1 Introduction
Spirocyclic systems containing one carbon atom common to two rings are structurally interesting [1,2]. The presence of the sterically constrained spiro structure in various natural products also adds to the interest in the investigations of spiro compounds [3]. Spiro compounds represent an important class of naturally occurring substances characterised by their highly pronounced biological properties [4,5]. Perimidines (peri-naphtho-fused pyrimidine systems) are an important class of heterocyclic compounds, since many of this heterocyclic system exhibit biological and pharmaceutical activity [6]. The interest in pyrido-fused perimidine derivatives stems from the appearance of these heterocyclic systems in many biologically active compounds [6,7]. Considering the important biological properties of perimidines, a number of methods have been reported for the synthesis of these heterocycles [6–10]. However, the preparation of perimidine derivatives using naphthalene-1,8-diamine with ketones has not been much developed. To our knowledge, in several methods, protonic acids have been used for this transformation as catalysts [11]. However, their strong acidity led to the occurrence of side reactions and substrates. Reddy and Rao reported synthesis of 2-methyl-2-aryl-2,3-dihydro-1H-perimidines using naphthalene-1,8-diamine with aromatic ketones in acetic acid or methanol with moderate product yields (60 %) [11c]. The methods of acid-catalyzed reactions are not very efficient. Therefore, there is a need for a new efficient catalyst for this organic transformation. Very recently, synthesis of perimidine derivatives has been reported using catalytic amounts of BiCl3 [12], RuCl3 [13] and Yb(OTf)3 [14].
In recent years, indium (III) chloride (InCl3) has received considerable attention as an inexpensive, non-toxic, readily available catalyst for various transformations under mild and convenient conditions, affording the corresponding products in excellent yields with high selectivity [15].
Due to the biological activity of perimidine derivatives, and our interest in the synthesis of heterocyclic compounds [16–31], herein, we report a simple and efficient method for the preparation of spiro-perimidines in water. In fact, as clearly stated by Sheldon [32], it is generally recognized that “the best solvent is no solvent and if a solvent (diluent) is needed it should preferably be water”. The use of water as the reaction medium represents a remarkable benefit since this green solvent is highly polar and therefore immiscible with most organic compounds; moreover, the water soluble catalyst resides and operates in the aqueous phase and separation of the organic materials is thus easy.
2 Results and discussion
To achieve suitable catalyst and solvent for the synthesis of spiro-perimidines, various solvent and catalysts have been investigated in the reaction of naphthalene-1,8-diamine 1 and isatin 2a as a model reaction at room temperature (Fig. 4). As could be seen in Fig. 4, the best result was obtained with a 20 mol % of InCl3 as the catalyst in water at room temperature (Fig. 4). Using a lower amount of catalyst resulted in lower yields, while a higher amount of catalyst did not affect reaction times and yields (Fig. 4). When this reaction was carried out without any catalyst, TLC and 1H NMR spectra of the reaction mixture showed a combination of starting materials and numerous products, the yield of the expected product was very poor (Fig. 4, entry 5). Fig. 4 demonstrates that water was the best choice of solvent and the use of InCl3 in water improved the yield of the product.
Effect of reaction conditions.
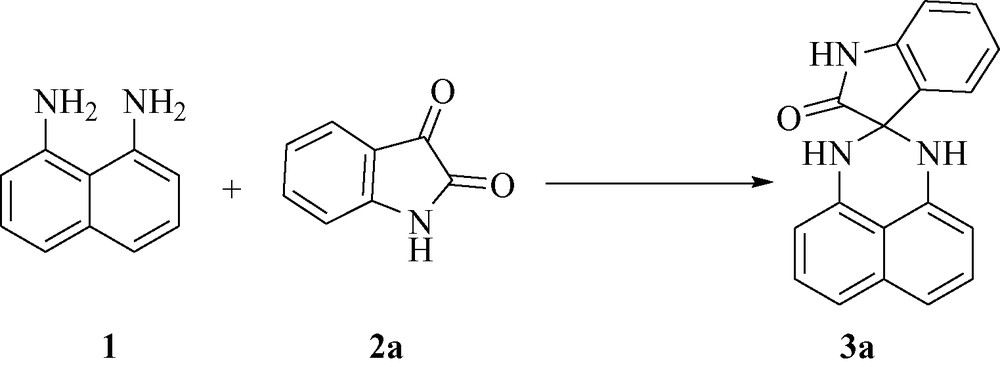
Encouraged by this success, we extended the reaction of naphthalene-1,8-diamine 1 with various isatins 2a-h under similar conditions (H2O/InCl3), furnishing the respective spiro[indoline-3,2′-perimidin]-2-ones 3a-h in good yields. The optimized results are summarized in Fig. 5.
