Initial metal ion concentration (mg/L)
CeResidual metal ion concentration (mg/L)
mMass of adsorbent (g)
VSolution volume (L)
qeEquilibrium metal uptake by the adsorbent (mg/g)
ηAdsorption efficiency
BETBrunauer-Emmett-Teller
MICMontmorillonite-illite clay
MIC(AA)Montmorillonite-illite clay (acid activated)
FTIRFourier transform infrared
RCorrelation coefficient
1 Introduction
Heavy metals accumulate in the food chain and persist in nature due to their discharge by many industrial activities. There are many treatment processes that can be used for the removal of metal ions from wastewater and the cost of operation plays an important role for determining which one is to be applied. As a result, in the last few decades, different adsorbents for the treatment of heavy metal contamination have been investigated. Despite the fact that single toxic metallic species rarely exist in wastewater, they are mostly analyzed in a single model system, possibly for the reason of better knowledge of adsorption phenomena. Some researchers investigated the adsorption from binary or multi-metal systems [1–4]. Besides heavy metal ions, industrial metal-bearing wastewater also contains other materials, such as supplementary cations, chelating agents, and other organic materials. All these can produce three possible types of behavior: synergism (the interaction of two different ions to produce an effect greater than the sum of their individual effects), antagonism (two or more different ions in combination have an overall effect which is less than the sum of their individual effects) and non-interaction. Thus, any study of adsorption characteristics of adsorbents in single metal solutions may only be considered as preliminary. Also, the literature is still scarce to cover the problem of multi-metal adsorption from real wastewaters. Some of the work available in the literature on multi-metal adsorption is cited in references [5–12].
In this work, isotherm studies on an acid-activated montmorillonite-illite clay [MIC(AA)] available in the Gulbarga region of Karnataka state, India, in removing heavy metal ions from industrial wastewater were examined.
Four isotherm models, viz: Freundlich [13], Langmuir [14], Brunauer-Emmett-Teller (BET) [15], and competitive Langmuir [16] (two competing ions) were applied to describe adsorption in this study. For modelling heavy metal adsorption from wastewater, these models have been previously applied by some researchers [1,17]. The isotherm parameters and correlation coefficients (R) were computed from the linearized forms of these isotherm equations.
Langmuir (monolayer) and Freundlich (multilayer) models were applied since they are simple and widely used for adsorption studies in aqueous solutions. The BET model represents an extension of the Langmuir model for multilayer adsorption. It is based on the assumption that each adsorbate in the first adsorbed layer serves as an adsorption site for the second layer, and so on. The difficulties in describing the adsorption of metal ions from wastewater resulted from the presence of several different components, causing interference and competition on adsorption sites. Therefore, it was necessary to use the competitive Langmuir model for two competing ions, in order to express the relationships between the quantity of the first component adsorbed and the concentration of the second component. All isotherms were fitted to experimental data, and the goodness of their fit was compared.
2 Materials and methods
2.1 Clay adsorbent
A sample of 20 g of raw clay montmorillonite-illite clay (MIC) in the particle size range 425 μm to 212 μm was mixed with 400 mL of H2SO4 solution which had 45% of H2SO4 by mass of the clay. The acid activation of the clay was performed by heating for six hours in a shaking water bath at 97 °C with reflux condensing of the vapors [18]. The activated clay sample was then washed several times with distilled water to remove sulfate ions. The sample was then dried in an air oven for 24 hours at 105 °C and stored in air tight polythene sachets. The acid-activated clay fraction in the size range 212 μm to 102 μm was used for experimentation.
2.2 Wastewater
Multi-metal ions containing industrial wastewater was collected from an electroplating factory in Bangalore, Karnataka, India. It was filtered through a qualitative filter paper and stored at 5 °C for further use. Chemical oxygen demand (COD) in wastewater was determined by means of dichromate method [19] and biochemical oxygen demand (BOD) by standard method [20].
2.3 Batch adsorption studies
Batch adsorption experiments were carried out at constant room temperature of 38 °C by adding different amounts of adsorbent to flasks containing wastewater, without adjusting pH. The flasks were shaken on a laboratory shaker for three hours. This contact time was sufficient to achieve equilibrium in the system for all heavy metal ions present in wastewater [21]. After that, the adsorbent was separated by vacuum filtration through a Gooch G3 crucible and the filtrate was analyzed for metal ions.
