1 Introduction
Plants (fruits, legumes, grains, medicines and aromatic herbs) produce a wide variety of secondary metabolites (SM) which may play numerous biological activities, such as protection against herbivores, microbes, or competing plants, adaptation and pollination [1]. These metabolites can be divided into two main groups:
- (a) those without nitrogen-like terpenoids (mono-, sesqui-, di-, tri-, and tertraterpenes; saponines; steroids; carotenoids; and cardiac glycosides), polyketides (anthraquinones), phenolic compounds (flavonoids, anthocyanins, galloyl and catechol tannins, phenolic acids, lignans, lignins, stilbenes, and coumarins), polyacetylenes and phenylpropanes;
- (b) those with nitrogen in their structures including alkaloids, amines, nonprotein aminoacids, cyanogenic glycosides, glucosinolates, protease inhibitors and lectins [2].
The polyphenol compounds play a wide range of biological effects including antibacterial, anti-inflammatory, antiallergic, hepatoprotective, antithrombotic, antiviral, anticarcinogenic and cardioprotective and vasodilatory effects [3]. These functions have been attributed to their antioxidant activity by several mechanisms such as free radical scavengers, reducing agents, complexers of pro-oxidant metals, quenchers of the formation of singlet oxygen and stimulating the antioxidative defence enzymes activities [4]. These mechanisms will be led by two types of reactions: hydrogen atom transfer and single electron transfer [5].
In the human body and during the normal conditions, the unlimited number of the physiological and biochemical processes leads to the production of two radical groups. The first group is the reactive oxygen species (ROS): superoxide radical (
Atriplex halimus is a shrub succulent halophyte widely distributed in semi-arid Mediterranean areas [8], in the high plateaus and on the littoral where favourable conditions are regrouped with an intra- and interindividual polymorphism for several floral morphological characters including styles, ovule types and radicle orientation according to salinity [9]. This plant represent a potential source for economical utilisation; it can provide forage sources with a good nutritive value during the dry seasons [10] and periods of shortage of grazing resources. Also, it can contribute to the valorisation of marginal and degraded soils and the improvement of the vegetable and animal productions in several stripped areas [11]. Moreover, the high biomass production has a good digestibility, but an abundance of sodium chloride and anti-nutritional factors reduce its palatability [8]. In traditional medicine, a cocktail of minerals in A. halimus is used to benefit glycaemic control in diabetic patients [12]. Like other halophytes, it can combat internal parasites in veterinary use [13].
The study of the chemical composition shows the presence of secondary metabolites in A. halimus such as tannins, flavonoids, saponins, alkaloids and resins [13], and it contains up 10% sodium chloride [14]. It is characterized by its high ash and crude fiber, moderate crude protein and low crude fat contents [15].
In our study, we investigate for the first time the polyphenol content and antioxidant capacity with DPPH radical scavenging and reducing power assay in leaves and stems from A. halimus of methanolic extract of polyphenols, tannins, alkaloids, saponins and ethyl acetate and butanolic fractions of flavonoids.
2 Materials and methods
2.1 Plant material
The aerial part (leaves, stems) of A. halimus was harvested in March 2008 from Bechar, Algeria. The plants collected were identified by the Vegetable Ecological Laboratory and the voucher specimens have been deposited at the Herbarium of the Department of Molecular and Cellular Biology, Tlemcen University, Algeria. Plant samples were dried in the share and conserved for future use.
2.2 Extractions of chemical compounds from the leaves and the stems
2.2.1 Methanolic extracts
The leaves and stems of A. halimus (1 g) were powdered and extracted for 24 h with 20 ml of methanol 96.6° at room temperature, followed by rapid paper filtration through Whatman No 0.45 μm filter paper. The resulting solutions were evaporated under vacuum at 60 °C by Buchi Rotavapor R-200 to dryness. The residues were then dissolved in 3 ml of methanol.
2.2.2 Ethyl acetate and butanolic fractions
The leaves and stems dry residues obtained by the same procedure for methanolic extracts extraction were treated with 10 ml of boiling water to dissolve the flavonoids. Further filtration through filter paper No 0.45 μm, afforded the aqueous solution that was firstly extracted with 10 ml of ethyl acetate, then with 10 ml of butanol-1. The two extracts were evaporated and weighed, then dissolved in 3 ml of methanol.
