration of the double bond yielding 3-pyrroline 9 (Scheme 4). Unexpectedly, when pure diastereomer cis-6e and a catalytic amount of TsOH were mixed in CDCl3 in a NMR tube for 20 min at rt, 1H NMR monitoring showed that the dehydrated product 8e was formed and the 8e/cis-6e ratio was 67:33 (Table 3, entry 5). This unexpected result can be attributed to the presence of a small amount of strongly acidic impurity in CDCl3.
These results suggest that cis-6 is the thermodynamically more stable diastereomer, which is in agreement with our computation prediction [16]. The computational studies showed that after chelation of Mg with two vicinal oxygens, the cis-addition (leading to trans-diastereomer) is kinetically more favorable over the corresponding trans-addition (leading to the cis-diastereomer), while the formation of the latter is more exothermic, demonstrating that it is the more thermodynamically stable isomer [16]. The different reactivities of the diastereomeric N,O-acetals under acidic conditions have been observed previously in a tricyclic oxazolidinolactam system [17]. This phenomenon can be attributed to a stereoelectronic effect [17,18].
Another feature of the dehydration reaction is that the reaction is highly stereoselective, and only (E)-enamides 8 were obtained. The stereochemistry of 8b was determined by NOESY experiments. The formation of enamides 8 was considered to proceed in two steps, namely dehydroxylation leading to an N-acyliminium intermediate C followed by a deprotonation-driven double-bond migration (Scheme 5).
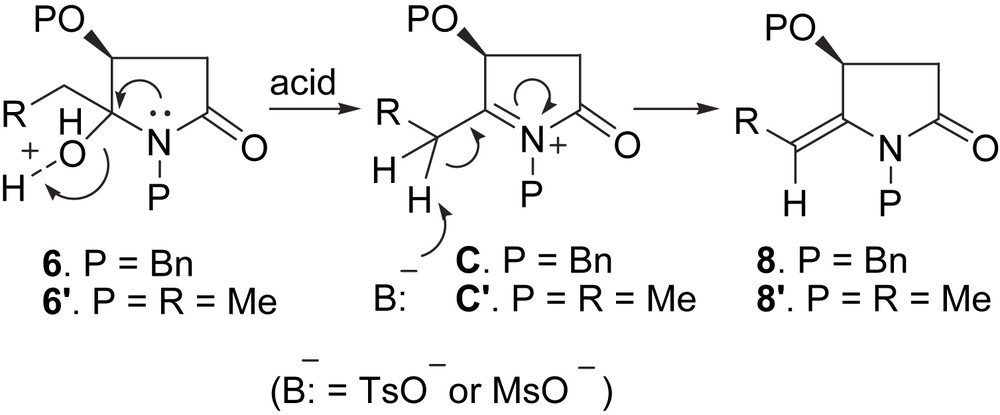
The exclusive formation of the (E)-enamides 8 could be understood in the light of the computational studies. It has been shown [16] that the delocalization of ð-electrons of the amide group (partial structure in the imide system) results in a C2–N1–C5 plane, which in turn gives rise to the rigidity of the five-membered ring, and hinders rotation of the RO–C3 around the C2–C3 bond. Because 8 is geometrically similar to a protected malimide, steric constrain between the alkyl group and N-Bn group in the (Z)-enamide is much more important compared with that between the alkyl group and O-Bn group in the (E)-enamide. As a result, (E)-enamide is the more thermodynamically stable isomer. This is confirmed by computational results, which shows that (E)-enamide 8f is more stable than (Z)-enamide 8f by 3.80 kcal/mol.
To get an insight into the stereoselective enamides formation, four possible transition states of mesylate promoted deprotonative double bond migration of model N-acyliminium intermediate C′ (Scheme 5) were located. As shown in Fig. 1, due to the steric interactions between MeO and mesylate in (E)-TS2 and (Z)-TS2, and R-C5 and the N-methyl group in (Z)-TS1, the relative energies increased in the sequence (E)-TS1 < (Z)-TS1 < (E)-TS2 < (Z)-TS2, and the (E)-TS1 is the preferred transition state. Consequently, the formation of the (E)-enamide is both thermodynamically and dynamically favored.
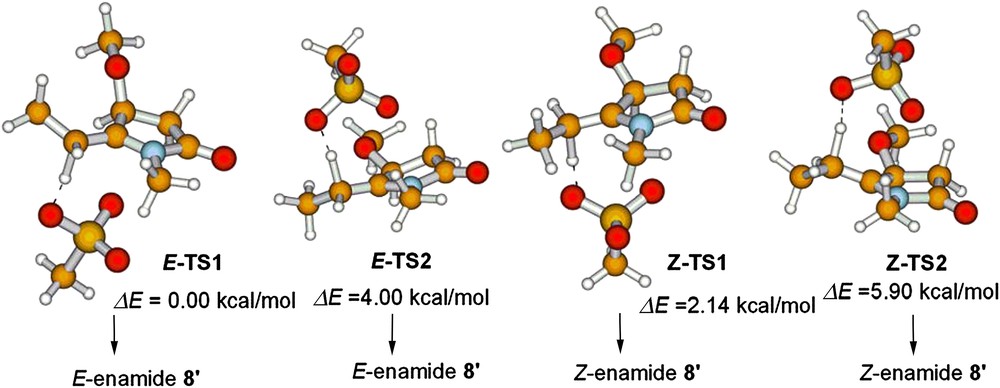
B3LYP/6-31G∗-optimized structures and relative energies of the transition states of mesylate-promoted deprotonative double-bond migration from the model N-acyliminium intermediate C′.
Next, the one-pot epoxidation-ring opening reactions of compounds 8 were investigated by using the method of Nagasaka and co-workers [15]. Thus when 8b was treated with 3 equiv MCPBA in a mixed solvent system of absolute MeOH and CH2Cl2 at −78 °C for 1 h, then warmed-up and stirred at rt for 10 h, the desired products 11b were obtained as a mixture of four diastereomers with a combined yield of 83% (Scheme 6). To confirm the structures of the products, flash column chromatography separation of a sample of diastereomeric mixture of 11b was undertaken, two pure diastereomers, and an inseparable mixture of the other two diastereomers were isolated and characterized.

The diastereomeric mixture of 11b (four diastereomers) was then subjected to Lewis acid mediated ionic hydrogenation (F3B·OEt2, Et3SiH, CH2Cl2, −78 °C to rt) [19,14], which gave two separable diastereomers trans-2b in 1:2 ratio with a combined yield of 78%. The fact that the reductive demethoxylation of a mixture of four diastereomers (11b) led to only two diastereomers (2b) might implicate that the transformation of 11b to 2b proceeded via the intermediacy of N-acyliminium ion [20] D (R = n-Pr), and the stereoselectivity at the C-5 of the 2-pyrrolidinone ring was higher than 95%. Both the two diastereomers of 2b were assigned to trans according to the observed vicinal coupling constant [21,13] (both J4,5 = ca. 0 Hz). To further confirm this point, a diastereomeric mixture of 2b (in 1:2 ratio) was treated with PCC (CH2Cl2, rt, 4 h, yield: 70%) (Scheme 7), and indeed, ketone (4S,5R)-12 was obtained as the sole diastereomer (J4,5 = 2.0 Hz) [21]. It allows us to conclude that the ionic hydrogenation resulted in high trans-stereoselectivity at the C-5 of 2-pyrrolidinone (2) and low selectivity at the C-1′. The stereochemistries at the C-1′of the two diastereomers of 2b were not determined.

With the MCPBA epoxidation-ring opening of 8b and the subsequent reductive demethoxylation reactions secured, the syntheses of other homologues or analogues of 2b were investigated and the results were outlined in Table 4. The results displayed in Table 4 showed that the one-pot epoxidation-ring openings of other enamides 8 worked similarly as 8b did in terms of chemical yields and diastereoselectivities, which demonstrated the flexibility of the method. Surprisingly, although the MCPBA epoxidation–MeOH ring opening reaction of 8a proceeded smoothly to give the desired N,O-acetal 11a in excellent yield, the subsequent reductive demethoxylation of 11a gave 2a in only 82:18 trans/cis diastereoselectivity.