The concentrations of heavy metal ions before and after adsorption experiments were determined using an inductively coupled plasma atomic emission spectrometer (ICPAES), Thermo Electron IRIS INTREPID II XSP DUO instrument. Adsorption efficiency (η) was expressed as a percentage of adsorbed metal compared to initial metal concentration as:
| (1) |
| (2) |
3 Results and discussion
3.1 Characteristics of adsorbent and wastewater
Acid treatment of clays creates new pores resulting in an increase of surface acidity through the replacement of cations, like Al3+, Fe3+ and Ca2+ from the structure with H+ [22]. During clay activation with mineral acids, the acid first dissolves part of Al2O3 as well as CaO and MgO from the lattice, which leads to opening of the crystal lattice and an increase in internal surface area. Then, there is gradual exchange of the Ca and Mg ions located at the surface of the crystal against hydrogen ions from the mineral acid. Acid-activated clay is almost saturated with H+ ions and exhibits acidic character and better adsorption property.
X-ray fluorescence instrument (ARL/XRF – 8600) was used to identify the oxides composition of the MIC and MIC(AA) (Table 1). The physical specifications of the activated clay adsorbents used in the study are also given in Table 1. Major oxides in the adsorbent were SiO2, Al2O3, Fe2O3 and CaO. Properties of the adsorbents, such as specific gravity, pH and loss on ignition, were determined by standard procedures [23].
Oxides composition and specifications of the raw and acid-activated clay.
| Oxides | Weight (%) | |
| MIC | MIC(AA) | |
| SiO2 | 48.523 | 53.996 |
| Al2O3 | 9.682 | 8.937 |
| Fe2O3 | 18.809 | 14.093 |
| CaO | 17.042 | 16.751 |
| MgO | 3.971 | 2.863 |
| K2O | 1.768 | 2.265 |
| Na2O | 0.139 | 0.140 |
| SO3 | 0.065 | 0.955 |
| Specification | ||
| Particle size range | 212 μm to 106 μm | 212 μm to 106 μm |
| Average particle size | ∼160 μm | ∼160 μm |
| Specific gravity | 2.4 | 1.78 |
| pH | 8 | 4.15 |
| Langmuir surface area | 156 m2/g | 251 m2/g |
| Loss on ignition | 11.1% | 6.94% |
Mineral phase analysis of the raw and acid-activated clay was carried out in a Bruker AXS D8 powder XRD instrument. Some of the calcium, potassium and magnesium were removed from the clay as their corresponding sulfates during acid activation, which led to the disappearance of the montmorillonite and illite phases in raw clay. Also, there was inversion of quartz to quartz (low) due to acid activation. The XRD patterns are shown in Fig. 1 and the mineral phase compositions are given in Table 2.

XRD of raw and acid-activated clays.
XRD phase analysis of raw and acid-activated clay.
| Adsorbent | Compounds | Chemical formula |
| MIC | Quartz | SiO2 |
| Montmorillonite | (Na,Ca)0.3(Al,Mg)2.Si4O10(OH)2.xH2 O | |
| Illite | KAl2(Si3Al)O10(OH)2 | |
| MIC(AA) | Quartz (low) | SiO2 |
| Sodium Aluminum Silicate | Na8Al4Si4O18 | |
| Beidellite | Na0.3Al2(Si,Al)4O10(OH)2.2H2O |
The characteristics of the wastewater after filtration are presented in Table 3. FTIR spectrum of the wastewater (obtained with Thermo Nicolet Avatar 370 instrument), showing the significant absorption bands with the respective assignments, is given in Fig. 2.
Characteristics of wastewater.