Synthesis of spiro[indoline-3,2′-perimidin]-2-ones 3.
Table 1 compares efficiency of InCl3 (time, yield, reaction conditions) with the efficiency of other catalysts in the synthesis of perimidine derivatives obtained by other catalysts. It is clear from Fig. 5 that InCl3 is comparable to the catalysts used previously for the synthesis of perimidines.
Comparison of efficiency of various catalysts in synthesis of perimidines.
| Catalyst | Conditions | Yield (%) | Time (h) | Ref. |
| InCl3 | H2O/r.t. | 77–91 | 3–4 | This work |
| BiCl3 | EtOH/r.t | 79–92 | 0.5–12 | [12] |
| RuCl3 | EtOH/r.t | 78–91 | 0.5–24 | [13] |
| Yb(OTf)3 | EtOH/r.t | 75–93 | 0.5–32 | [14] |
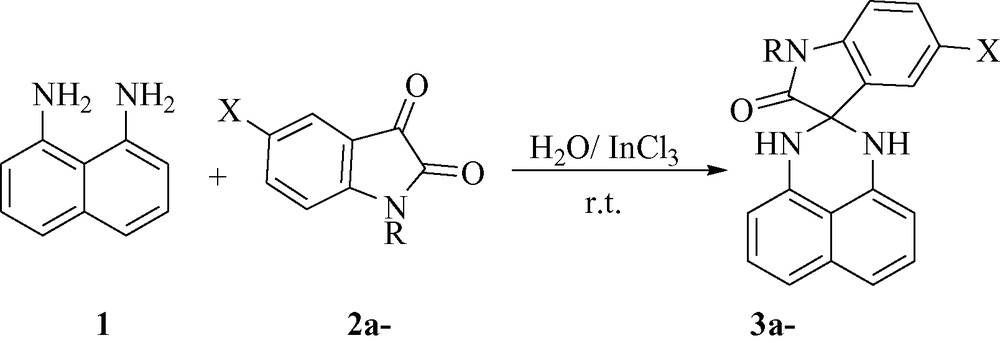
As expected, when the isatin 2 was replaced by acenaphthylene-1,2-dione 4, 1′,3′-dihydro-2H-spiro[acenaphthylene-1,2′-perimidin]-2-one 5 was obtained in 78 % yield under the same reaction conditions (Fig. 1).
.
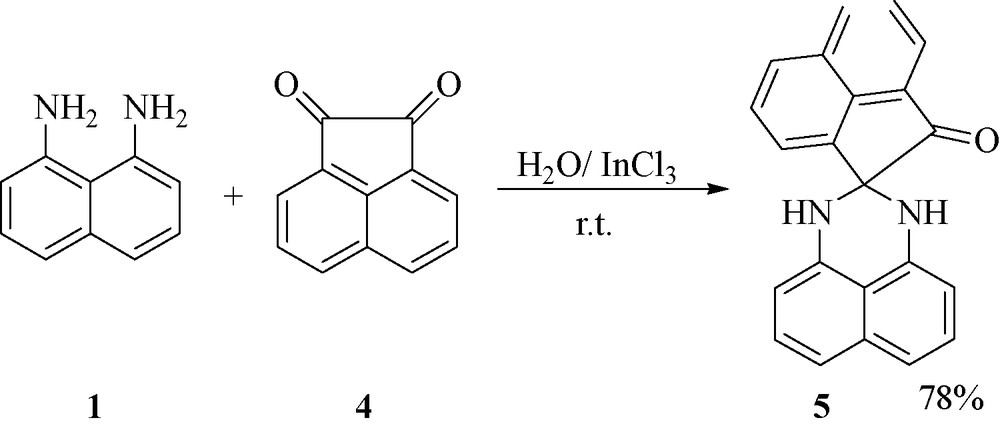
To further explore the potential of this protocol for perimidines synthesis, we investigated reaction of naphthalene-1,8-diamine 1 with 2-indanone 6 or pyrimidine-tetraone 7 and obtained tetrahydrospiroindene-perimidine 8 and 1,3-dihydro-1′H-spiro[perimidine-2,5′-pyrimidine]-2′,4′,6′(3′H)-trione 9 in excellent yields (Fig. 2).