Results of the MCPBA-mediated epoxidation–ring-opening reactions of 8 and the subsequent reductive demethoxylation reactions leading to 2
| Entry | Starting material | Product 11 (yield %)a | Product 2 (yield %)a | Stereoselectivity at C-1′ |
| 1 | 8a | 11a (91) | 2a (93)b,c | − |
| 2 | 8b | 11b (83) | 2b (78) | 1:2c |
| 3 | 8c | 11c (80) | 2c (85) | 1:4d |
| 4 | 8d | 11d (86) | 2d (81) | 1:4c |
| 5 | 8e | 11e (86) | 2e (78) | 1:1c |
| 6 | 8f | 11f (85) | 2f (85) | 1:1.6d |
| 7 | 8g | 11g (90) | 2g (74) | 1:2c |
| 8 | 8h | 11h (93) | 2h (98) | 1:1.5c |
| 9 | 8i | 11i (90) | 2i (98) | 1:2.6e |
a Combined yield of the diastereomers.
b Two diastereomers (trans/cis = 82:18) were obtained.
c Diastereomeric ratio determined by chromatography separation.
d diastereomeric ratio determined by 1H NMR.
e Ratio determined by HPLC.
As can be seen from Scheme 6, the diastereoselectivity at the C-1′ during the transformation of 8 to 2 is determined in the epoxidation step. The observed low diastereoselectivity of the epoxidation might be attributed to two plausible competing transition states E and F (Scheme 8). While transition state E is favoured by avoiding the steric interaction between the incoming MCPBA and the C-4 benzyloxy group, possible hydrogen bond formation in the transition state F recompenses the steric interaction and the resultant hinge effect led the epoxidation to occur from the β-face. On the basis of these mechanistic considerations, investigations were carried out to improve the diastereoselectivity of the epoxidation by trying to alert the hydrogen bond formation. First, the reaction was performed at rt and in CH2Cl2 (instead of using a mixed solvent system: MeOH/CH2Cl2) for 2 h. However, only degradation [22] product 5 was obtained in 71% yield. When running the reaction at −78 °C for 1 h, then warmed-up and stirred at rt for 10 h, namely under the standard conditions (vide supra) except CH2Cl2 was used as the solvent, 5 was obtained once again in 72% yield. Next, we also tried the Ag2O/I2 system [23] under two types of conditions. Unfortunately, all led to complex mixture of products.

3 Conclusions
In summary, a flexible four-step trans-diastereoselective approach to (4S,5R)-N-benzyl-4-benzyloxy-5-hydroxyalkyl-2-pyrrolidinones 2 has been developed starting from (S)-N,O-dibenzyl malimide (5). To the best of our knowledge, this represents the first flexible asymmetric approach to the substituted 2-pyrrolidinone derivatives 2, and provides an alternative solution to the challenging problems showed retrosynthetically in Scheme 1. Importantly, the observed higher reactivity of the trans-diastereomer of 6 towards the dehydration reaction, the isomerization of trans-diastereomers to cis-diastereomers, as well as the exclusive formation of the (E)-enamides 8, provides experimental proofs to our previous predictions made on the basis of computational studies, or has been rationalized by calculations.
4 Experimental
4.1 General
Melting points were determined on a Yanaco MP-500 micromelting point apparatus and were uncorrected. Infrared spectra were measured with a Nicolet Avatar 330 FT-IR spectrometer using the film KBr pellet technique. 1H NMR spectra were recorded in CDCl3 on a Bruker AV400 or a Varian Unity+ 500 spectrometer with tetramethylsilane as an internal standard. Chemical shifts are expressed in δ (ppm) units downfield from TMS. Mass spectra were recorded by Bruker Dalton Esquire 3000 plus LC-MS apparatus. Optical rotations were measured with a PerkinElmer 341 automatic polarimeter. Flash column chromatography was carried out on silica gel (300–400 mesh). THF was distilled over sodium. Dichloromethane was distilled over P2O5.
4.2 General procedure for the preparation of 6a–i from (S)-N,O-dibenzyl malimide (5)
To a cooled (−15 to −10 °C) solution of N,O-dibenzylmalimide 5 (1.0 mmol) in dry CH2Cl2 (10 mL) was added dropwise a Grignard reagent (3.0 mmol) in diethyl ether under nitrogen atmosphere. After being stirred at the same temperature for 4 h, the reaction was quenched with a saturated aqueous solution of ammonium chloride (6 mL) and extracted with dichloromethane (3 × 30 mL). The combined extracts were dried over anhydrous Na2SO4, filtered and concentrated in vacuum. Filtration through a short pad of SiO2 eluting with ethyl acetate–petroleum ether gave a mixture of two diastereomers 6 (yield: 81–99%), which, without separation, was used in the next step as it was. The diastereomeric ratios were determined either by flash chromatographic separation or by 1H NMR spectroscopy of the crude mixture.
4.3 (S)-1-Benzyl-4-(benzyloxy)-5-methylenepyrrolidin-2-one (8a)
To a solution of 6a (1.0 mmol) and DMAP (0.05 mmol) in CH2Cl2 (10 mL) were added pyridine (0.8 mL, 10.0 mmol) and Ac2O (0.47 mL, 5.0 mmol). The mixture was heated at reflux for 2 days, then cooled to room temperature, diluted with CH2Cl2, and washed successively with 1.0 M HCl and water. The organic layer was separated, dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was purified by column chromatography on silica gel eluting with ethyl acetate/PE (1:2) to give 8a (yield 78%) as a colorless oil: [α]D20 +60.0 (c 0.4, CHCl3); IR (film): 3030, 2923, 1722, 1648, 1449, 1394, 1341, 1211, 1070 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.65 (dd, J = 3.0, 17.5 Hz, 1H, COCH2), 2.79 (dd, J = 7.3, 17.5 Hz, 1H, COCH2), 4.40 (dd, J = 1.4, 1.9 Hz, 1H, CH2), 4.45 (dd, J = 1.0, 1.9 Hz, 1H, CH2), 4.51–4.53 (m, 1H, BnOCH), 4.54 (d, J = 11.6 Hz, 1H, PhCH2O), 4.59 (d, J = 11.6 Hz, 1H, PhCH2O), 4.65 (d, J = 15.5 Hz, 1H, PhCH2N), 4.75 (d, J = 15.5 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 37.2, 43.4, 70.5, 72.6, 89.5, 127.1, 127.4, 127.9, 128.0, 128.5, 128.6, 135.6, 137.4, 146.4, 173.3 ppm; MS (ESI, m/z): 316 (M + Na+, 6), 294 (M + H+, 100); Anal. calcd for C19H19NO2: C, 77.79; H, 6.53; N, 4.77. Found: C, 77.74; H, 6.39; N, 4.42.
4.4 Representative procedure for the dehydration of N,O-acetals 8b–i
To a solution of 6b (62 mg, 0.2 mmol) in CH2Cl2 (3 mL) was added a catalytic amount of p-toluenesulfonic acid monohydrate. The mixture was stirred at rt for 2 h. The reaction was quenched by a saturated aqueous NaHCO3 and extracted with CH2Cl2 (3 × 5 mL). The combined extracts were washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was purified by column chromatography on silica gel eluting with ethyl acetate/PE (1:2) to give 8b (44 mg, yield 74%) as a colorless oil.
4.4.1 (S,E)-1-Benzyl-4-(benzyloxy)-5-butylidenepyrrolidin-2-one (8b)
Compound 8b: yield, 74%. Colorless oil; [α]D20 +62.0 (c 0.4, CHCl3); IR (film): 3060, 3023, 2957, 2929, 2870, 1719, 1674, 1496, 1454, 1409, 1340, 1230, 1201, 1070, 1028 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.80 (t, J = 7.3 Hz, 3H, CH3), 1.22–1.38 (m, 2H, MeCH2), 1.94–2.12 (m, 2H, EtCH2), 2.68 (dd, J = 1.7, 17.8 Hz, 1H, COCH2), 2.78 (dd, J = 7.0, 17.8 Hz, 1H, COCH2), 4.42 (d, J = 11.2 Hz, 1H, PhCH2O), 4.53 (d, J = 11.2 Hz, 1H, PhCH2O), 4.70 (s, 2H, PhCH2N), 4.74 (dd, J = 1.7, 7.0 Hz, 1H, BnOCH), 4.84 (t, J = 7.5 Hz, 1H, CH), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 13.6, 23.3, 28.7, 36.6, 43.4, 69.9, 70.2, 108.0, 127.0, 127.2, 128.0, 128.1, 128.3, 128.4, 128.5, 135.8, 137.3, 138.9, 173.1 ppm; MS (ESI, m/z): 336 (M + H+, 100); Anal. calcd for C22H25NO2: C, 78.77; H, 7.51; N, 4.18. Found: C, 78.81; H, 7.47; N, 4.00.