2.2.3 Total alkaloids
They were obtained by triple liquid–liquid extraction according to the method of Harbone [16], the leaves and of the stems of A. halimus were extracted with the Soxhlet by 150 ml from absolute ethanol during 5 h. The ethanolic extracts were then evaporated under vacuum at 40 °C by Buchi Rotavapor R-200. The dry residues were taken again by 20 ml of chloroform and acidify by HCl at 5% to pH 3; they were let rest during 30 min at the ambient temperature. The acid aqueous phases were extracted by 20 ml of chloroform, basified by NaHCO3 at 5% to pH 9 and let rest during 15 min at the ambient temperature. The chloroformic phases were evaporated and the dry residues, made up of total alkaloids, were weighed then dissolved in 3 ml methanol.
2.2.4 Saponins
They were extracted according to the method worked out by Bouchelta et al. [17]. The leaves and stems powdered were delipided during 2 h by 150 ml of n-hexane. After elimination of the organic phases, the precipitates obtained were macerated in 50 ml of absolute ethanol under magnetic agitation at the ambient temperature during 24 h. The ethanolic phases were evaporated at 40 °C by the rotavapor. The dry residues were extracted three times by 50 ml from distilled water/petroleum ether mixture (V/V) heated at 50 °C in water bath during 30 min. The aqueous phases were mixed then treated by 10 ml of butanol during 30 min. The organic phases, evaporated at 40 °C, were weighed and dissolved in 3 ml methanol.
2.2.5 Tannins
The extraction of tannins from A. halimus was obtained according to the method of Zhang et al. [18]. Five grams of each part (leaves, stems) was milled into powder. The powder was extracted with 100 ml acetone–water (70/30, V/V), and the mixture was stirred continuously for 72 h at room temperature. Then, the mixture was filtrated and evaporated under vacuum at 40 °C to remove acetone. The remaining solution was washed with 30 ml dichlomethane to remove lipid-soluble substances. After that, the solution was further extracted with ethyl acetate at a ratio of 30/30 (V/V). The water layer was separated and extracted twice more similarly. Then the resulting water layer was evaporated to dryness, and the resulting substance was weighed and dissolved in methanol.
2.2.6 Determination of total phenolic content
The total phenolic in leaves and stems methanolic extracts content was determined by spectrometry using “Folin-Ciocalteu” reagent assay [19]. A volume of 200 μl of the extract was mixed with 1 ml of Folin-Ciocalteu reagent diluted 10 times with water and 0.8 ml of a 7.5% sodium carbonate solution in a test tube. After stirring and 30 min later, the absorbance was measured at 765 nm by using a Jenway 6405 UV-vis spectrophotometer. Gallic acid was used as a standard for the calibration curve. The total phenolic content was expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g DW).
2.3 Antioxidant activity
2.3.1 Reducing power assay
The reducing power of the extract was determined according to the method of Oyaizu [20]. Various concentrations of the extracts (mg/ml) in distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and 1% of potassium ferricyanide water solution (2.5 ml, K3[Fe(CN)6]). The mixture was incubated at 50 °C for 20 min. Aliquots of trichloracetic acid (2.5 ml, 10% aqueous solution) were added to the mixture which was then centrifuged at 3000 rpm for 10 min. The supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and a freshly prepared FeCl3 solution (0.5 ml, 0.1%). The absorbance was measured at 700 nm. Ascorbic acid was used as a positive control. In this method, the higher the absorbance, the higher the reducing power.
2.3.2 DPPH scavenging assay
The hydrogen atom donation ability of chemical compounds in leaves and stems was measured on the basis to scavenge the 2,2-diphenyl-1-picrylhydrazil free radical [21]. Fifty microliter of various concentrations of the extracts in methanol were added to 1950 μl of a 0.025 g/l methanol solution DPPH. After a 30-min incubation period at room temperature, the absorbance was read against a blank at 515 nm. DPPH free radical scavenging activity in percentage (%) was calculated using the following formula:
Extract concentration providing 50% inhibition (EC50) was calculated form the graph plotted of inhibition percentage against extract concentrations. The ascorbic acid methanol solution was used as positive control.
2.3.3 Statistical analysis
All evaluations of antioxidant activity were performed in twice. Data were expressed as means ± standard derivation (S.D.). Correlation coefficient of antioxidant activity was determined using Excel programme and Origin 6.
3 Results and discussion
3.1 Extract yields and phenolic contents
The specific extractions of A. halimus leaves and stems have makes to determine the yield of chemical groups which more used in therapy. The obtained results are shown in Table 1. The two parts of the plant were characterized by the presence of the flavonoids, the tannins, the alkaloids and the saponins where the leaves exhibited the higher yields. These molecules were known to show medicinal activity as well as exhibiting physiological activity.
