1 Introduction
Marine sponges and tunicates are known as rich sources of novel microorganisms showing a vast array of biological activities [1,2], many of which can be used for drug development. Continuing our search of new bioactive compounds from plants and marine organisms collected from the Tunisian coast [3–11], we have isolated from ethyl ether extracts four marine organisms: the glycosphingolipids phallusides 1-3 (1), the furanosesquiterpene Fasciculatin (2) Acanthellin (3), Axisonitrile (4), Oroïdin (5) and a new bromopyrrole derivative named Axinellizine (6) (Fig. 1). Fasciculatin, previously isolated from Ircinia fasciculata, was reported to have a moderate cytotoxicity and inhibition of lymphocyte proliferation [12]. Preliminary work on Acanthellin and Axisonitrile, indicated their utility as antimalarial drugs [13]. In this study, we report the investigation of the effects of the bioactive secondary metabolites indicated above towards five pathogenic fungi as well as insecticidal activity of Acanthellin against Tribolium confusum Duv larvae.
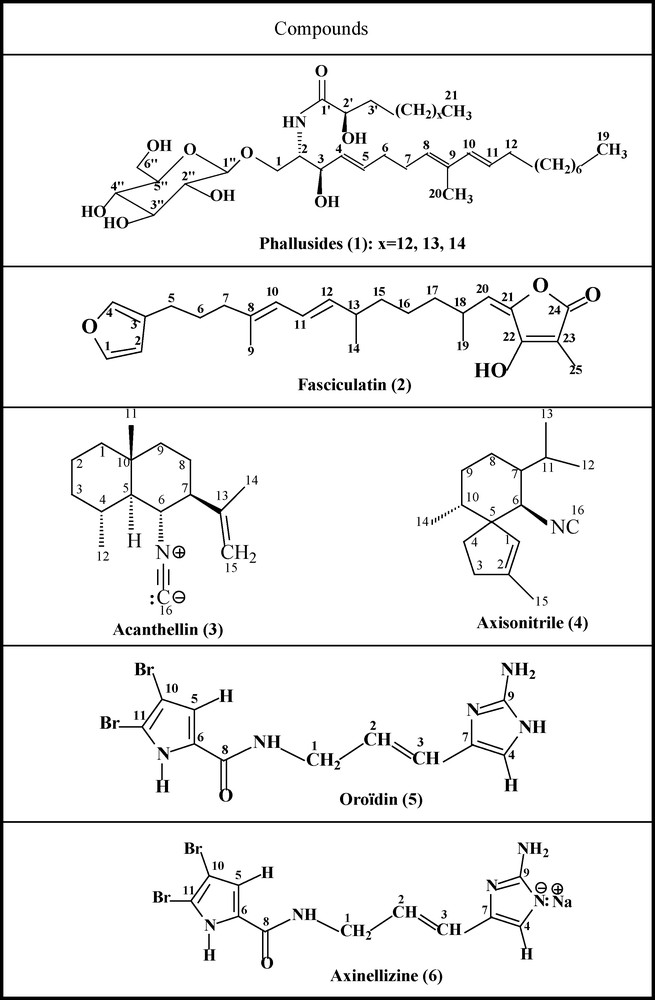
Natural substances evaluated for their antifungal effects.
2 Material and methods
2.1 Animal material
The tunicate Sidnyum turbinatum (polyclinidae family, ascidiaceae order) and the sponges Ircinia variabilis, Acanthella acuta and Axinella damicornis (Axinellidae) were collected by hand at depths of 10 m, 18 m and 25 m, from sidi Elghdamssi island in Monastir region (Center East coast of Tunisia) in August 2003 and were stored in a freezer (−20 °C) until extraction. Voucher specimens were deposited in the Laboratorio de Sostanze Naturali Consiglio Nazionale delle Ricerche, Instituto di Chimica Biomolecolare Pozzuoli, Napolie, Italy.
2.2 Extraction and isolation
2.2.1 Sidnyum turbinatum
The frozen organism (33.9 g dry weight after extraction) was exhaustively extracted with acetone in an ultrasound apparatus at room temperature and the extract was filtered and concentrated by rotary evaporation. The resulting water residue was extracted subsequently with diethyl ether and butanol yielding 560 and 60 mg, respectively.
2.2.2 Ircinia variabilis, Acanthella acuta and Axinella damicornis
Ethyl ether extracts from three sponges were prepared in the same way as previously described for the tunicate.
2.3 Fungitoxicity assay
2.3.1 Fungal isolate
Five phytopatogenic fungal species were used for the antifungal testing, namely: Fusarium oxysporum f. sp. Niveum (Boughalleb and El Mahjoub 2005) [14], Fusarium solani f. sp. Cucurbitae (Boughalleb et al. 2005) [15], Pythium ultimum and Alternaria solani. Samples of each isolated and identified fungi were deposited in the collection bank at the plant pathology laboratory institut supérieur agronomique de Chott Mériem, université du centre, Sousse, Tunisia.