4.4.2 (S,E)-1-Benzyl-4-(benzyloxy)-5-(2-methylpropylidenepyrrolidin-2-one (8c)
Compound 8c: yield, 83%. Colorless oil; [α]D20 +38.8 (c 1.0, CHCl3); IR (film): 3030, 2957, 2867, 1718, 1673, 1410, 1339 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.88 (d, J = 6.5 Hz, 3H, CH3), 0.96 (d, J = 6.5 Hz, 3H, CH3), 2.48–2.58 (m, 1H, Me2CH), 2.68 (dd, J = 2.0, 17.9 Hz, 1H, COCH2), 2.78 (dd, J = 7.0, 17.9 Hz, 1H, COCH2), 4.44 (d, J = 11.2 Hz, 1H, PhCH2O), 4.52 (d, J = 11.2 Hz, 1H, PhCH2O), 4.67 (dd, J = 2.0, 7.0 Hz, 1H, BnOCH), 4.68 (s, 2H, PhCH2N), 4.77 (d, J = 7.0, 1.8 Hz, 1H, CH), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 23.6, 23.7, 26.7, 36.5, 43.4, 69.9, 70.4, 115.5, 127.0, 127.2, 127.9, 128.0, 128.4, 135.8, 137.1, 137.3, 173.0 ppm; MS (ESI, m/z): 337 [(M + 2H)+, 24], 358 (M + Na+, 36), 336 (M + H+, 100); Anal. calcd for C22H25NO2: C, 78.77; H, 7.51; N, 4.18. Found: C, 78.21; H, 7.37; N, 4.36.
4.4.3 (S,E)-1-Benzyl-4-(benzyloxy)-5-heptylidenepyrrolidin-2-one (8d)
Compound 8d: yield, 63%. Colorless oil; [α]D20 +68.7 (c 0.9, CHCl3); IR (film): 3030, 2925, 2857, 1720, 1674, 1495, 1453, 1409, 1338, 1204, 1073 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.85 (t, J = 7.0 Hz, 3H, CH3), 1.10–1.32 (m, 8H, Me(CH2)5), 1.96–2.12 (m, 2H, Me(CH2)5), 2.68 (dd, J = 2.0, 17.9 Hz, 1H, COCH2), 2.77 (dd, J = 7.0, 17.9 Hz, 1H, COCH2), 4.44 (d, J = 11.2 Hz, 1H, PhCH2O), 4.53 (d, J = 11.2 Hz, 1H, PhCH2O), 4.69 (s, 2H, PhCH2N), 4.74 (dd, J = 2.0, 7.0 Hz, 1H, BnOCH), 4.83 (td, J = 7.3, 1.0 Hz, 1H, CH), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 14.0, 22.6, 26.8, 28.8, 30.1, 31.6, 36.6, 43.5, 69.9, 70.2, 108.3, 127.0, 127.2, 128.0, 128.1, 128.4, 128.5, 135.9, 137.3, 138.7, 173.1; MS (ESI, m/z): 400 (M + Na+, 11), 379 [(M + 2H)+, 28], 378 (M + H+, 100) ppm; Anal. calcd for C25H31NO2: C, 79.54; H, 8.28; N, 3.71. Found: C, 79.25; H, 8.29; N, 3.84.
4.4.4 (S,E)-1-Benzyl-4-(benzyloxy)-5-pentylidenepyrrolidin-2-one (8e)
Compound 8e: yield, 69%. Colorless oil; [α]D20 +51.6 (c 0.5, CHCl3); IR (film): 3030, 2926, 2861, 1719, 1675, 1496, 1453, 1409, 1338, 1202, 1071, 1027 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.2 Hz, 3H, CH3), 1.15–1.35 (m, 4H, Me(CH2)3), 1.98–2.12 (m, 2H, Me(CH2)3), 2.68 (dd, J = 1.7, 17.8 Hz, 1H, COCH2), 2.78 (dd, J = 7.1, 17.8 Hz, 1H, COCH2), 4.45 (d, J = 11.2 Hz, 1H, PhCH2O), 4.54 (d, J = 11.2 Hz, 1H, PhCH2O), 4.70 (s, 2H, PhCH2N), 4.76 (dd, J = 1.7, 7.1 Hz, 1H, BnOCH), 4.84 (t, J = 7.5 Hz, 1H, CH), 7.16–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 13.9, 22.2, 26.5, 32.3, 36.6, 43.5, 69.9, 70.2, 108.2, 127.0, 127.2, 128.0, 128.1, 128.4, 128.5, 135.9, 137.3, 138.7, 173.0 ppm; MS (ESI, m/z): 351 [(M + 2H)+, 27], 350 (M + H+, 100); Anal. calcd for C23H27NO2: C, 79.05; H, 7.79; N, 4.01. Found: C, 78.57; H, 7.69; N, 4.20.
4.4.5 (S,E)-1-Benzyl-4-(benzyloxy)-5-ethylidenepyrrolidin-2-one (8f)
Compound 8f: yield, 67%. Colorless oil; [α]D20 +99.7 (c 0.3, CHCl3); IR (film): 3031, 2923, 2861, 1718, 1677, 1496, 1412, 1337, 1205, 1069 cm−1; 1H NMR (500 MHz, CDCl3) δ 1.70 (d, J = 7.1 Hz, 3H, CH3), 2.70 (dd, J = 1.9, 17.9 Hz, 1H, COCH2), 2.80 (dd, J = 7.1, 17.9 Hz, 1H, COCH2), 4.44 (d, J = 11.3 Hz, 1H, PhCH2O), 4.54 (d, J = 11.3 Hz, 1H, PhCH2O), 4.70 (d, J = 15.6 Hz, 1H, PhCH2N), 4.74 (d, J = 15.6 Hz, 1H, PhCH2N), 4.78 (dd, J = 1.9, 7.1 Hz, 1H, BnOCH), 4.90 (qd, J = 7.1, 1.1 Hz, 1H, CH), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 12.0, 36.5, 43.4, 70.0, 70.1, 101.9, 127.0, 127.2, 127.9, 128.0, 128.4, 128.5, 135.9, 137.3, 139.6, 173.0 ppm; MS (ESI, m/z): 330 (M + Na+, 90), 308 (M + H+, 100); Anal. calcd for C20H21NO2: C, 78.15; H, 6.89; N, 4.56. Found: C, 77.99; H, 6.83; N, 4.84.
4.4.6 (S,E)-1-Benzyl-4-(benzyloxy)-5-propylidenepyrrolidin-2-one (8g)
Compound 8g: yield, 79%. Colorless oil; [α]D20 +42.7 (c 0.3, CHCl3); IR (film): 3030, 2962, 2927, 2867, 1717, 1673, 1496, 1450, 1409, 1338, 1256, 1200, 1072, 1016 cm−1; 1H NMR (500 MHz, CDCl3) δ 0.60 (t, J = 7.5 Hz, 3H, CH3), 2.02–2.14 (m, 2H, MeCH2), 2.66 (dd, J = 1.8, 17.8 Hz, 1H, COCH2), 2.76 (dd, J = 7.1, 17.8 Hz, 1H, COCH2), 4.44 (d, J = 11.2 Hz, 1H, PhCH2O), 4.52 (d, J = 11.2 Hz, 1H, PhCH2O), 4.69 (s, 2H, PhCH2N), 4.76 (dd, J = 1.8, 7.1 Hz, 1H, BnOCH), 4.83 (td, J = 7.5, 1.0 Hz, 1H, CH), 7.18–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 14.9, 20.2, 36.6, 43.4, 69.9, 70.2, 109.6, 127.0, 127.2, 127.9, 128.0, 128.4, 128.5, 135.9, 137.3, 138.5, 173.1 ppm; MS (ESI, m/z): 321 (M+, 100); Anal. calcd for C21H23NO2: C, 78.47; H, 7.21; N, 4.36. Found: C, 78.07; H, 7.13; N, 4.68.
