1 Introduction
Owing to their unique properties, acidic ionic liquids (ILs) have attracted increasing attention in many fields [1]. Although acidic ILs were initially introduced as alternative green media because they are low-melting salts that are nonvolatile, thermally stable, recyclable, and easy to handle, there is currently a realization that there is a vast range of novel applications of ILs, such as in electrochemistry [2], extraction [3], organic synthesis [4], biphasic catalysis [5], physical chemistry [6], media for crystal growth [7], and materials science. These ILs, which are nonflammable and nonvolatile, can also be used as lubricants [8] and heat transfer fluids. A number of ILs with unique properties have been developed and used as catalysts in many types of reactions. However, as is true for conventional organic solvents, not all ILs are appropriate for a particular reaction, and a single IL will not always be the best for every reaction [9]. These ILs can be considered as a unique subclass called task-specific ILs [10] which otherwise are not present in ordinary ILs. Therefore, it is worthwhile to design and synthesize task-specific ILs for particular chemical processes [11].
An acidic IL based on the benzimidazolium cation 1-ethylbenzimidazolium tetrafluoroborate ([H-ebim]BF4) was reported as an efficient medium for the preparation of arylic esters [12]. In this article we present the synthesis and characterization of five novel ILs based on the benzimidazolium cation. Furthermore, we prepared a crystal of 1-butyl-2-methylbenzimidazolium tetrafluoroborate and determined its crystal structure by single-crystal X-ray diffraction. The preparation (Scheme 1) and characterization are described. These new ILs show good catalytic activity in the esterification of carboxylic acids with alcohols, and they maintain good catalytic performance after recycling at least six times.
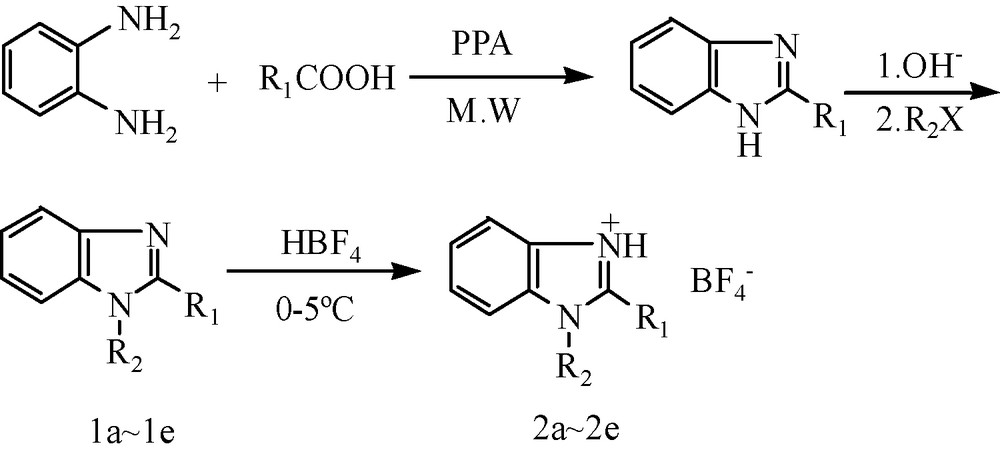
Synthesis of 1-R2-2-R1benzimidazolium tetrafluoroborate, R1 = Me, R2 = Me, Et, Pr-n, Bu-n, Pen-n.
2 Experimental
2.1 General remarks
All chemicals and solvents used were AR grade without further purification and commercially available. Acidic ILs, based on the benizimidazolium cation (Bim) and inorganic anion BF4−, were prepared by acid-base neutralization at 0–5 °C. Elemental analyses of carbon, hydrogen, and nitrogen were carried out with a Perkin-Elmer model 240C instrument. Infrared spectra on KBr pellets were recorded on a Bruker EQUINOX-55 FTIR spectrophotometer in the range 4000–400 cm−1. 1HNMR spectra in propanone-d6 solution were taken with a Varian INOVA-400 spectrometer. The differential scanning calorimetry experiment and the thermal stability of each IL was performed using Q1000DSC + LNCS + FACS Q600SDT at a heating rate of 5 °C min−1 with nitrogen as the purge gas. The esterification reactions were carried out in a glass reactor fitted with a reflux condenser and oil bath. The reaction was monitored by GC-MS (AligentGC: 6890N, MS: 5793N). The melting points were determined by Melting Point Apparatus XT-4.