.
When the piperidin-4-one 10, cyclododecanone 11 and 11H-indeno[2,1-b]quinoxalin-11-one 12 was selected as active carbonyl compounds (Fig. 3), the desired spiro-perimidines 13, 14 and 15 were obtained in good yields.
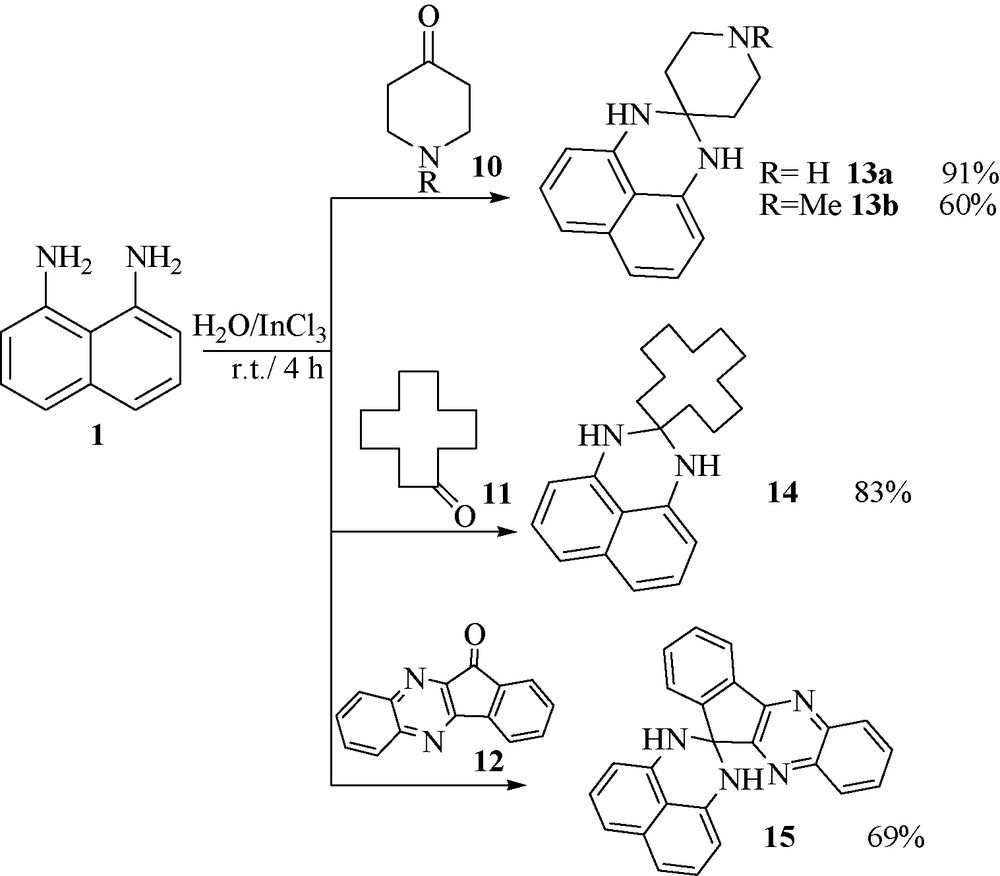
.