4.4.7 (S,E)-1-Benzyl-4-(benzyloxy)-5-(2-phenylethylidene)pyrrolidin-2-one (8h)
Compound 8h: yield, 55%. Colorless oil; [α]D20 +85.7 (c 0.6, CHCl3); IR (film): 3028, 2920, 1719, 1672, 1495, 1449, 1406, 1337, 1205, 1070 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.73 (dd, J = 2.3, 17.8 Hz, 1H, COCH2), 2.82 (dd, J = 7.0, 17.8 Hz, 1H, COCH2), 3.38 (dd, J = 8.1, 16.0 Hz, 1H, PhCH2CH), 3.44 (dd, J = 7.8, 16.0 Hz, 1H, PhCH2CH), 4.44 (d, J = 11.3 Hz, 1H, PhCH2O), 4.54 (d, J = 11.3 Hz, 1H, PhCH2O), 4.69 (d, J = 16.4, 1H, PhCH2N), 4.74 (d, J = 16.4, 1H, PhCH2N), 4.82 (dd, J = 2.3, 7.0 Hz, 1H, BnOCH), 5.01 (td, J = 7.8, 1.2 Hz, 1H, CH), 7.00–7.40 (m, 15H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 32.4, 36.4, 43.5, 70.2 (2C), 106.0, 126.0, 127.1, 127.3, 128.0, 128.1, 128.2, 128.3, 128.5, 128.6, 135.7, 137.1, 140.0, 140.5, 173.0 ppm; MS (ESI, m/z): 385 [(M + 2H)+, 23], 384 (M + H+, 100). Anal. calcd for C26H25NO2: C, 81.43; H, 6.57; N, 3.65. Found: C, 81.23; H, 6.88; N, 3.69.
4.4.8 (S,E)-1-Benzyl-5-benzylidene-4-(benzyloxy)pyrrolidin-2-one (8i)
Compound 8i: yield, 77%. Colorless oil; [α]D20 +276.7 (c 0.7, CHCl3); IR (film): 3029, 2924, 1718, 1652, 1495, 1449, 1410, 1344, 1222, 1283, 1064 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.72 (d, J = 17.8 Hz, 1H, COCH2), 2.76 (dd, J = 1.7, 17.8 Hz, 1H, COCH2), 4.35 (d, J = 11.1 Hz, 1H, PhCH2O), 4.38 (d, J = 11.1 Hz, 1H, PhCH2O), 4.74 (d, J = 15.8, 1H, PhCH2N), 4.80 (d, J = 15.8, 1H, PhCH2N), 4.86 (m, 1H, BnOCH), 5.90, (s, 1H, CH), 7.10–7.30 (m, 15H, Ar); 13C NMR (125 MHz, CDCl3) δ 36.2, 43.9, 69.6, 71.0, 108.9, 126.5, 127.0, 127.5, 128.0, 128.1, 128.2, 128.4, 128.7, 135.4, 135.5, 136.9, 141.0, 173.1; MS (ESI, m/z): 371 (M + 2H+, 29), 370 (M + H+, 100); Anal. calcd for C25H23NO2: C, 81.27; H, 6.27; N, 3.79. Found: C, 80.99; H, 6.40; N, 3.93.
4.5 General procedure for preparation of 11 from 8 utilizing MCPBA as an oxidant
To a solution of 8 (1.0 mmol) in abs. MeOH (20 mL) and dry CH2Cl2 (10 ml) was added dropwise a solution of MCPBA (3.0 mmol) in CH2Cl2 (10 ml) at −78 °C under nitrogen atmosphere. After being stirred for 1 h at the same temperature, and then at room temperature overnight, the reaction was quenched by addition of a solution of aqueous 10% Na2S2O3 and saturated NaHCO3. The mixture was extracted with CH2Cl2 (3 × 40 mL). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuum. Filtration through a short pad of SiO2 eluting with ethyl acetate–petroleum ether gave a mixture of diastereomers 11. The diastereomeric ratios were determined either by flash chromatographic separation or by 1H NMR spectra of the crude mixture.
4.5.1 (4S,5R/S,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxybutyl)-5-methoxy-pyrrolidin-2-one (11b)
The oxidation of 8b (470 mg, 1.4 mmol) under the conditions described in the general procedure and subsequent chromatography on silica gel eluting with ethyl acetate/PE (1:2) gave four diastereomers 11b (combined yield 83%). The first diastereomer: Rf: 0.50 (AcOEt: PE = 1:2). White crystals, mp 88–89 °C (ethyl acetate/PE). [α]D20 −33.2 (c 0.2, CHCl3). IR (KBr pellet): 3438, 2955, 2925, 1696, 1505, 1398, 1356, 1088 cm−1. 1H NMR (500 MHz, CD3CN) δ 0.64 (t, J = 6.8 Hz, 3H, CH3), 0.98–1.16 (m, 2H, Me(CH2)2), 1.26–1.38 (m, 2H, Me(CH2)2), 2.49 (dd, J = 6.6, 17.8 Hz, 1H, COCH2), 2.88 (dd, J = 9.3, 17.8 Hz, 1H, COCH2), 2.95 (d, J = 4.4 Hz, 1H, OH), 3.26 (s, 3H, CH3O), 3.60 (ddd, J = 1.6, 4.4, 10.4 Hz, 1H, CHOH), 4.13 (d, J = 15.2 Hz, 1H, PhCH2N), 4.32 (dd, J = 6.6, 9.3 Hz, 1H, BnOCH), 4.62 (d, J = 11.6 Hz, 1H, PhCH2O), 4.73 (d, J = 11.6 Hz, 1H, PhCH2O), 5.00 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.50 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) δ 14.7, 20.7, 34.4, 38.7, 44.7, 52.8, 73.5, 74.5, 76.3, 97.9, 128.5, 129.1, 129.4, 129.7, 129.8, 129.9, 140.0, 140.1, 175.0 ppm; MS (ESI, m/z): 384 (M + H+, 9), 406 (M + Na+, 100); Anal. calcd for C23H29NO4: C, 72.04; H, 7.62; N, 3.65. Found: C, 71.72; H, 7.70; N, 3.47. The second diastereomer: Rf: 0.26 (AcOEt:PE = 1:2). Colorless oil; [α]D20 +24.7 (c 0.4, EtOH). IR (film): 3454, 3031, 2958, 2870, 1699, 1496, 1455, 1400, 1355, 1096, 1029 cm−1. 