2.2 Synthesis of ionic liquids
The synthesis process was carried out according to the literature [13]. A crystal suitable for single-crystal X-ray analysis was grown by diffusion of Et2O in a mixture of ethanol and chloroform at room temperature.
2.2.1 Typical procedure of preparation for the ionic liquids
2-methylbenzimidazole (13.2 g, 0.1 mol), 50 mL of 50% sodium hydroxide (aqueous solution), and methyl iodide (8.7 mL, 0.11 mol) were placed in a three-necked 250 mL flask equipped with a mechanical stirrer, reflux condenser and thermometer. The mixture was stirred for 10 min; when it solidified, the temperature of the water bath was raised to 30–40 °C and the contents became liquid. After 5 min, an exothermic effect was observed and the temperature of the mixture was maintained at 30–40 °C for 10 min. The organic layer was extracted with CHCl3 (3 × 30 mL), washed with water, and dried with anhydrous sodium sulfate. The solvent was evaporated in a vacuum, and the oil obtained was purified by distillation.
To a solution of 7.2 g (0.05 mol) of 1,2-bismethylbenzimidazole in 50 mL of ethanol was added, drop-wise and with continuous stirring, 11.0 g (0.05 mol) of 40% w/w aqueous tetrafluoroboric acid. The mixture was stirred for 3 h before most of the ethanol and water was removed under vacuum in a rotary evaporator. The viscous residue was dehydrated at 80 °C in a vacuum oven for 2 h. The dehydrated residue solidified on cooling to a colorless crystalline solid.
Yield: 11.7 g (0.05 mol, 100%); IR, ν(KBr): 3308, 3027, 2996, 2839, 1627, 1569, 1502, 1468, 1379, 1313, 1082(broad, BF4−), 766 cm−1. 1HNMR (CD3COCD3/TMS), δ: 7.96–7.98 (m, 1H, Ar-H), 7.86–7.88 (m, 1H, Ar-H), 7.63–7.67 (m, 2H, Ar-H), 4.19 (s, 3H, N-CH3), 3.05 (s, 3H, =C–CH3). Elemental analysis calculated (%) for C9H11N2BF4 (234.00): C, 46.19; H, 4.74; N, 11.97. Found: C, 46.25; H, 4.68; N, 12.03. UV, λmax (water): 275.5 nm.
2.2.2 1-ethyl-2-methylbenzimidazolium tetrafluoroborate, [H-emBim]BF4
Yield: 12.4 g (0.05 mol, 100%); IR, ν(KBr): 3308, 3021, 2978, 2889, 1625, 1565, 1501, 1465, 1448, 1383, 1320, 1082 (broad, BF4−), 763 cm−1. 1HNMR (CD3COCD3/TMS), δ: 8.02–8.04 (m, 1H, Ar-H), 7.86–7.88 (m, 1H, Ar-H), 7.63–7.67 (m, 2H, Ar-H), 4.68 (q, J(H,H) = 7.4 Hz, 2H, N-CH2), 3.07 (s, 3H), 1.59 (t, J(H,H) = 7.4 Hz, 3H). Elemental analysis calcd (%) for C10H13N2BF4 (248.03): C, 48.42; H, 5.28; N, 11.29. Found C, 48.55; H, 5.36; N, 11.37. UV, λmax (water): 275.5 nm.