Yields of some bioactive compounds from Atriplex halimus leaves and stems.
| Bioactive compounds | Yields (%) | |
| Leaves | Stems | |
| Methanolic extract | 24 ± 1.41 | 7.5 ± 0.70 |
| Ethyl acetate fraction | 2.66 ± 0.57 | 1 ± 0.00 |
| Butanolic fraction | 1 ± 0.00 | 1 ± 0.00 |
| Tannins | 0.5 ± 0.14 | 0.3 ± 0.14 |
| Alkaloids | 0.25 ± 0.07 | 0.2 ± 0.00 |
| Saponins | 0.33 ± 0.14 | 0.25 ± 0.00 |
The methanolic extract of the leaves exhibited the higher content (24 ± 1.41%), as compared to the stems extract (7.5 ± 0.70%). Concerning, the distribution of secondary metabolites, we record a high yields of ethyl acetate (2.66 ± 0.57%) and butanolic fractions (1%). Thus, the leaves presented the higher yields of flavonoids followed by tannins (0.5 ± 0.14%) and saponins (0.33 ± 0.14%). With regard to the alkaloids, the yields varied between 0.2 ± 0.00% in the stems to 0.25 ± 0.07% in the leaves. This distribution was characterized by weaker yields for the stems. These findings agree with previous reports indicating that secondary metabolites distribution may fluctuate between different plant organs [22,23].
Table 2 summarizes the contents of total phenolics of the methanolic extracts of the two parts of A. halimus. These contents varied from 3.77 ± 0.06 mg GAE/g DW in stems to 10.12 ± 2.24 mg GAE/g DW in leaves. This result proves the data quoted in Table 1, where we recorded high yields of the secondary metabolites without nitrogen in the leaves such as flavonoids characterized by the two fractions, saponins and the tannins.
Total phenolics in leaves and stems methanolic extracts of Atriplex halimus.
| Plant part | Methanolic extract | |
| Leaves | Stems | |
| Total phenolics (mg GAE/g DW) | 10.12 ± 2.24 | 3.77 ± 0.06 |
The diversity of secondary metabolites in A. halimus has been reported in the literature [13]. In fact, our results are partially in agreement with those of El-Adawy et al. [24] who showed that the total extractable phenolic compounds, saponins and the alkaloids of the leaves of A. halimus were 11.3, 12.38 and 0.23% respectively. El-Waziry [25] reported the presence a lower contents of totals phenols and tannins in Atriplex fresh and silage. These variations in the distribution of the secondary metabolites can be partially due to genotypic factors that control accumulation of these compounds in the plant [25]. Moreover, other studies suggested that the biotic conditions (species, organ and physiological stage) and abiotic stresses (salinity, luminosity, water deficit and edaphic factors) widely present in the arid zone may enhance the phenolic metabolism as a response to oxidative stress [26]. This proposition was confirmed by the studies of Ksouri et al. [27], who found the augmentation in polyphenol content in Cakile maritime leaves challenged with 0, 100 and 400 mM NaCl.
The predominance of the polyphenols family in particular the flavonoids found in our results is in agreement with the studies of Al-Jaber et al. [28]. It is noted in A. hortensis, the flavonoid content in shoot part was equal to 68.5 mg/100 g fresh weight [29]. The naringin and naringenin 7-O-glucoside are very common in species of Chenopodiaceae [28]. Moreover, the flavonol class forms the major chemical compounds of Atriplex species [30]. However, triterpene saponins occur in some species [31]. Also, Chenopodiaceae family is characterised by the presence of glycinebetaine as an alternative osmolyte to protect from salt and water stress.
3.2 Antioxidant activity
The diversity of nature and the complexity of phytochemical compounds of plant extracts impose the development of many methods to evaluate the antioxidant activity and to estimate the effectiveness of these substances. The majority of these methods are based on the colouring or the discolouration of a reagent in the reactional medium. They can be classified into two groups: those assays used in food and biological system to evaluate lipid peroxidation while measuring the degree of oxidation inhibition [32] and those assays used to measure free radical scavenging ability [6]. Some can be based on metal reducing power (ferric reducing antioxidant power, FRAP), peroxyl radical scavenging (oxygen radical absorbance capacity, ORAC; total radical trapping antioxidant parameter, TRAP), hydroxyl radical scavenging (deoxyribose assay), organic radical scavenging like ABTS and DPPH (trolox equivalent antioxidant capacity, TEAC), quantification of products formed during the lipid peroxidation (thiobarbituric acid reactive substance, TBARS; LDL oxidation) [6]. Reducing power and DPPH have been used in the present investigation.