1H NMR (500 MHz, CD3CN) δ 0.80 (t, J = 7.2 Hz, 3H, CH3), 1.04–1.14 (m, 1H, Me(CH2)2), 1.33–1.60 (m, 3H, Me(CH2)2), 2.62 (dd, J = 5.7, 17.0 Hz, 1H, COCH2), 2.78 (dd, J = 7.6, 17.0 Hz, 1H, COCH2), 2.91 (d, J = 8.0 Hz, 1H, OH), 3.10 (s, 3H, CH3O), 3.88 (dt, J = 8.0, 1.4 Hz, 1H, CHOH), 4.36 (dd, J = 5.7, 7.6 Hz, 1H, BnOCH), 4.46 (d, J = 15.6 Hz, 1H, PhCH2N), 4.58 (d, J = 11.6 Hz, 1H, PhCH2O), 4.62 (d, J = 15.6 Hz, 1H, PhCH2N), 4.70 (d, J = 11.6 Hz, 1H, PhCH2O), 7.20–7.50 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) δ 14.7, 20.7, 34.4, 38.7, 44.7, 52.8, 73.5, 74.5, 76.3, 97.9, 128.5, 129.1, 129.4, 129.7, 129.8, 129.9, 140.0, 140.1, 175.0 ppm; MS (ESI, m/z): 384 (M + H+, 23), 406 (M + Na+, 100). HRESIMS calcd for [C23H29NO4+H]+: 384.2169; found: 384.2165. The third fraction of diastereomers (including two inseparable diastereomers): Rf: 0.38 (AcOEt:PE = 1:2). IR (KBr pellet): 3527, 3031, 2958, 2871, 1699, 1496, 1453, 1403, 1354, 1211, 1112 cm−1. Major diastereomer: 1H NMR (500 MHz, CD3CN) δ 0.92 (t, J = 7.3 Hz, 3H, CH3), 1.22–1.40 (m, 2H, Me(CH2)2), 1.44–1.63 (m, 2H, Me(CH2)2), 2.56 (dd, J = 3.7, 17.6 Hz, 1H, COCH2), 2.80 (dd, J = 7.2, 17.6 Hz, 1H, COCH2), 2.86 (s, 3H, CH3O), 3.30 (d, J = 9.5 Hz, 1H, OH), 3.87 (dt, J = 9.5, 2.4 Hz, 1H, CHOH), 4.40 (dd, J = 3.7, 7.2 Hz, 1H, BnOCH), 4.45 (d, J = 15.3 Hz, 1H, PhCH2N), 4.53 (d, J = 11.2 Hz, 1H, PhCH2O), 4.60 (d, J = 15.3 Hz, 1H, PhCH2O), 4.68 (d, J = 15.3 Hz, 1H, PhCH2N), 7.20–7.50 (m, 10H, Ar) ppm. Minor diastereomer: 1H NMR (500 MHz, CD3CN) δ 0.92 (t, J = 6.8 Hz, 3H, CH3), 1.22–1.40 (m, 2H, Me(CH2)2), 1.64–1.81 (m, 2H, Me(CH2)2), 2.50 (dd, J = 7.5, 17.1 Hz, 1H, COCH2), 2.82 (d, J = 4.3 Hz, 1H, OH), 2.84 (dd, J = 8.9, 17.1 Hz, 1H, COCH2), 3.07 (s, 3H, CH3O), 3.70 (ddd, J = 2.0, 4.3, 6.4 Hz, 1H, CHOH), 4.34 (d, J = 15.1 Hz, 1H, PhCH2N), 4.36, (dd, J = 7.5, 8.9 Hz, 1H, BnOCH), 4.38 (d, J = 11.3 Hz, 1H, PhCH2O), 4.56 (d, J = 15.1 Hz, 1H, PhCH2N), 4.65 (d, J = 11.3 Hz, 1H, PhCH2O), 7.20–7.50 (m, 10H, Ar) ppm. 13C NMR (125 MHz, CD3CN) for the two diastereomers: δ 14.8 (2C), 20.8, 21.0, 34.2, 35.1, 38.1 (2C), 43.6, 44.7, 50.8, 52.5, 72.6, 72.9, 73.0, 73.1, 74.4, 78.6, 98.3, 99.1, 128.3, 128.9, 129.3, 129.4, 129.5, 129.6, 129.7, 129.8, 130.0, 130.1, 138.8, 139.6, 139.8, 175.0, 177.4 ppm; MS (ESI, m/z): 384 (M + H+, 13), 406 (M + Na+, 100). HRESIMS calcd for [C23H29NO4+H]+: 384.2169; found: 384.2165.
4.6 General procedure for preparation of 2 from 11 utilizing Et3SiH/F3B·OEt2
To a cooled (−78 °C) solution of diastereomer mixture 11 (1.0 mmol) in dry dichloromethane (10 ml) was added dropwise triethylsilane (10 mmol) and boron trifluoride etherate (10.0 mmol) under nitrogen atmosphere. After being stirred for 6 h at the same temperature, the reaction was allowed to warm up and stirred at rt overnight. The reaction was quenched by saturated aqueous sodium bicarbonate and extracted with dichloromethane (3 × 20 ml). The combined extracts were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuum. The crude was purified by flash column chromatography on silica gel eluting with ethyl acetate–petroleum ether to give 2.
4.6.1 (4S,5S)-1-Benzyl-4-(benzyloxy)-5-hydroxymethyl-pyrrolidin-2-one (cis-2a), (4S,5R)-1-Benzyl-4-(benzyloxy)-5-hydroxymethyl-pyrrolidin-2-one (trans-2a)
Reduction of 8a gave cis-2a and trans-2a in 18:82 ratio with a combined yield of 93%. (4S,5R)-trans-2a (major diastereomer): Rf: 0.12 (AcOEt:PE = 1:1). White crystals, mp 89–90 °C (ethyl acetate/PE). [α]D20 +66.7 (c 0.6, CHCl3). IR (KBr pellet): 3261, 2916, 2865, 1659, 1453, 1345, 1298, 1261, 1083, 1051 cm−1. 1H NMR (500 MHz, CDCl3) δ 2.52 (dd, J = 1.7, 17.3 Hz, 1H, COCH2), 2.67 (br s, 1H, OH), 2.80 (dd, J = 6.7, 17.3 Hz, 1H, COCH2), 3.54–3.60 (m, 2H, BnNCH, CH2OH), 3.72 (dd, J = 3.4, 12.6 Hz, 1H, CH2OH), 4.16 (dd, J = 1.7, 6.7 Hz, 1H, BnOCH), 4.22 (d, J = 15.3 Hz, 1H, PhCH2N), 4.43 (d, J = 11.7 Hz, 1H, PhCH2O), 4.50 (d, J = 11.7 Hz, 1H, PhCH2O), 4.93 (d, J = 15.3 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 38.1, 44.4, 60.5, 65.4, 70.8, 74.5, 127.6, 127.7, 127.9, 128.5, 128.9, 136.4, 137.6, 174.0 ppm; MS (ESI, m/z): 334 (M + Na+, 21), 313 [(M + 2H)+, 24], 312 (M + H+, 100); Anal. calcd for C19H21NO3: C, 73.29; H, 6.80; N, 4.50. Found: C, 73.20; H, 6.97; N, 4.60. (4S,5S)-cis-2a (minor diastereomer): Rf: 0.24 (AcOEt:PE = 1:1). White crystals, mp 65–67 °C (ethyl acetate/PE). [α]D20 −15.6 (c 0.4, CHCl3). IR (KBr pellet): 3404, 3031, 2927, 1679, 1495, 1447, 1355, 1259, 1109, 1069 cm−1. 1H NMR (500 MHz, CDCl3) δ 2.58 (br s, 1H, OH), 2.60 (d, J = 6.6 Hz, 2H, COCH2), 3.56–3.60 (m, 1H, BnNCH), 3.80–3.85 (m, 2H, CH2OH), 4.02 (d, J = 15.2 Hz, 1H, PhCH2N), 4.34 (dt, J = 6.8, 6.6 Hz, 1H, BnOCH), 4.46 (d, J = 11.6 Hz, 1H, PhCH2O), 4.64 (d, J = 11.6 Hz, 1H, PhCH2O), 5.12 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 37.1, 44.3, 59.2, 60.4, 71.9, 74.1, 127.6, 127.7, 128.0, 128.3, 128.7, 136.3, 136.7, 172.3 ppm; MS (ESI, m/z): 334 (M + Na+, 18), 312 (M + H+, 100); Anal. calcd for C19H21NO3: C, 73.29; H, 6.80; N, 4.50. Found: C, 73.30; H, 7.04; N, 4.53.