2.2.3 1-propyl-2-methylbenzimidazolium tetrafluoroborate, [H-pmBim]BF4
Yield: 13.1 g (0.05 mol, 100%); IR, ν(KBr): 3444, 3018, 2930, 2883, 1647, 1567, 1516, 1465, 1390, 1311, 1087 (broad, BF4−), 751 cm−1. 1HNMR (CD3COCD3/TMS), δ: 7.89–8.02 (m, 1H, Ar-H), 7.81–7.83 (m, 1H, Ar-H), 7.44–7.57 (m, 2H, Ar-H), 4.50 (t, J(H,H) = 7.2 Hz, 2H, N-CH2), 2.94 (s, 3H), 1.95–2.03 (m, 2H), 1.04 (t, J(H,H) = 7.2 Hz, 3H). Elemental analysis calcd (%) for C11H15N2BF4 (262.05): C, 50.42; H, 5.77; N, 10.69. Found: C, 50.54; H, 5.74; N, 10.74. UV, λmax (water): 275.5 nm.
2.2.4 1-butyl-2-methylbenzimidazolium tetrafluoroborate, [H-bmBim]BF4
Yield: 13.8 g (0.05 mol, 100%); IR, ν(KBr): 3274, 3027, 2962, 2894, 1648, 1561, 1501, 1466, 1381, 1318, 1078 (broad, BF4−), 761 cm−1. 1HNMR (CDCl3/TMS), δ: 7.76–7.78 (m, 1H, Ar-H), 7.49–7.58 (m, 3H, Ar-H), 4.35 (t, J(H,H) = 8 Hz, 2H, N-CH2), 2.91 (s, 3H), 1.84–1.92 (m, 2H), 1.42–1.47 (m, 2H), 1.00 (t, J(H,H) = 7.2 Hz, 3H). Elemental analysis calcd (%) for C12H17N2BF4 (276.08): C, 52.21; H, 6.21; N, 10.15. Found: C, 52.34; H, 6.26; N, 10.26. UV, λmax (water): 275.5 nm.
2.2.5 1-pentyl-2-methylbenzimidazolium tetrafluoroborate, [H-pemBim]BF4
Yield: 14.5 g (0.05 mol, 100%); IR, ν(KBr): 3443, 3034, 2958, 2889, 1647, 1564, 1516, 1467, 1383, 1311, 1089 (broad, BF4−), 754 cm−1. 1HNMR (CD3COCD3/TMS), δ: 7.88–8.02 (m, 1H, Ar-H), 7.78–7.82 (m, 1H, Ar–H), 7.46–7.53 (m, 2H, Ar-H), 4.52 (t, J(H,H) = 5.6 Hz, 2H, N-CH2), 2.92 (s, 3H), 1.92–2.00 (m, 2H), 1.38–1.48 (m, 4H), 0.90 (t, J(H,H) = 3.6 Hz, 3H). Elemental analysis calcd (%) for C13H19N2BF4 (290.11): C, 53.82; H, 6.60; N, 9.66. Found: C, 53.84; H, 6.57; N, 9.71. UV, λmax (water): 275.5 nm.
2.3 Typical preparation for esterification in novel ionic liquids (demonstrated by the example of 1-butyl-2-methylbenzimidazolium tetrafluoroborate [H-bmBim]BF4)
Acetic acid (0.01 mol), equivalent ethanol (0.01 mol) and ILs [H-bmBim]BF4 (0.005 mol) were added to a flask with a reflux condenser in an oil bath. The mixture was stirred for 2 h with the oil bath at 80 °C and progress of the reaction was monitored by GC-MS. Then the mixture was decanted and the ester isolated, and the IL [H-bmBim]BF4 was reused after removal of water under vacuum (0.01 Torr) at 80 °C for 2 h.
2.4 Crystal structure determination
X-ray diffraction data were collected with graphite-monochromated Mo Kα radiation (λ = 0.071073 nm) using a BRUKER SMART APEX CCD diffractometer for the compound at 296(2)K. The structure of [H-bmBim]BF4 was determined by direct methods and refined by full-matrix least-squares methods on F2 with the SHELXTL-97 program [14]. All hydrogen atoms were added according to theoretical models. Further details on crystal data, structure refinement and selected bond lengths and angles are given in Section 3.5.