When this reaction was carried out with ninhydrine 16, benzoquinone 17 and naphthoquinone 18 in same conditions (H2O/InCl3), TLC and 1H NMR spectra of the reaction mixture showed a combination of starting materials and numerous products, the yield of the expected product was very poor.
All synthesized compounds are stable solids whose structures are fully supported by IR, 1H and 13C NMR spectroscopy, mass spectrometry, and elemental analysis.
3 Conclusions
In conclusion, InCl3 has been found to be an efficient catalyst for the preparation of spiro-perimidine derivatives via a cyclo-condensation reaction in water. This protocol includes some important aspects like the use of water as a “green” reaction medium, high atom economy and mild reaction conditions.
4 Experimental
4.1 Materials and techniques
Melting points were taken on an Electrothermal 9100 apparatus and left uncorrected. IR spectra were obtained on a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer at 300.13 and 75.47 MHz. NMR spectra were obtained on solutions in DMSO using TMS as internal standard. All of the chemicals were purchased from Fluka, Merck and Aldrich and used without purification.
4.2 General procedure for the preparation of spiro-perimidines
A mixture of naphthalene-1,8-diamine (1 mmol), active carbonyl compounds (1 mmol) and InCl3 (20 mol %) in water (5 ml) was stirred at room temperature for appropriate time (the progress of reaction was monitored by TLC). After completion of the reaction, the reaction mixture was filtered and the precipitate washed with water and then ether to afford the pure product.
4.2.1 1′,3′-Dihydrospiro[indoline-3,2′-perimidin]-2-one 3a
White powder; m.p. 244–246 °C. IR (KBr): 3361, 3211, 1722, 1597/cm. 1H NMR (300 MHz, DMSO-d6): δ = 6.44–7.35 (12H, m, H-Ar and 2NH), 10.24 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): 67.9, 105.5, 110.2, 111.8, 116.0, 122.3, 125.8, 127.2, 130.2, 130.8, 133.9, 141.0, 143.0, 177.9. MS, m/z: 287 (M+). Analytically calculated for C18H13N3O: C 75.25; H 4.56; N 14.63 %. Found: C 75.16; H 4.51; N 14.55 %.
4.2.2 5-Bromo-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3b
Cream powder; m.p. 236 °C (dec). IR (KBr): 3362, 3047, 1723, 1599/cm. 1H NMR (300 MHz, DMSO-d6): δ = 6.43–7.34 (11H, m, H-Ar and 2NH), 10.24 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ = 67.9, 105.4, 110.1, 111.8, 116.0, 122.3, 125.7, 127.2, 130.2, 130.8, 133.9, 141.0, 143.0, 177.9. MS, m/z: 367 (M+), 365 (M+). Analytically calculated for C18H12BrN3O: C 59.03; H 3.30; N 11.47 %. Found: C 59.9; H 3.24; N 11.40 %.
4.2.3 5-Nitro-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3c
Light brown powder; m.p. 263 °C (dec). IR (KBr): 3345, 3054, 2917, 1745, 1624/cm. 1H NMR (300 MHz, DMSO-d6): δ = 6.47–8.33 (11H, m, H-Ar and 2NH), 11.02 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ = 67.7, 106.0, 110.6, 111.8, 116.7, 120.9, 127.3, 127.9, 131.1, 133.9, 140.1, 142.6, 149.6, 178.0. MS, m/z: 332 (M+). Analytically calculated for C18H12N4O3: C 65.06; H 3.64; N 16.86 %. Found: C 65.15; H 3.71; N 16.93 %.
4.2.4 1-Methyl-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3d
Light pink powder; m.p. 281 °C (dec). IR (KBr): 3356, 3069, 2917, 1705, 1603/cm. 1H NMR (300 MHz, DMSO-d6): δ = 3.10 (3H, s, CH3), 6.43–7.46 (12H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 26.1, 67.8, 105.5, 109.0, 111.8, 116.1, 123.0, 125.3, 127.2, 129.5, 130.9, 133.9, 140.8, 144.4, 175.9. MS, m/z: 301 (M+). Analytically calculated for C19H15N3O: C 75.73; H 5.02; N 13.94 %. Found: C 75.62; H 5.07; N 13.86 %.