3.2.1 Reducing power
Fig. 1. depicts the reducing power of chemical compounds from leaves and stems. All compounds showed the presence the reductive effects, which increased with an increase in concentration. However, when compared to leaves and stems, flavonoids in ethyl acetate fraction was more potent on reducing power in the two part of plant, followed by leaves butanolic fraction and stems methanolic extract. Alkaloids appear to be less reductive effect compared to saponins and tannins. At 0.4 mg/ml, the absorbance values of ethyl acetate fraction, butanolic fraction, saponins, methanolic extract, tannins and alkaloids at 700 nm were 0.13 ± 0.00; 0.11 ± 0.00; 0.06 ± 0.00; 0.06 ± 0.00; 0.05 ± 0.00; 0.03 ± 0.00 respectively in leaf chemicals, they were 0.11 ± 0.00; 0.03 ± 0.00; 0.03 ± 0.00; 0.07 ± 0.00; 0.03 ± 0.00; 0.02 ± 0.00 in stem chemicals.
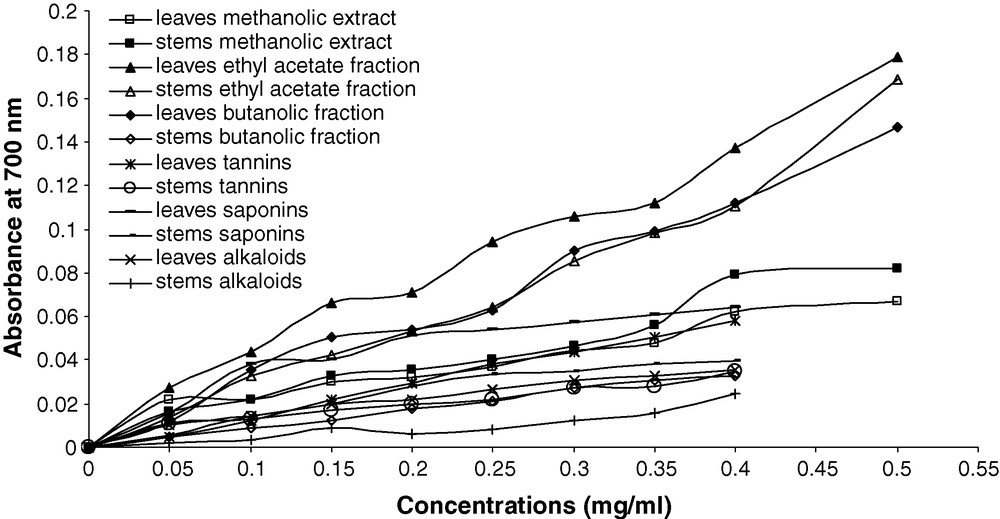
Correlation between the sample concentrations and absorbance of reducing power of bioactive substances extracts from leaves and stems.
The use of EC50 parameter is an index to compare and to express the reducing power ability of the bioactive substances (Table 3). The weak value of EC50, the higher reducing power was observed in ethyl acetate and butanolic fractions (1.51 ± 0.01 and 1.76 ± 0.00 mg/ml respectively). The EC50 concentrations were ranged between 3.31 ± 0.22 mg/ml in tannins to 6.71 ± 0.44 mg/ml in alkaloids from leaves, and 1.60 ± 0.05 mg/ml in ethyl acetate fraction to 9.06 ± 0.45 mg/ml in alkaloids from stems. These capacities of all extracts were less than that ascorbic acid (0.06 ± 0.00 mg/ml).
EC50 concentrations of reducing power from bioactive compounds.
| Bioactive compounds | EC50 (mg/ml) Leaves | R2 | EC50 (mg/ml) Stems | R2 |
| Methanolic extract | 4.55 ± 0.79 | 0.89 | 3.24 ± 0.23 | 0.93 |
| Ethyl acetate fraction | 1.51 ± 0.01 | 0.96 | 1.60 ± 0.05 | 0.96 |
| Butanolic fraction | 1.76 ± 0.00 | 0.98 | 5.96 ± 0.44 | 0.96 |
| Tannins | 3.31 ± 0.22 | 0.98 | 7.61 ± 0.08 | 0.97 |
| Alkaloids | 6.71 ± 0.44 | 0.98 | 9.06 ± 0.45 | 0.85 |
| Saponins | 4.02 ± 0.30 | 0.86 | 5.12 ± 0.03 | 0.93 |
| Ascorbic acid | 0.06 ± 0.00 | 0.99 |
3.2.2 DPPH radical scavenging activity
The results of DPPH radical scavenging activity are shown in Figs. 2 and 3.