4.6.2 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-n-butyl)pyrrolidin-2-one (2b)
Combined yields of the diastereomers: 83% (11b); 78% (2b), separable diastereomeric mixture in 1.0:2.0 ratio. 2b (major diastereomer): Rf: 0.29 (AcOEt:PE = 1:1.5). Colorless oil. [α]D20 +44.2 (c 1.0, CHCl3). IR (film): 3378, 3063, 3031, 2928, 2868, 1671, 1494, 1452, 1353, 1259, 1085, 1020 cm−1. 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 7.1 Hz, 3H, CH3), 1.22–1.48 (m, 4H, Me(CH2)2), 1.69 (br s, 1H, OH), 2.50 (dd, J = 1.4, 17.5 Hz, 1H, COCH2), 2.78 (dd, J = 6.8, 17.5 Hz, 1H, COCH2), 3.40 (s, 1H, BnNCH), 3.78–3.84 (m, 1H, CHOH), 4.18 (d, J = 15.3 Hz, 1H, PhCH2N), 4.19 (dd, J = 1.4, 6.8 Hz, 1H, BnOCH), 4.40 (d, J = 11.8 Hz, 1H, PhCH2O), 4.48 (d, J = 11.8 Hz, 1H, PhCH2O), 5.00 (d, J = 15.3 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (100 MHz, CDCl3) δ 13.9, 19.3, 34.8, 38.6, 44.2, 68.0, 67.9, 70.4, 71.9, 127.7, 127.8, 128.4, 128.8, 136.2, 137.5, 174.3 ppm; MS (ESI, m/z): 355 [(M + 2H)+, 22], 354 (M + H+, 84), 376 (M + Na+, 100); Anal. calcd for C22H27NO3: C, 74.76; H, 7.70; N, 3.96. Found: C, 74.28; H, 7.52; N, 3.98. 2b (minor diastereomer): Rf: 0.36 (AcOEt:PE = 1:1.5). White crystals, mp 77–79 °C (ethyl acetate/PE). [α]D20 +13.9 (c 0.4, CHCl3). IR (KBr pellet): 3394, 3062, 3031, 2928, 2869, 1669, 1495, 1452, 1357, 1259, 1175, 1073 cm−1. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.3 Hz, 3H, CH3), 1.10–1.32 (m, 3H, Me(CH2)2), 1.42–1.52 (m, 1H, Me(CH2)2), 2.33 (br s, 1H, OH), 2.51 (d, J = 17.7 Hz, 1H, COCH2), 2.75 (dd, J = 6.4, 17.7 Hz, 1H, COCH2), 3.58 (d, J = 4.6 Hz, 1H, BnNCH), 3.61–3.65 (m, 1H, CHOH), 4.02 (d, J = 6.4 Hz, 1H, BnOCH), 4.18 (d, J = 15.2 Hz, 1H, PhCH2N), 4.42 (s, 2H, PhCH2O), 5.02 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 13.8, 19.2, 34.8, 38.2, 45.9, 67.8, 70.2, 71.3, 73.8, 127.5, 127.6, 127.7, 127.9, 128.4, 128.6, 136.3, 137.6, 174.3 ppm; MS (ESI, m/z): 354 (M + H+, 67), 376 (M + Na+, 100); Anal. calcd for C22H27NO3: C, 74.76; H, 7.70; N, 3.96. Found: C, 74.77; H, 7.94; N, 4.02.
4.6.3 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-i-butyl)pyrrolidin-2-one (2c)
Combined yields of the diastereomers: 80% (11c); 85% (2c), inseparable diastereomeric mixture in 1.0:4.0 ratio. Rf: 0.45 (AcOEt:PE = 1:1). White crystals, mp 65–66 °C (ethyl acetate/PE). IR (KBr pellet): 3376, 3031, 2957, 2871, 1672, 1451, 1353, 1312, 1257, 1074, 1027 cm−1. 2c (major diastereomer): 1H NMR (500 MHz, CDCl3) δ 0.76 (d, J = 6.6 Hz, 3H, CH3), 0.98 (d, J = 6.6 Hz, 3H, CH3), 1.68–1.72 (m, 1H, Me2CH), 2.52 (dd, J = 1.5, 17.5 Hz, 1H, COCH2), 2.78 (dd, J = 6.7, 17.5 Hz, 1H, COCH2), 3.10 (br s, 1H, OH), 3.35–3.39 (m, 1H, CHOH), 3.62 (s, 1H, BnNCH), 4.10 (d, J = 15.2 Hz, 1H, PhCH2N), 4.18 (dd, J = 1.5, 6.7 Hz, 1H, BnOCH), 4.36 (d, J = 11.7 Hz, 1H, PhCH2O), 4.49 (d, J = 11.7 Hz, 1H, PhCH2O), 5.01 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 2c (minor diastereomer): 1H NMR (500 MHz, CDCl3) δ 0.82 (d, J = 6.6 Hz, 3H, CH3), 0.94 (d, J = 6.6 Hz, 3H, CH3), 1.58–1.68 (m, 1H, Me2CH), 2.50 (dd, J = 3.8, 17.3 Hz, 1H, COCH2), 2.86 (dd, J = 5.9, 17.3 Hz, 1H, COCH2), 3.20 (br s, 1H, OH), 3.22–3.26 (m, 1H, CHOH), 3.67 (d, J = 5.6 Hz, 1H, BnNCH), 3.95 (dd, J = 3.8, 5.9 Hz, 1H, BnOCH), 4.09 (d, J = 15.5 Hz, 1H, PhCH2N), 4.37 (d, J = 11.8 Hz, 1H, PhCH2O), 4.44 (d, J = 11.8 Hz, 1H, PhCH2O), 5.27 (d, J = 15.5 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) for the mixture: δ 18.0, 19.1, 19.6, 19.9, 30.4, 31.5, 37.9, 38.5, 44.2, 46.5, 64.7, 66.7, 70.3, 70.5, 72.0 (2C), 73.9, 79.0, 127.4, 127.5, 127.7, 127.8, 127.9, 128.4, 128.6, 128.8, 136.2, 137.6, 174.3, 175.4 ppm; MS (ESI, m/z): 355 [(M + 2H)+, 23], 354 (M + H+, 100); Anal. calcd for C22H27NO3: C, 74.76; H, 7.70; N, 3.96. Found: C, 75.04; H, 7.80; N, 3.91.
4.6.4 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-n-heptyl)pyrrolidin-2-one (2d)
Combined yields of the diastereomers: 86% (11d); 81% (2d), separable diastereomeric mixture in 1.0:4.0 ratio. 2d (major diastereomer): Rf: 0.14 (AcOEt:PE = 1:2). White crystals, mp 77 °C (ethyl acetate/PE). [α]D20 +34.1 (c 0.4, CHCl3). IR (KBr pellet): 3374, 3031, 2927, 2859, 1674, 1451, 1352, 1082 cm−1. 1H NMR (500 MHz, CD3CN) δ 0.94 (t, J = 7.0 Hz, 3H, CH3), 1.22–1.50 (m, 10H, Me(CH2)5), 2.38 (d, J = 17.3 Hz, 1H, COCH2), 2.74 (dd, J = 6.6, 17.3 Hz, 1H, COCH2), 3.16 (d, J = 4.6 Hz, 1H, BnNCH), 3.40 (s, 1H, OH), 3.82–3.87 (m, 1H, CHOH), 4.07 (d, J = 15.6 Hz, 1H, PhCH2N), 4.18 (d, J = 6.6 Hz, 1H, BnOCH), 4.44 (d, J = 11.9 Hz, 1H, PhCH2O), 4.53 (d, J = 11.9 Hz, 1H, PhCH2O), 5.02 (d, J = 15.6 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) δ 14.9, 23.8, 27.2, 30.3, 32.9, 34.4, 39.7, 44.7, 69.3 (2C), 71.3, 73.8, 128.8, 129.1, 129.2, 129.3, 129.8, 130.1, 138.7, 139.8, 174.4 ppm; MS (ESI, m/z): 418 (M + Na+, 16), 397 [(M + 2H)+, 29], 396 (M + H+, 100); Anal. calcd for C25H33NO3: C, 75.91; H, 8.41; N, 3.54. Found: C, 75.81; H, 8.55; N, 3.64. 2d (minor diastereomer): Rf: 0.21 (AcOEt:PE = 1:2). White crystals, mp 49–50 °C (ethyl acetate/PE). [α]D20 +11.5 (c 0.7, CHCl3). IR (KBr pellet): 3395, 2926, 2857, 1672, 1450, 1356, 1257, 1078 cm−1. 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H, CH3), 1.06–1.44 (m, 10H, Me(CH2)5), 1.85 (br s, 1H, OH), 2.55 (d, J = 17.7 Hz, 1H, COCH2), 2.76 (dd, J = 6.4, 17.7 Hz, 1H, COCH2), 3.57 (d, J = 4.6 Hz, 1H, BnNCH), 3.62–3.68 (m, 1H, CHOH), 4.04 (d, J = 6.4 Hz, 1H, BnOCH), 4.23 (d, J = 15.2 Hz, 1H, PhCH2N), 4.44 (s, 2H, PhCH2O), 5.00 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 14.0, 22.5, 26.0, 29.1, 31.7, 32.7, 38.2, 46.0, 67.8, 70.3, 71.7, 73.8, 127.5, 127.6, 127.7, 127.8, 128.4, 128.6, 128.7, 136.4, 137.6, 174.2 ppm; MS (ESI, m/z): 418 (M + Na+, 5), 397 [(M + 2H)+, 25], 396 (M + H+, 100); Anal. calcd for C25H33NO3: C, 75.91; H, 8.41; N, 3.54. Found: C, 75.60; H, 8.70; N, 3.28.