4.2.5 1-Ethyl-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3e
Brown powder; m.p. 250–252 °C. IR (KBr): 3374, 3048, 1707, 1598/cm. 1H NMR (300 MHz, DMSO-d6): δ = 1.15 (3H, bs, CH3), 3.60 (2H, bs, CH2), 6.43–7.44 (12H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 12.9, 34.2, 67.7, 105.5, 109.1, 111.8, 116.1, 122.8, 125.5, 127.2, 129.7, 130.9, 133.9, 140.9, 143.5, 175.5. MS, m/z: 315 (M+). Analytically calculated for C20H17N3O: C 76.17; H 5.43; N 13.32 %. Found: C 76.10; H 5.38; N 13.41 %.
4.2.6 1-Benzyl-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3f
Dark pink powder; m.p. 168 °C. IR (KBr): 3332, 1750, 1682, 1587/cm. 1H NMR (300 MHz, DMSO-d6): δ = 4.79 (2H, s, CH2), 6.47–7.35 (17H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 42.8, 67.8, 105.6, 109.7, 111.8, 116.2, 123.1, 125.6, 127.2, 127.7, 129.0, 129.5, 130.8, 133.9, 136.5, 140.8, 143.5, 176.0. MS, m/z: 377 (M+). Analytically calculated for C25H19N3O: C 79.55; H 5.07; N 11.13 %. Found: C 79.66; H 5.14; N 11.08 %.
4.2.7 5-Bromo-1-methyl-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3g
Dark brown powder; m.p. 265–258 °C (dec). IR (KBr): 3343, 1693, 1603/cm. 1H NMR (300 MHz, DMSO-d6): δ = 3.05 (3H, s, CH3), 6.42–7.64 (11H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 26.3, 67.8, 105.7, 111.2, 111.8, 114.6, 116.4, 126.8, 127.2, 131.8, 133.5, 133.9, 140.4, 143.7, 175.3. MS, m/z: 381 (M+), 379 (M+). Analytically calculated for C19H14BrN3O: C 60.02; H 3.71; N 11.05 %. Found: C 60.10; H 3.77; N 12.99 %.
4.2.8 1-Methyl-5-nitro-1′,3′-dihydrospiro[indoline-3,2′-perimidin]-2-one 3h
Dark yellow powder; m.p. 274 °C (dec). IR (KBr): 3347, 3043, 2927, 1728, 1605/cm. 1H NMR (300 MHz, DMSO-d6): δ = 3.15 (3H, s, CH3), 6.46–8.43 (11H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 26.7, 67.4, 106.1, 109.5, 116.9, 120.3, 127.3, 128.0, 130.5, 133.6, 133.9, 140.0, 143.1, 150.6, 176.2. MS, m/z: 346 (M+). Analytically calculated for C19H14N4O3: C 65.89; H 4.07; N 16.18 %. Found: C 65.81; H 4.15; N 16.11 %.
4.2.9 1′,3′-Dihydro-2H-spiro[acenaphthylene-1,2′-perimidin]-2-one 5
Brown powder; m.p. 279 °C (dec). IR (KBr): 3450, 3043, 2933, 1677, 1629/m. 1H NMR (300 MHz, DMSO-d6): δ = 7.27–8.82 (14H, m, H-Ar and 2NH). MS, m/z: 322 (M+). Analytically calculated for C22H14N2O: C 81.97; H 4.38; N 8.69 %. Found: C 81.88; H 4.31; N 8.77 %.
Due to very low solubility of the product 5, we cannot report the 13C NMR data for this product.