2.3.2 Effect on mycelial growth of fungi
Fungitoxicity of the indicated pure natural products was assessed using the disk diffusion method [16,17]. Fungal broth culture aliquots were added to potato dextrose agar medium (PDA) and distributed uniformly in 9 cm Petri dishes. Once the substrate solidified, four Wattman disks were placed in Petri dishes. Each one was moistened with 20 μg of pure compound dissolved in the appropriate solvent at a concentration of 1 mg mL−1.
A control was prepared by moistening a small disk with the same volume of SDW + Tween 80 (10%). Inhibition zone diameters around the disks were measured after cultivation at 25 °C for eight days. Each experiment was performed in triplicate.
2.4 Insecticidal assay
2.4.1 Insecticidal activity
Three millimeter long larvae of the T. confusum insect were obtained from same-age cultures. All insects were fed with white wheat flour and beer yeast (95:5) and incubated at a constant temperature of 32 °C, in darkness. Parent adults were provided by the laboratory of entomology reserve, école supérieure d’horticulture et d’élevage, Chott Mériem, Sousse, Tunisia.
2.4.2 Bioassays
Acanthellin was tested for its toxic and larval growth inhibition effects. In fact, 5 μL of the compound (solution of 10 mg/mL) were mixed with discs weighing about 20 mg and having 1 cm diameter, dried at 32 °C during 24 h and then weighed before being afforded to larvae inside 4 cm diameter glass Petri dishes. Each test is done in three replications for 10 insects. A control was prepared in the same way using only the dissolving solvent. Larval growth inhibition was obtained by measuring length growth, recorded 16 days after treatment compared with the control. Percentage of alive larvae (CI) was calculated using the following formula:
In the precedent described Petri dishes, mortality was determined every four days during the essay (16 days) [10].
3 Results and discussion
Phallusides (1), Fasciculatin (2), Acanthellin (3), Axisonitrile 3 (4), Oroïdin (5) and the Novel Alcaloïd (6) were isolated from diethylether extracts of marine organisms collected from the Tunisian coast. Structural elucidation of all the compounds was established using 1D and 2D NMR spectra [9]. In the present work, we report the evaluation of their antifungal effects against the filamentous fungi: F. oxysporum f. sp. Niveum, F. solani f. sp. Cucurbitae, P. ultimum and A. solani at a concentration of 20 μg/disc as well as effects of Acanthellin on larvae of the stored product pest T. confusum Duv (Table 1).
Fungi used to evaluate activity of the pure indicated compounds.
| Fungus | Origin | Plant part sampled | Location | Collection date |
| F. Oxysporum f. sp. niveum | Watermelon | Roots | Skhira | 23/05/2001 |
| F. Solani f. sp. cucurbitae | Watermelon | Roots | Skhira | 23/05/2001 |
| Pythium ultimum | Watermelon | Roots and stems | Regueb | 23/05/2001 |
| Alternaria solani | Tomato | Leaves | Chott Mariem | 12/11/2002 |
3.1 Antifungal activity of six pure compounds
Antifungal evaluation showed some interesting results and the inhibition zones of fungal growth are presented in Table 2. Thus, Oroïdin, Phallusides and the new Axinellizine have shown antifungal activities against A. solani, Axisonitrile was found to be the most active compound inhibiting the growth of Fusarium oxysporom, Fusarium solani and A. solani fungi. None of the tested compounds exhibited activity towards Pythium ultimum.
Antifungal test results.
| Organism | Phallusides | Fasciculatine | Acanthelline | Axisonitrile | Oroïdin | Axinellizine | |
| Filamentous fungi | |||||||
| Fusarium oxysporum f. sp. Niveum | – | (22.5) | (23) | (13.5) | – | – | |
| Fusarium solani f. sp | – | – | – | (15) | – | – | |
| Pythium ultimum | – | – | – | – | – | – | |
| Alternaria solani | (25) | – | – | (16) | (16) | (11.5) |
3.2 Insecticidal activity of Acanthellin (3)
3.2.1 Grain contact toxicity
The percentage of alive larvae were determined each 4 days of insect exposure, indicated that a dose of 0.025 mg/grain of the oil was able to induce 35 and 45% mortality of insects within 4 days and 8 days of exposure, respectively (Fig. 2).