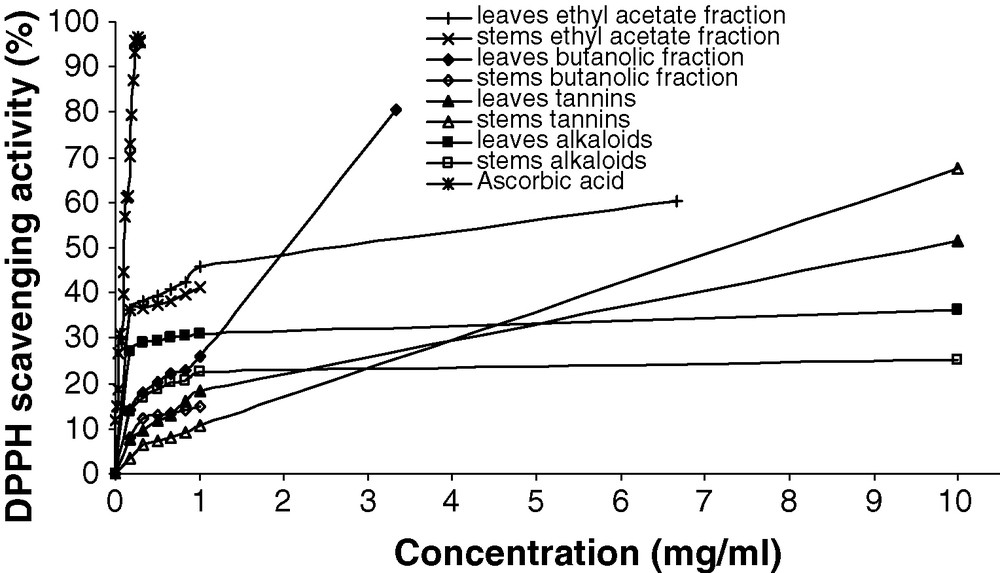
DPPH radical scavenging activities (%) of ethyl acetate fraction, butanolic fraction, tannins and alkaloids from Atriplex halimus leaves and stems. Ascorbic acid was used as positive control.
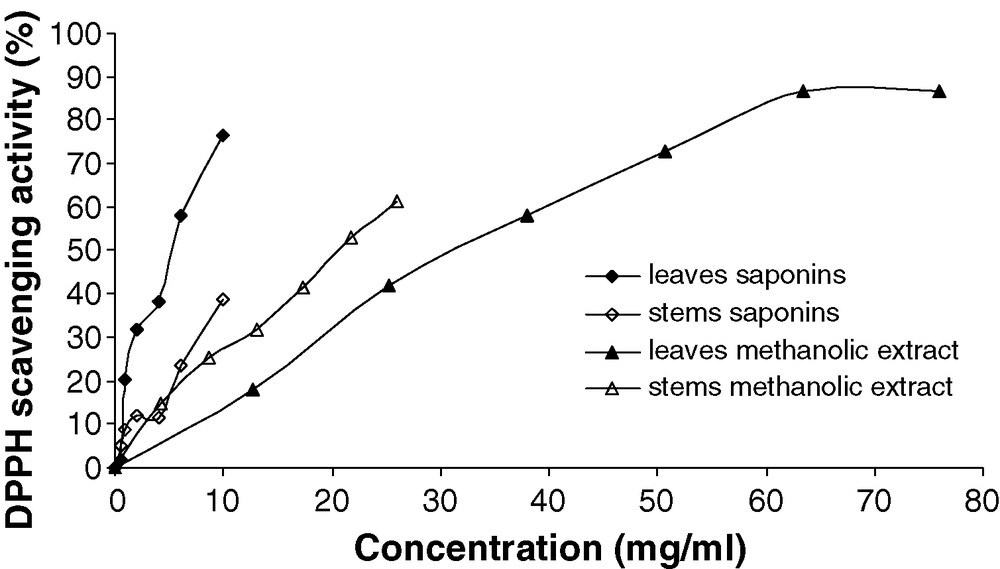
DPPH radical scavenging activities (%) of methanolic extracts and saponins from Atriplex halimus leaves and stems.