3 Results and discussion
3.1 Esterification in the novel ionic liquids
The novel ILs were used as catalysts in esterification resulting in high yields. The esterification of a variety of acids with common alcohols was carried out using the synthesized ILs as catalysts (Table 1). Good yields were obtained in all cases. Because the tetrafluoroborate salts reported here are miscible with water but immiscible with ethers, esterification proceeded smoothly to completion without removal of the water produced, even though esterification is a reversible reaction. Table 2 shows the results of esterification of acetic acid and butanol isomeride in [H-bmBim]BF4 at 120 °C.
Results of esterification for different acids and alcohols in ionic liquids (ILs).
| Entry | Acid | Alcohol | ILs | Time (h) | T (° C) | Yield (%) |
| 1 | Acetic | Ethanol | [H-bmBim]BF4 | 2.0 | 80 | 95 |
| 2 | Acetic | 1-butanol | [H-bmBim]BF4 | 2.0 | 120 | 95 |
| 3 | Oxalic acid | Ethanol | [H-bmBim]BF4 | 4.0 | 80 | 90 |
| 4 | Oxalic acid | 1-butanol | [H-bmBim]BF4 | 4.0 | 120 | 90 |
| 5 | Benzoic acid | Ethanol | [H-bmBim]BF4 | 8.0 | 80 | 85 |
| 6 | Benzoic acid | 1-butanol | [H-bmBim]BF4 | 8.0 | 120 | 88 |
| 7 | Acetic | Ethanol | [H-emBim]BF4 | 2.0 | 80 | 96 |
| 8 | Oxalic acid | 1-butanol | [H-emBim]BF4 | 8.0 | 120 | 89 |
| 9 | Benzoic acid | Ethanol | [H-emBim]BF4 | 8.0 | 80 | 84 |
Results of esterification for acetic acids and butanol isomeride in [H-bmBim]BF4 at 120 °C.
| Entry | Butanol isomer | Time (h) | Yield (%) |
| 1 | 1-butanol | 2.0 | 95 |
| 2 | iso-butyl alcohol | 2.0 | 89 |
| 3 | sec-butyl alcohol | 2.0 | 70 |
| 4 | tert-butyl alcohol | 2.0 | 53 |
The results in Table 1 show that the esterification of aliphatic acids with primary alcohols was very satisfactory, followed by secondary alcohols and tertiary alcohols due to an increase in steric interaction. The length of alkyl chains of the primary alcohols did not affect the conversion. In the reaction of oxalic acid, no monoesterification products were detected when the molar ratio of acid to alcohol was 1:2. Esterification of aromatic acids gave lower yields compared with those of aliphatic acids; it is possible that the positive charge of the carbon cation formed during reaction was delocalized to benzene.
After the reaction, the ILs could be easily recycled. The mixture was decanted and the ILs separated and regenerated by distillation in a vacuum at 80 °C for 2 h. The IL [H-bmBim]BF4 was recycled six times in the esterification of acetic acid with ethanol, and its catalytic activity was unchanged (Table 3).
Reuse of [H-bmBim]BF4 in the synthesis of acetic acetate.
| Run | Time (h) | T (° C) | Yield (%) |
| 1 | 2.0 | 80 | 95 |
| 2 | 2.0 | 80 | 95 |
| 3 | 2.0 | 80 | 95 |
| 4 | 2.0 | 80 | 95 |
| 5 | 2.0 | 80 | 95 |
| 6 | 2.0 | 80 | 95 |
Compared with the inorganic acid catalyzed esterification, these new ILs showed good catalytic activity under mild reaction conditions, no corrosion and no waste acid. At the same time, several noteworthy features of this approach as follows: (1) they could maintain good catalytic performance after recycling at least six times; (2) the esters produced can be separated by easy decantation with high yields. These advantages make this methodology became a green alternative to the conventional mineral acid catalyzed esterification.