4.6.5 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-n-pentyl)pyrrolidin-2-one (2e)
Combined yields of the diastereomers: 86% (11e); 78% (2e), separable diastereomeric mixture in 1.0:1.0 ratio. 2e (major diastereomer): Rf: 0.12 (AcOEt:PE = 1:2). White crystals, mp 53–55 °C (ethyl acetate/PE). [α]D20 +29.6 (c 1.1, CHCl3). IR (KBr pellet): 3383, 3031, 2931, 2864, 1675, 1451, 1352, 1255, 1081 cm−1. 1H NMR (500 MHz, CD3CN) δ 0.93 (t, J = 7.0 Hz, 3H, CH3), 1.22–1.54 (m, 6H, Me(CH2)3), 2.39 (d, J = 17.2 Hz, 1H, COCH2), 2.76 (dd, J = 6.6, 17.2 Hz, 1H, COCH2), 3.28 (d, J = 4.6 Hz, 1H, BnNCH), 3.41 (s, 1H, OH), 3.84–3.88 (m, 1H, CHOH), 4.08 (d, J = 15.5 Hz, 1H, PhCH2N), 4.20 (d, J = 6.6 Hz, 1H, BnOCH), 4.46 (d, J = 11.7 Hz, 1H, PhCH2O), 4.54 (d, J = 11.7 Hz, 1H, PhCH2O), 5.04 (d, J = 15.5 Hz, 1H, PhCH2N), 7.24–7.44 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) δ 14.8, 23.8, 29.5, 34.1, 39.7, 44.7, 69.3, 69.4, 71.3, 73.8, 128.8, 129.1, 129.2, 129.3, 129.8, 130.1, 138.7, 139.8, 174.8 ppm; MS (ESI, m/z): 369 [(M + 2H)+, 27], 368 (M + H+, 100); Anal. calcd for C23H29NO3: C, 75.17; H, 7.95; N, 3.81. Found: C, 75.09; H, 8.02; N, 3.72. 2e (minor diastereomer): Rf: 0.19 (AcOEt:PE = 1:2). Colorless oil. [α]D20 +11.7 (c 0.6, CHCl3). IR (film): 3396, 3031, 2932, 2865, 1672, 1451, 1356, 1256, 1075 cm−1. 1H NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.4 Hz, 3H, CH3), 1.04–1.13 (m, 1H, Me(CH2)3), 1.20–1.46 (m, 5H, Me(CH2)3), 2.30 (br s, 1H, OH), 2.50 (d, J = 17.6 Hz, 1H, COCH2), 2.76 (dd, J = 6.2, 17.6 Hz, 1H, COCH2), 3.57 (d, J = 4.4 Hz, 1H, BnNCH), 3.62–3.66 (m, 1H, CHOH), 4.02 (d, J = 6.2 Hz, 1H, BnOCH), 4.20 (d, J = 15.3 Hz, 1H, PhCH2N), 4.41 (d, J = 12.0 Hz, 1H, PhCH2O), 4.44 (d, J = 12.0 Hz, 1H, PhCH2O), 5.01 (d, J = 15.3 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 13.9, 22.5, 28.2, 32.5, 38.2, 46.0, 67.8, 70.2, 71.7, 73.9, 127.4, 127.6, 127.7, 127.8, 128.4, 128.6, 136.3, 137.6, 174.4 ppm; MS (ESI, m/z): 369 [(M + 2H)+, 28], 368 (M + H+, 100); Anal. calcd for C23H29NO3: C, 75.17; H, 7.95; N, 3.81. Found: C, 75.34; H, 8.12; N, 3.98.
4.6.6 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxyethyl)pyrrolidin-2-one (2f)
Combined yields of the diastereomers: 85% (11f); 85% (2f), inseparable diastereomeric mixture in 1.0:1.6 ratio. Rf: 0.31 (AcOEt:PE = 2:1). White crystals, mp 59–60 °C (ethyl acetate/PE). IR (KBr pellet): 3391, 3031, 2973, 2929, 1671, 1495, 1450, 1356, 1257, 1093 cm−1. 2f (major diastereomer): 1H NMR (500 MHz, CD3CN) δ 1.10 (d, J = 7.5 Hz, 3H, CH3), 2.33 (d, J = 17.4 Hz, 1H, COCH2), 2.72 (dd, J = 6.6, 17.4 Hz, 1H, COCH2), 3.22 (d, J = 5.3 Hz, 1H, BnNCH), 3.38 (s, 1H, OH), 4.02–4.12 (m, 1H, CHOH), 4.13 (d, J = 15.8 Hz, 1H, PhNCH2), 4.18 (d, J = 6.6 Hz, 1H, BnOCH), 4.42 (d, J = 12.5 Hz, 1H, PhCH2O), 4.50 (d, J = 12.5 Hz, 1H, PhCH2O), 4.98 (d, J = 15.8 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 2f (minor diastereomer): 1H NMR (500 MHz, CD3CN) δ 1.04 (d, J = 7.5 Hz, 3H, CH3), 2.33 (d, J = 17.4 Hz, 1H, COCH2), 2.72 (dd, J = 6.6, 17.4 Hz, 1H, COCH2), 3.30 (d, J = 4.9 Hz, 1H, BnNCH), 3.48 (d, J = 5.4 Hz, 1H, OH), 3.84–3.94 (m, 1H, CHOH), 4.11 (d, J = 15.8 Hz, 1H, PhCH2N), 4.19 (d, J = 6.6 Hz, 1H, BnOCH), 4.42 (d, J = 12.5 Hz, 1H, PhCH2O), 4.50 (d, J = 12.5 Hz, 1H, PhCH2O), 4.98 (d, J = 15.8 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) for the mixture: δ 19.1, 19.5, 38.9, 39.2, 44.4, 46.0, 65.1, 67.3, 69.2, 69.7, 70.7, 70.9, 73.4, 74.5, 128.3, 128.5, 128.6, 128.7, 128.8, 128.9, 129.3, 129.4, 129.5, 129.6, 138.3, 139.4, 174.4 (2C) ppm; MS (ESI, m/z): 326 (M + H+, 25), 348 (M + Na+, 100); Anal. calcd for C20H23NO3: C, 73.82; H, 7.12; N, 4.30. Found: C, 73.89; H, 7.21; N, 4.49.
4.6.7 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-n-propyl)pyrrolidin-2- one (2g)
Combined yields of the diastereomers: 90% (11g); 74% (2g), separable diastereomeric mixture in 1.0:2.0 ratio. 2g (major diastereomer): Rf: 0.19 (AcOEt:PE = 1:1). White crystals, mp 66–67 °C (ethyl acetate/PE). [α]D20 +51.4 (c 0.4, CHCl3). IR (KBr pellet): 3379, 3031, 2929, 2873, 1673, 1452, 1353, 1258, 1085 cm−1. 1H NMR (500 MHz, CD3CN) δ 0.90 (t, J = 7.4 Hz, 3H, CH3), 1.34–1.50 (m, 2H, MeCH2), 2.32 (dd, J = 1.1, 17.3 Hz, 1H, COCH2), 2.71 (dd, J = 6.7, 17.3 Hz, 1H, COCH2), 3.24 (d, J = 4.6 Hz, 1H, BnNCH), 3.40 (s, 1H, OH), 3.70–3.76 (m, 1H, CHOH), 4.03 (d, J = 15.5 Hz, 1H, PhCH2N), 4.14 (dd, J = 1.1, 6.7 Hz, 1H, BnOCH), 4.40 (d, J = 11.9 Hz, 1H, PhCH2O), 4.48 (d, J = 11.9 Hz, 1H, PhCH2O), 5.00 (d, J = 15.5 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CD3CN) δ 11.4, 27.4, 39.7, 44.6, 69.1, 71.0, 71.3, 74.0, 128.7, 129.0, 129.1, 129.2, 129.7, 130.0, 138.7, 139.8, 174.7 ppm; MS (ESI, m/z): 341 [(M + 2H)+, 23], 340 (M + H+, 100); Anal. calcd for C21H25NO3: C, 74.31; H, 7.42; N, 4.13. Found: C, 74.53; H, 7.57; N, 4.15. 2g (minor diastereomer): Rf: 0.21 (AcOEt:PE = 1:1). White crystals, mp 93–94 °C (ethyl acetate/PE). [α]D20 +25.8 (c 0.8, CHCl3). IR (KBr pellet): 3381, 2924, 1667, 1448, 1355, 1067 cm−1. 1H NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.4 Hz, 3H, CH3), 1.20–1.50 (m, 2H, MeCH2), 2.30 (s, 1H, OH), 2.54 (d, J = 17.7 Hz, 1H, COCH2), 2.78 (dd, J = 6.3, 17.7 Hz, 1H, COCH2), 3.54–3.64 (m, 2H, CHOH, BnNCH), 4.04 (d, J = 6.3 Hz, 1H, BnOCH), 4.20 (d, J = 15.5 Hz, 1H, PhCH2N), 4.42 (s, 2H, PhCH2O), 5.04 (d, J = 15.5 Hz, 1H, PhCH2N), 7.20–7.40 (m, 10H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 10.5, 25.8, 38.2, 46.0, 67.5, 70.2, 73.3, 74.0, 127.4, 127.6, 127.7, 127.8, 128.4, 128.6, 128.7, 136.3, 137.6, 174.3 ppm; MS (ESI, m/z): 340 (M + H+, 50), 362 (M + Na+, 100); Anal. calcd for C21H25NO3: C, 74.31; H, 7.42; N, 4.13. Found: C, 73.97; H, 7.76; N, 4.00.