| Parameter | Value |
| pH | 2.40 |
| COD (mg O2/L) | 690.00 |
| BOD (mg O2/L) | 275.00 |
| Cu (mg/L) | 280.32 |
| Zn (mg/L) | 382.72 |
| Pb (mg/L) | 17.00 |
| Total dissolved solids (mg/L) | 425.00 |
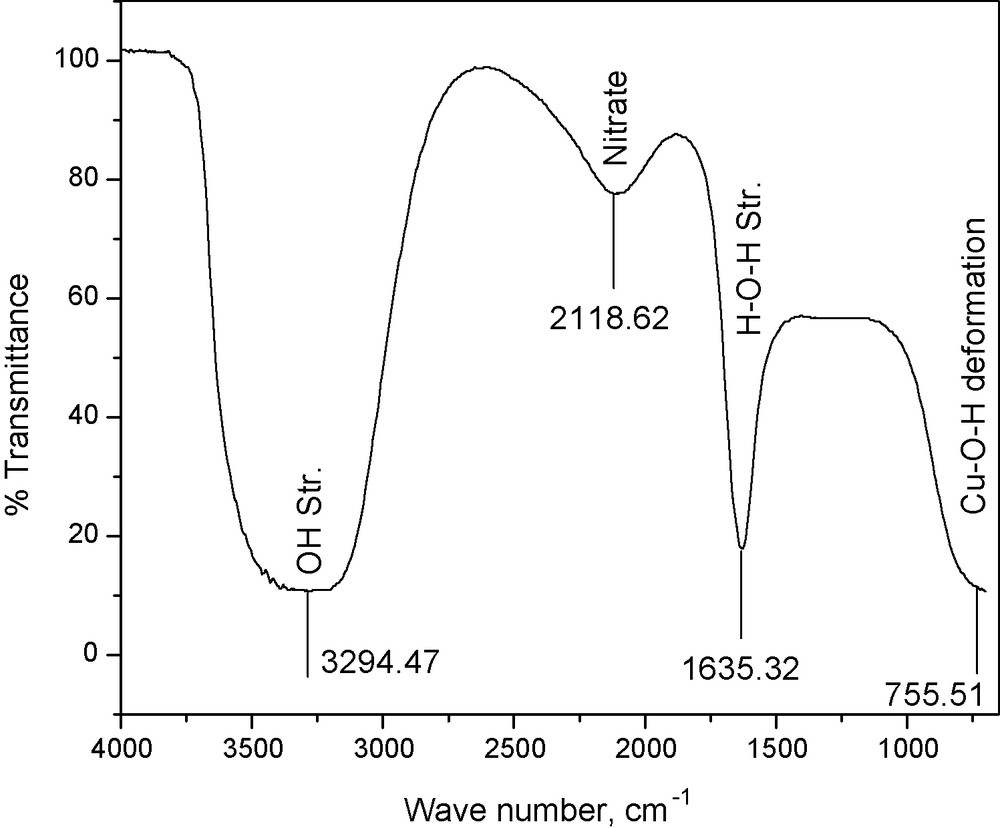
FTIR of wastewater.
3.2 Isotherm analysis
The acid-activated clay was tested for simultaneous adsorption of Cu(II) and Zn(II) from the industrial wastewater. The wastewater was filtered and diluted (4×) so as to reduce the Pb(II) concentration to a low level. Batch adsorption studies were performed with the diluted wastewater at room temperature. Fig. 3 shows the adsorption efficiencies achieved. As the solid/liquid ratio (m/V) was increased, the adsorption efficiency increased for Zn(II) as expected. However, it was observed that Zn(II) was a competing ion for Cu(II). Fig. 4 shows the adsorption isotherms for Cu(II) and Zn(II) on the acid-activated clay. The shapes of the adsorption isotherms indicated that the adsorption of two or more metals from wastewater was much complex than mono-metal adsorption. Investigations of adsorption from binary metal systems show different curves of adsorption plots influenced by competition phenomena [1]. The shapes of the adsorption isotherms obtained indicated that the adsorption from wastewater was multilayer. Table 4 gives the isotherm equations and Table 5 gives the values of the isotherm parameters and correlation coefficient (R) obtained by linear fitting using Origin 6® software.