3.2 Synthesis of ionic liquids
The tetrafluoroborate salts were prepared by simple acid-base neutralization from the corresponding 1-alkylbenzimidazole with tetrafluoroboric acid. The acid-base neutralization is an atom-economical reaction – both atoms in the tetrafluoroboric acid and all atoms in the corresponding 1-alkylbenzimidazole molecules are involved in the final products. The atom economy for these reactions was 100%. Ethanol was used as the solvent, though not stoichiometrically due to its high solubility and easy reuse. The reactions were carried out in an ice bath and required little time or effort. The molecular structures were characterized by 1HNMR and FTIR spectroscopy and elemental analysis. Single-crystal X-ray crystallography of [H-bmBim]BF4 was obtained from a prepared crystal. All characterization data accord with expected compositions and structures.
3.3 Solubility
Due to their polarity and/or hydrogen bonding, polar solvents such as methanol show good solubility in ILs, while ILs are insoluble in nonpolar solvents. The novel ILs were completely soluble in ethanol, water, acetonitrile, methanol and acetone, but insoluble in toluene, benzene, ethyl acetate, cyclohexane and diethyl ether. Chloroform appeared to be borderline, as it is partly soluble in the novel ILs. The solubility in chloroform can be used to purify the products. We studied 1HNMR spectra in a propanone-d6 solution rather than in chloroform, as some novel ILs are immiscible with chloroform.
3.4 Thermal stability and differential scanning calorimietry measurements
Thermogravimetric data of the new ILs are shown in Table 4. Although research indicates that the new salts begin to decompose at slightly lower temperatures than the imidazole series [15], they also display high thermal stability. In each case, the samples showed no weight loss below 250 °C and some weight loss for 250–280 °C. Thorough degradation was observed until the temperature reached ∼300 °C. Therefore, the salts have potential usage as alternatives to conventional organic solvents due to their solubility and thermal stability.
Thermal analysis data of novel benzimidazolium salts.
| Entry | ILs | MP (°) | Td (°) |
| 1 | [HmmBim]BF4 | 125.5 | 274 |
| 2 | [HemBim]BF4 | 127.9 | 260 |
| 3 | [HpmBim]BF4 | 120.0 | 266 |
| 4 | [HbmBim]BF4 | 110.9 | 260 |
| 5 | [HpemBim]BF4 | 106.5 | 257 |
3.5 Description of the structure of [H-bmBim]BF4
The crystal data and structural details of this new IL, [H-bmBim]BF4, as determined by single-crystal X-ray diffraction, are presented in Table 5. Selected bond lengths and bond angles are listed in Table 6. The structure determined from these data is shown in Fig. 1 which shows that the atom B1 with the benzimidazole moiety is co-planar and the tetrafluoroborate anion and the stretched n-butyl group are on the same side of the benzimidazole plane.
Selected crystallographic data for the compound.
| Compound | [H-bmBim]BF4 |
| Formula | C12H17BF4N2 |
| Formula weight | 276.09 |
| T/K | 296(2) |
| Crystal system | Orthorhombic |
| Space group | Pna2(1) |
| Crystal size/mm | 0.31 × 0.24 × 0.15 |
| a/Å | 13.566(4) |
| b/Å | 15.593(4) |
| c/Å | 6.8193(17) |
| β/(°) | 90 |
| V/Å3 | 1442.5(6) |
| Z | 4 |
| Dc/(g cm−3) | 1.271 |
| Refl. No. | 7034 |
| θ/(°) | 1.99–25.09 |
| μ/mm−1 | 0.111 |
| F(000) | 576 |
| Limiting indices | −16 ≤ h ≤ 13 |
| −18 ≤ k ≤ 18 | |
| −7 ≤ l ≤ 8 | |
| Goodness-of-fit on F2 | 0.967 |
| R1a, b [I > 2σ(I)] | 0.0684, 0.1690 |
| R1, (all data) | 0.1812, 0.2298 |
| Largest diff. peak and hole (e Å−3) | 0.198 and −0.165 |
a R = ∑ ||F0| − |Fc||/∑ |F0|.
b .