4.2.10 1,1′,3,3′-Tetrahydrospiro[indene-2,2′-perimidine] 8
Light pink powder; m.p. 282 °C (dec). IR (KBr): 3339, 3038, 2938, 1597/cm. 1H NMR (300 MHz, DMSO-d6): δ = 3.12 (4H, s, 2CH2), 6.42–7.18 (12H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 46.5, 74.9, 105.2, 113.0, 115.4, 125.3, 126.9, 127.4, 134.8, 140.7, 142.4. MS, m/z: 272 (M+). Analytically calculated for C19H16N2: C 83.79; H 5.92; N 10.29 %. Found: C 83.72; H 5.86; N 10.35 %.
4.2.11 1,3-Dihydro-1′H-spiro[perimidine-2,5′-pyrimidine]-2′,4′,6′(3′H)-trione 9
Brown powder; m.p. 201 °C (dec). IR (KBr): 3412, 3327, 2927, 1754, 1687 cm−1. 1H NMR (300 MHz, DMSO-d6): δ = 6.78-7.59 (8H, m, H-Ar and 2NH), 9.46 (2H, bs, 2NH). MS, m/z: 282 (M+). Analytically calculated for C14H10N4O3: C 59.57; H 3.57; N 19.85 %. Found: C 59.50; H 3.51; N 19.79 %.
Due to very low solubility of the product 9, we cannot report the 13C NMR data for this product.
4.2.12 1,3-Dihydrospiro[perimidine-2,4′-piperidine] 13a
Light pink powder; m.p. 282 °C (dec). IR (KBr): 3364, 3206, 2938, 1599/cm. 1H NMR (300 MHz, DMSO-d6): δ = 1.87 (4H, bs, 2CH2), 3.26 (4H, bs, 2CH2), 6.51–7.15 (8H, m, H-Ar and 2NH), 9.13 (1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δ = 33.3, 48.7, 62.1, 105.1, 112.4, 115.5, 127.5, 134.5, 140.9. MS, m/z: 239 (M+). Analytically calculated for C15H17N3: C 75.28; H 7.16; N 17.56 %. Found: C 75.17; H 7.11; N 16.63 %.
4.2.13 1′-Methyl-1,3-dihydrospiro[perimidine-2,4′-piperidine] 13b
Dark brown powder; m.p. 115 °C (dec). IR (KBr): 3301, 3027, 1603/cm. 1H NMR (300 MHz, DMSO-d6): δ = 1.91 (4H, bs, 2CH2), 2.28 (3H, s, CH3), 2.79 (4H, bs, 2CH2), 6.50–7.54 (8H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 33.5, 42.7, 49.9, 61.5, 105.3, 112.4, 115.7, 127.3, 134.7, 140.8. MS, m/z: 253 (M+). Analytically calculated for C16H19N3: C 75.85; H 7.56; N, 16.59 %. Found: C 75.93; H 7.64; N 16.65 %.
4.2.14 1′,3′-dihydrospiro[cyclododecane-1,2′-perimidine] 14
Pink powder; m.p. 185 °C. IR (KBr): 3365, 3038, 2926, 1605 cm−1. 1H NMR (300 MHz, DMSO-d6): δ = 1.35–1.60 (22H, m, 11CH2), 6.14–7.11(8H, m, H-Ar and 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 19.0, 22.1, 22.5, 26.0, 26.2, 33.2, 39.1, 39.4, 40.2, 40.5, 40.7, 68.5, 104.6, 112.9, 114.7, 127.4, 134.6, 141.9. MS, m/z: 322 (M+). Analytically calculated for C22H30N2: C 81.94; H 9.38; N 8.69 %. Found: C 81.86; H 9.43; N 8.76 %.
4.2.15 1′,3′-dihydrospiro[indeno[2,1-b]quinoxaline-11,2′-perimidine] 15
Brown powder; m.p. Greater than 290 °C. IR (KBr): 3369, 3033, 2917, 1598/cm. 1H NMR (300 MHz, DMSO-d6): δ = 6.42–8.11 (16H, m, H-Ar and 2NH). MS, m/z: 372 (M+). Analytically calculated for C25H16N4: C 80.63; H 4.33; N 15.03 %. Found: C 80.52; H 4.39; N 15.11 %.
Due to very low solubility of the product 15, we cannot report the 13C NMR data for this product.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.