4.6.8 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxy-phenylethyl)pyrrolidin-2-one (2h)
Combined yields of the diastereomers: 93% (11h); 98% (2h), separable diastereomeric mixture in 1.0:1.5 ratio. 2h (major diastereomer): Rf: 0.31 (AcOEt:PE = 1:1). Colorless oil; [α]D20 +38.0 (c 0.3, CHCl3). IR (film): 3360, 3029, 2928, 2860, 1673, 1495, 1451, 1352, 1310, 1256, 1087 cm−1. 1H NMR (500 MHz, CDCl3) δ 2.50 (br s, 1H, OH), 2.51 (d, J = 17.4 Hz, 1H, COCH2), 2.62 (dd, J = 5.2, 13.8 Hz, 1H, PhCH2CH), 2.72 (dd, J = 8.5, 13.8 Hz, 1H, PhCH2CH), 2.81 (dd, J = 6.8, 17.4 Hz, 1H, COCH2), 3.44 (s, 1H, BnNCH), 4.04–4.06 (m, 1H, CHOH), 4.06 (d, J = 15.4 Hz, 1H, PhCH2N), 4.30 (d, J = 6.8 Hz, 1H, BnOCH), 4.38 (d, J = 11.7 Hz, 1H, PhCH2O), 4.50 (d, J = 11.7 Hz, 1H, PhCH2O), 4.98 (d, J = 15.4 Hz, 1H, PhCH2N), 7.00–7.40 (m, 15H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 38.4, 39.5, 44.2, 67.7, 69.5, 70.4, 71.9, 126.6, 127.6, 127.7, 127.8, 128.5, 128.6, 128.7, 129.1, 136.0, 137.5, 137.7, 174.1 ppm; MS (ESI, m/z): 424 (M + Na+, 15), 403 [(M + 2H)+, 29], 402 (M + H+, 100); Anal. calcd for C26H27NO3: C, 77.78; H, 6.78; N, 3.49. Found: C, 77.87; H, 6.80; N, 3.61; 2h (minor diastereomer): Rf: 0.48 (AcOEt:PE = 1:1). Colorless oil; [α]D20 −25.7 (c 0.3, CHCl3). IR (film): 3382, 3030, 2925, 2868, 1672, 1495, 1449, 1356, 1259, 1173, 1084 cm−1. 1H NMR (500 MHz, CDCl3) δ 1.80 (br s, 1H, OH), 2.39 (dd, J = 10.7, 13.2 Hz, 1H, PhCH2CH), 2.57 (d, J = 17.7 Hz, 1H, COCH2), 2.68 (dd, J = 2.1, 13.2 Hz, 1H, PhCH2CH), 2.80 (dd, J = 6.6, 17.7 Hz, 1H, COCH2), 3.74 (d, J = 4.8 Hz, 1H, BnNCH), 3.86 (ddd, J = 2.1, 4.8, 10.7 Hz, 1H, CHOH), 4.18 (d, J = 6.6 Hz, 1H, BnOCH), 4.42 (d, J = 15.1 Hz, 1H, PhCH2N), 4.47 (d, J = 12.0 Hz, 1H, PhCH2O), 4.50 (d, J = 12.0 Hz, 1H, PhCH2O), 4.90 (d, J = 15.1 Hz, 1H, PhCH2N), 6.90–7.40 (m, 15H, Ar) ppm; 13C NMR (125 MHz, CDCl3) δ 38.2, 38.9, 46.3, 68.0, 70.3, 72.3, 73.3, 126.9, 127.5, 127.6, 127.7, 127.9, 128.1, 128.4, 128.7, 128.8, 129.1, 136.7, 137.3, 137.6, 174.1 ppm; MS (ESI, m/z): 403 [(M + 2H)+, 24], 402 (M + H+, 100); Anal. calcd for C26H27NO3: C, 77.78; H, 6.78; N, 3.49. Found: C, 77.69; H, 6.94; N, 3.48.
4.6.9 (4S,5R,1′R/S)-1-Benzyl-4-(benzyloxy)-5-(1-hydroxyphenymethyl) pyrrolidin-2-one (2i)
Combined yields of the diastereomers: 90% (11i); 98% (2i), inseparable diastereomeric mixture in 1.0:2.6 ratio. Rf: 0.31 (AcOEt:PE = 1:1). White crystals, mp 104–105 °C (ethyl acetate/PE). IR (KBr, Pellet): 3364, 3030, 2928, 1670, 1449, 1254, 1071 cm−1. 2i (major diastereomer): 1H NMR (500 MHz, CDCl3) δ 2.02 (s, 1H, OH), 2.46 (d, J = 17.5 Hz, 1H, COCH2), 2.78 (dd, J = 6.6, 17.5 Hz, 1H, COCH2), 3.66 (d, J = 2.3 Hz, 1H, BnNCH), 3.85 (d, J = 12.0 Hz, 1H, PhCH2O), 3.92 (d, J = 12.0 Hz, 1H, PhCH2O), 4.04 (d, J = 6.6 Hz, 1H, BnOCH), 4.14 (d, J = 15.2 Hz, 1H, PhCH2N), 4.94 (d, J = 2.3 Hz, 1H, CHOH), 5.60 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 15H, Ar) ppm; 2i (minor diastereomer): 1H NMR (500 MHz, CDCl3) δ 2.00 (br s, 1H, OH), 2.22 (dd, J = 5.8, 17.4 Hz, 1H, COCH2), 2.32 (d, J = 17.4 Hz, 1H, COCH2), 3.81 (d, J = 5.0 Hz, 1H, BnNCH), 3.96 (d, J = 5.8 Hz, 1H, BnOCH), 4.12 (d, J = 15.2 Hz, 1H, PhCH2N), 4.24 (d, J = 11.8 Hz, 1H, PhCH2O), 4.29 (d, J = 11.8 Hz, 1H, PhCH2O), 4.82 (d, J = 5.0 Hz, 1H, CHOH), 5.18 (d, J = 15.2 Hz, 1H, PhCH2N), 7.20–7.40 (m, 15H, Ar) ppm; 13C NMR (125 MHz, CDCl3) for the mixture: δ 37.3, 38.6, 44.7, 45.6, 54.6, 64.6, 69.8 (2C), 70.1, 70.6, 71.5, 74.0, 125.6, 125.8, 127.4, 127.9, 128.1, 128.3, 128.8, 136.3, 137.4, 137.5, 139.9, 140.2, 174.5, 174.6 ppm; MS (ESI, m/z): 389 [(M + 2H)+, 28], 388 (M + H+, 100); Anal. calcd for C25H25NO3: C, 77.49; H, 6.50; N, 3.61. Found: C, 77.61; H, 6.74; N, 3.83.
Acknowledgments
The authors are grateful to the NSF of China (20390050, 20572088, 20602028) for financial support. Partial support from the program for Innovative Research Team in Science and Technology in Fujian Province University is acknowledged.