Selected bond lengths (Å) and angles (°) of the compound.
| Bond | Dist. | Bond | Dist. |
| N(1)-C(1) | 1.400(6) | C(1)-C(2) | 1.380(7) |
| N(1)-C(7) | 1.321(6) | C(5)-C(6) | 1.381(7) |
| N(2)-H(2) | 0.8600 | C(7)-C(8) | 1.496(7) |
| N(1)-C(9) | 1.465(6) | B(1)-F(2) | 1.389(9) |
| N(2)-C(6) | 1.405(6) | C(1)-C(6) | 1.388(6) |
| N(2)-C(7) | 1.332(6) | ||
| Angle | (°) | Angle | (°) |
| F(1)-B(1)-F(4) | 110.7(9) | N(1)-C(1)-C(2) | 132.5(5) |
| C(7)-N(1)-C(1) | 109.1(4) | N(1)-C(7)-N(2) | 109.6(5) |
| C(7)-N(2)-C(6) | 109.0(4) | N(1)-C(9)-C(10) | 113.7(6) |
| C(7)-N(1)-C(9) | 127.0(5) | N(1)-C(7)-C(8) | 126.6(5) |
| N(1)-C(1)-C(6) | 106.6(5) | N(2)-C(6)-C(1) | 105.7(4) |

Crystal structure of [H-bmBim]BF4. The atom B1 with the benzimidazole moiety is co-planar. The tetrafluoroborate anion and the stretched n-butyl group are in the same side of benzimidazole plane.
The compound [H-bmBim]BF4 is composed of the 1-butyl-2-methylbenzimidazole cation and the tetrafluoroborate anion. The N1 atom adds a proton to form the cation, and the anion balances the charge. The bond lengths of C-N, N1-C7 and N2-C7 (1.321 Å and 1.332 Å, respectively) are greater than normal C-N bond lengths, indicating that they have double bond characteristics. However, they are different from those of other neutral substituted benzimidazole compounds (1.320(3) Å for N1-C7 and 1.359(2) Å for N2-C7 in 1,4-bis(2-benzimidazolyl) benzene [16]). The other two C-N bond lengths in the imidazole ring are in the range 1.401–1.405 Å, which is shorter than the single-bond length of 1.48 Å, but longer than the typical CN length of 1.28 Å, indicating appreciable charge delocalization in the ring.
The molecular structure is formed by weak π-π interactions and intermolecular hydrogen bonds between the benzimidazole rings, yielding a three-dimensional (3-D) net-like supramolecule. The benzimidazole moiety with the conjunction carbon atoms C8 and C9 are almost in the same plane, with the maximum departure of C8 0.023 Å and of C9 0.047 Å. The tetrafluoroborate anion and the stretched n-butyl group are on the same side of the benzimidazole plane. The distance between the atom B and the plane is 3.673 Å. The 1-butyl-2-methylbenzimidazole cation is connected with the tetrafluoroborate anion by hydrogen bonds as C–H···F and N–H···F, yielding a two-dimensional (2-D) sheet. All possible hydrogen bonding in the compound crystal between a cation and adjacent BF4− anions and symmetry codes is listed in Table 7. There are six kinds of intermolecular and no intramolecular hydrogen bonds in the packing structure. Furthermore, the neighboring 2-D sheets are extended through interlayer hydrogen bonds and π-π interactions between the benzimidazole rings [the centroid of the imidazolium ring (C1, N1, C7, C6, N2) to the centroid of the phenyl ring (C6, C5, C4, C3, C2, C1) distance is 3.672 Å (symmetry code 1-x, 1-y, 1/2 + z)], leading to a 3-D supramolecular structure shown in Fig. 2.