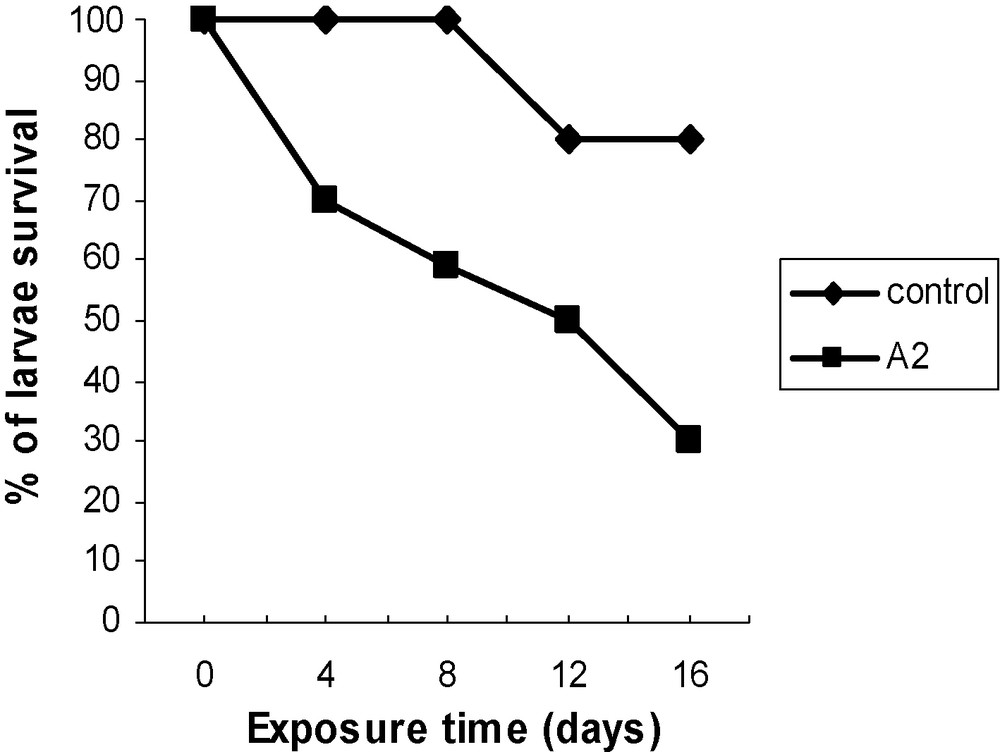
Toxicity effect of Acanthellin on Tribolium confusum larvae.
3.2.2 Insect growth inhibition bioassay
The evolution comparison of T. confusum larval growth index of treated discs with that of the control shows a larval growth inhibition of Acanthellin on T. confusum larvae (Fig. 3), the pure compound was tested at a concentration of 10 mg mL−1.
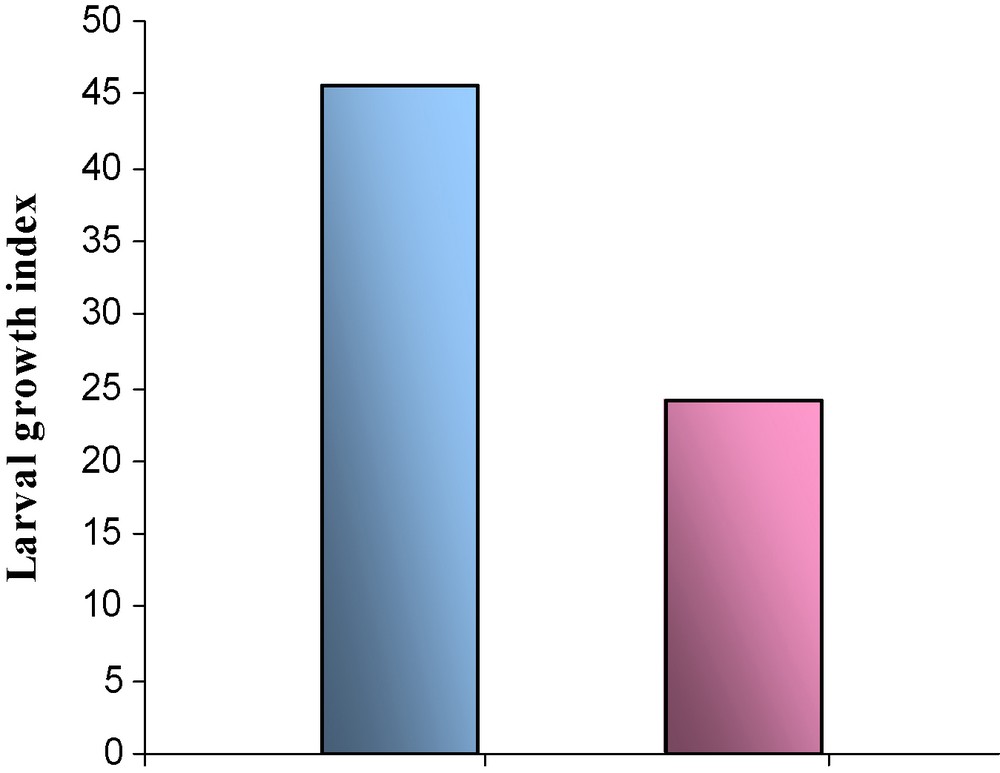
Effect of Acanthellin on Tribolium confusum larval growth.
Acknowledgements
Authors are grateful to Pr. Guido Cimino and Dr. Maria Letizia Ciavatta (Laboratorio Sostanze Naturali Consiglio Nazionale delle Ricerche Instituto di Chimica Biomolecolare Pozzuoli, Napolie, Italy) for their support in structural elucidation.


