1 Introduction
Ionic liquids are known for their high ionic conductivity and their wide electrochemical potentiality. They have recently been used as electrolytes in solar and fuel cells [1,2] and lithium batteries [3]. For such applications, these ionic liquids have been confined in a solid matrix [4,5]. However, the molecular dynamics of these liquid-like ions within a disordered solid matrix is still unknown. Here, we choose the (1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl) amide) (BMI)(TFSI) as an anion–cation pair of ionic liquid (Li+-ionogels) confined within a silica-like mesoporous matrices made by a sol-gel route from hydrophobic methyl groups precursors [4,5]. We present here, for the first time, the nuclear magnetic relaxation dispersion (NMRD) of the confined proton-bearing cation (BMI) (Fig. 1) in a large range of temperatures. One obtains almost similar NMRD data for the fluorine-bearing anion (TFSI) (Fig. 1). Several dynamical parameters have been determined from these proton and fluorine NMRD such as: translational correlation time, activation energy as well as a surface diffusion coefficient. These first results are in favour of a very-correlated dynamical motion of the anion–cation pair within the solid and disordered silica matrix.
Molecular structures of the proton-bearing cation (BMI) and fluorine-bearing anion (TFSI).
2 Experiments
2.1 Proton and fluorine frequency dependences of nuclear magnetic relaxation dispersion at various temperatures
We have measured the proton and fluorine nuclear magnetic relaxation dispersion (NMRD) of the confined proton-bearing cation (BMI) and fluorine-bearing anion (TFSI) in a large range of temperatures (2–90 °C) (Fig. 2a et b). These NMRD data were recorded using a fast field cycling FFC NMR spectrometer from Stelar s.r.l., Mede, Italy. We used a fast field cycling sequence to improve the signal to noise, where spins are polarized at 15 MHz and the free-induction decays are recorded following a single 90° excitation pulse of 5.8 μs duration applied at 11 MHz. The frequency dependences of 1/T1 behaves as a power law, 1/T1∼ω-1/2, over more than three orders of magnitude and tends to a plateau at low frequency. This suggests a translational diffusion of this confined cation and anion at proximity of the flat pore surface up to a point where all the dipolar correlations disappear. The slight but systematic deviations from such a power law, observed at high frequency for some temperatures, does not change the conclusion given above.

(a) Logarithmic plot of the frequency dependencies of the proton nuclear magnetic relaxation dispersion of the confined proton-bearing cation (BMI) in solid silica matrix at various temperatures. The continuous lines are the best fits obtained with the theoretical model outlined in the text [6]. (b) Logarithmic plot of the frequency dependencies of the fluorine nuclear magnetic relaxation dispersion of the confined fluorine-bearing anion (TFSI) in solid silica matrix at various temperatures.
The fact that 1H and 19F NMRD have the same frequency behavior over more than three orders of magnitude (Fig. 2) proves that fluctuations at the origin of the relaxation processes are effective on length-scales far above the molecular scale since the NMRD profiles follow this unique power law in a large time range between few ns up to few μs. It is why the BMI and TFSI NMRD profiles are so similar even if the molecular bonds of the hydrogen and fluorine are so obviously different.
3 Discussion
We have interpreted all the NMRD data within an original phenomenological model that describes the nuclear spin-lattice relaxation of proton or fluorine by considering the dipole–dipole interaction modulated by the dynamics in confinement. In this model, we have introduced two correlation times: a translational correlation time τm for the surface diffusion and a time of residence τs over which the ionic liquid stays correlated with the solid surface [6]. The main interest of this phenomenological model is to take into account two previous approaches considering either modulation of intramolecular or intermolecular dipolar interactions by bridge statistics [7,8] and explored diffusive volume [9], respectively. The NMRD data are reported in Fig. 2 in the large temperature range studied. The continuous lines on Fig. 2 represent the best fits of the NMRD data achieved with our model. This gives the temperature dependences of the two dynamical parameters reported in Fig. 3.
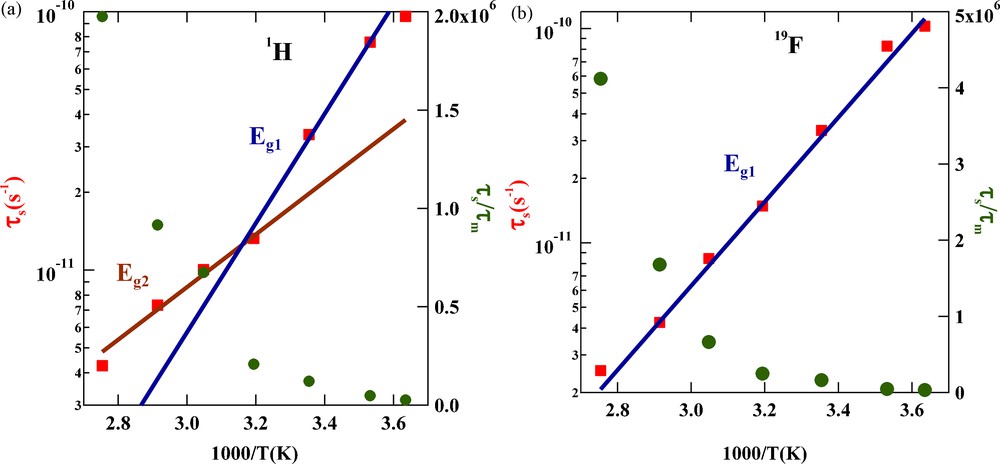
(a) Arrhenius plot representing the temperature dependencies of the translational correlation time τm and the ratio τs/τm for confined proton-bearing cation (BMI) in solid silica matrix. The slopes of the two continuous lines give the activation energies of 10 and 4.8 kcal/mol at low and high temperatures, respectively. (b) Arrhenius plot representing the temperature dependences of the translational correlation time τm and the ratio τs/τm for confined fluorine-bearing anion (TFSI) in solid silica matrix. The slope of the single continuous line gives activation energy of 10 kcal/mol.
Considering the temperature dependence of τm that describes the dynamics of the confined liquid, one notes below 40 °C, similar diffusive behaviours for the cation and anion characterized by an activation energy Eg1 = 10 kcal/mol and apparently a change of activation energy Eg2 = 4.8 kcal/mol for the cation above 40 °C in conformity with recent conductivity measurements [1]. The main difference between BMI cation and TFSI anion is the single diffusive regime for the anion of activation Eg1 = 10 kcal/mol over the whole temperature range. Now, the ratio τs/τm describes roughly the number of diffusion steps on the surface or a dynamical surface affinity. We note on Fig. 3 an increase of this ratio with temperature. This surprising result may be explained by a diminution of the polarisability when increasing temperature.
4 Conclusion
Fast field cycling relaxometry has proven useful to obtain dynamical information in favour of a very-correlated dynamical motion of anion–cation pair of ionic liquids (Li+-ionogels) confined within a silica-like mesoporous matrix designed for lithium batteries.
Acknowledgements
This work has been supported by a grant from the ANR project “LISSIL”.