Hydrogen bonding parameters for [H-bmBim]BF4 crystal system.
| D-H…Aα | D-H | H…A | D…A | ∠D-H…A |
| C(8)-H(8A)…F(3)a | 0.960 | 2.457 | 3.394 | 165.58 |
| C(9)-H(9B)…F(3)a | 0.970 | 2.447 | 3.346 | 154.15 |
| C(8)-H(8C)…F(1)b | 0.960 | 2.381 | 3.278 | 155.44 |
| C(10)-H(10A)…F(1)b | 0.960 | 2.641 | 3.508 | 149.02 |
| N(2)-H(2)…F(2)c | 0.860 | 1.977 | 2.813 | 163.45 |
| N(2)-H(2)…F(4)d | 0.860 | 2.459 | 3.166 | 139.81 |
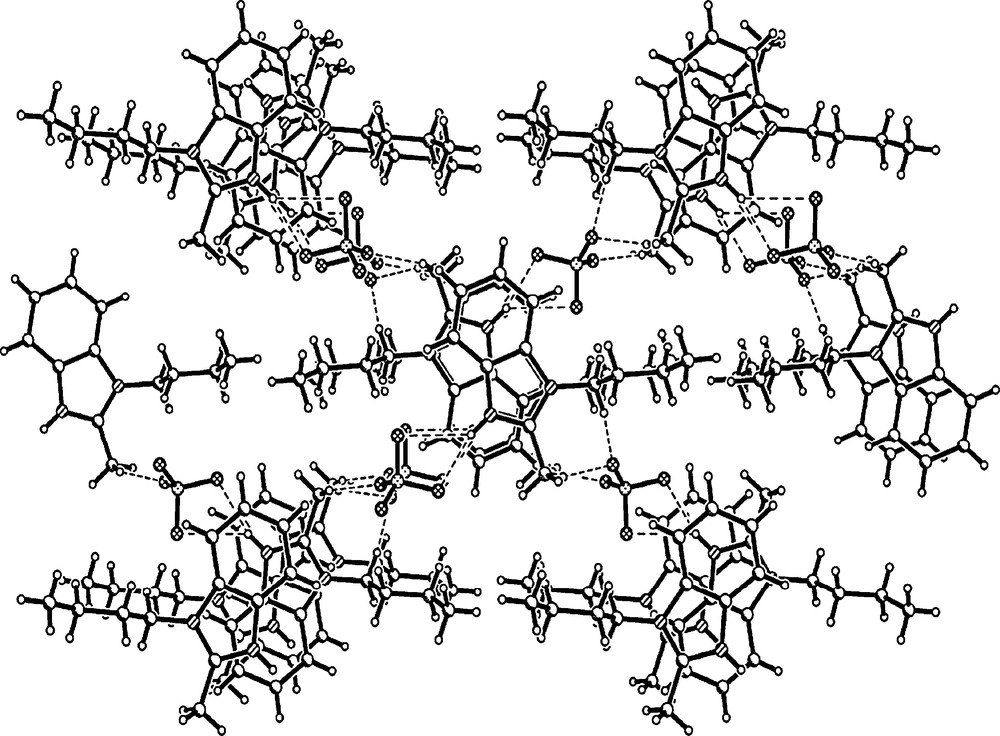
Packing in the crystal of [H-bmBim]BF4, with the view being down the crystallographic c axis.
4 Conclusions
In conclusion, several task-specific BrØnsted-acidic ILs based on the 1,2-bisalkylbenizimidazolium cation and inorganic anion BF4− have been designed, synthesized and fully characterized. These new ILs show good catalytic activity to esterification of carboxylic acids with alcohols, which could maintain good catalytic performance after recycling at least six times. Finally, we prepared a crystal of [H-bmBim]BF4, and the crystal structure has been determined by X-ray diffraction analysis.
Acknowledgments
This project was supported by the Natural Science Foundation of Shanxi Province (No. 03B19), China.
Supporting information: crystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication No. CCDC-743508. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336 033; e-mail: deposit@ccdc.cam.ac.uk).