All chemical compounds had the scavenger effect expressed in DPPH free radical scavenging activity (%). This activity increased with increasing of concentration. For some chemicals, we can calculate the EC50 (Table 4), but for others, it is impossible to determinate this parameter in this type of experimental conditions such as the leaves and stems alkaloids which presented the plateau state at percentage less to 50% inhibition. Though, the highest DPPH scavenging activity was found in butanolic and ethyl acetate fractions of the leaves, the EC50 values were 1.73 (R2 = 0.98) and 2.04 (R2 = 0.99) mg/ml respectively. Moreover, leaves saponins, leaves tannins and stems tannins are also able to donate hydrogen atoms to DPPH radicals. The lowest hydrogen atom donating ability was found in leaves and stems methanolic extract (31.83 and 20.58 mg/ml respectively). However, DPPH free radical scavenging of all secondary metabolites tested was less than that ascorbic acid, a synthetic antioxidant (0.11 mg/ml). This result might be explained that the additive or synergistic effects of polyphenols make the antioxidant activity of the methanolic extract feeble than that the isolated bioactive compounds. In addition, the total phenolic content in crude extract does not incorporate all the antioxidants [33].
EC50 concentrations of DPPH scavenging capacity from bioactive compounds.
| Bioactive compounds | EC50 (mg/ml) | R2 |
| Leaves ethyl acetate fraction | 2.04 | 0.99 |
| Leaves butanolic fraction | 1.73 | 0.98 |
| Leaves methanolic extract | 31.83 | 0.99 |
| Stems methanolic extract | 20.58 | 0.99 |
| Leaves saponins | 4.75 | 0.98 |
| Leaves tannins | 7.64 | 0.99 |
| Stems tannins | 6.31 | 0.99 |
| Ascorbic acid | 0.11 | 0.96 |
According to our knowledge, the chemical analysis of flavonoids of A. halimus from Algerian Sahara is not yet investigated. Both fractions ethyl acetate and butanolic from leaves and stems demonstrate the potent antioxidant properties and are related to their phenols including flavonoids. This family from polyphenols showed the higher redox potentials which allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers [34]. In A. halimus, this activity might be explained by the presence of flavonols, a class major in Atriplex species and the most abundant flavonoids in food. The flavonol aglycones: Kaempferol, quercetin, isorhamnetin, sometimes patuletin, spinacetin and tricin were detected in eight Atriplex species [30]. The presence of two new flavonoid sulphates: kaempferol 3-O-sul phate-7-O-arabinopyranoside and quercetin 3-O-sul phate-7-O-arabinopyranoside from leaves of A. hortensis L. was reported by Bylka et al. [35]. For A. littoralis, the new acetylated flavonol glycoside was isolated from the aerial part [36]. Other earlier works suggested the presence the naringin, naringenin 7-O-glucoside, isorhamnetin 3-O-rhamnosyl (1-6) glucopyranoside and isorhamnetin 7-O-glucopyranoside in A. farinosa [28].
It is known in the literature that only the flavonoids of certain molecular structure, particularly those with a certain number and configuration of hydroxyl groups will determine the presence of the antioxidant properties [37]. These properties were decreased by glycosylation and configuration of other substituents [2] and influenced hydrogen or electron donating ability. Flavonols are one group of flavonoids cited by the same authors to possess the highest radical scavenging activity in plant extract than flavonoids, because their structure contain the hydroxyl groups with ortho dihydroxy groups (catechol structure) in the B-ring, 3-hydroxyl group and/or galloyl group (catechol structure) in the C-ring and the 2,3-double bond in conjugation with 4-oxo function (carbonyl group) in the C-ring [37]. These three criteria are essential for strong antioxidant activity.
4 Conclusion
It may be suggested that like all the halophyte plants, A. halimus produce the polyphenols and other bioactive substances potentially useful for medicinal properties and as natural food preservation. The distribution of these molecules was unequal in different parts of plant. The leaves exhibited the higher phenolic content in comparison with the stems. However, the flavonoids in ethyl acetate and butanolic fractions possess potential antioxidant activity which explains the relation structure-activity. Further isolation and identification of potential bioactive compounds particularly flavonoids responsible for antioxidant activity are needed. Other compounds like peptide, organic acids and proline which exist in strong concentrations in halophytes plants to the abiotic constraints remain to be evaluated the in vitro their biological activity and to elucidate different antioxidant mechanisms.