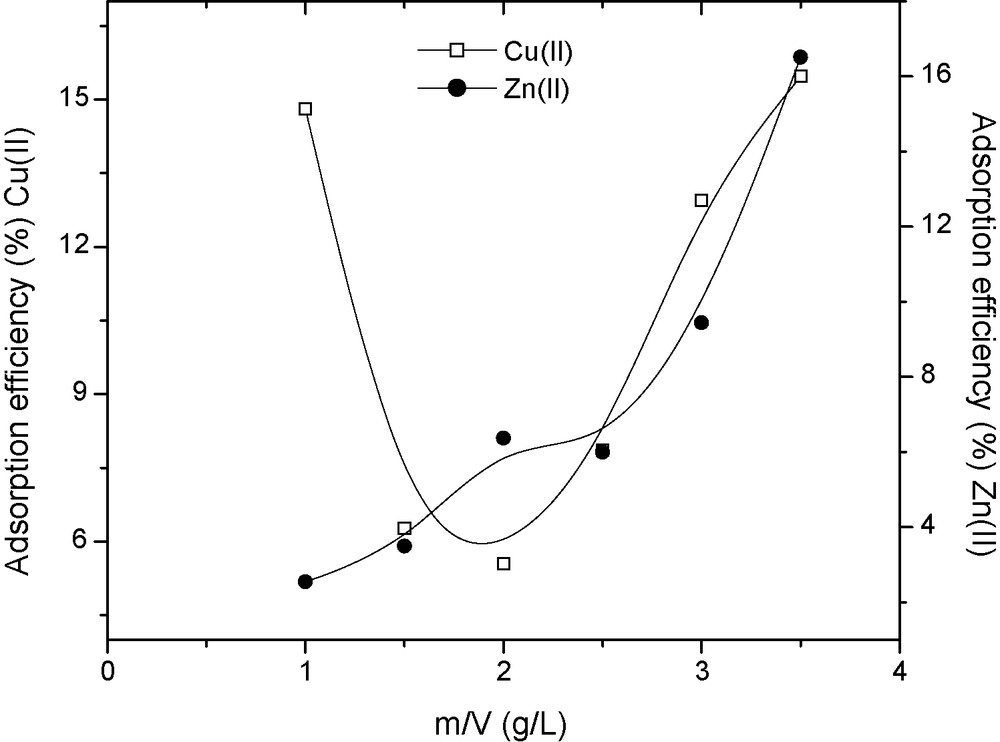
Adsorption efficiencies of Cu(II) and Zn(II) on acid-activated clay at 38 °C.

Adsorption isotherms at 38 °C, shaking time three hours.
Isotherm models.
| Model | Equation | Parameters |
| Freundlich | KF, n | |
| Langmuir | qm, KL | |
| BET | qm, k | |
| Competitive Langmuir | qm,i, Ki, Kj |
Parameter values and correlation coefficients of isotherm models.
| Isotherm model | Parameters | Values for the adsorption of | |
| Cu(II) | Zn(II) | ||
| Freundlich | KF | 3.46E14 | 9.43E08 |
| n | −0.1281 | −0.2284 | |
| R | −0.6496 | −0.9320 | |
| Langmuir | qm | 0.4076 | 0.5844 |
| KL | −0.0186 | −0.0144 | |
| R | 0.7843 | 0.9302 | |
| BET | qm | 5.5E-06 | 1.6E-06 |
| k | −3397.6 | −7583.7 | |
| R | 0.9142 | 0.8560 | |
| Competitive Langmuir | qm | 0.2573 | 0.4168 |
| K | −0.0720 | −0.0584 | |
| R | 0.8325 | 0.8882 |
Some researchers [1,2,10] observed that competitive adsorption was significant in binary or multi-metal systems when copper ions were present. This is a consequence of its paramagnetic nature and high electronegativity, and the pH values corresponding to bonding of aqua-hydroxocomplexes [24]. It was also observed that the copper and zinc ions have a synergistic effect. Hence, it is apparent that there is more than one factor playing important role in the adsorption process. Moreover, these factors may interact with each other and the conclusions extracted from the experiments with real wastewater are valid only for certain conditions and do not represent a general rule.
The Freundlich isotherm showed an inverse fit to the linearized equilibrium data for the adsorption of both Cu(II) and Zn(II) on the acid-activated clay due to the competing nature of the adsorption process. The linearized equilibrium data for the adsorption of both Cu(II) and Zn(II) on the acid-activated clay gave satisfactory fit to Langmuir, BET and competitive Langmuir models. The best fit, however, for Cu(II) was provided by BET model, and for Zn(II) by Langmuir model. Hence, it was confirmed that the Cu(II) adsorption from wastewater on the clay was multilayer, but Zn(II) adsorption was, probably, monolayer.
However, despite numerous investigations, the fundamental aspects of cation uptake from wastewaters are not well understood and conclusions about the mechanisms are divergent [9,25].
4 Conclusions
In this study, the ability of an acid-activated montmorillonite-illite type of clay to bind Cu(II) and Zn(II) from industrial wastewater was investigated. Results obtained were modelled using four adsorption models: Langmuir, Freundlich, BET and competitive Langmuir and the goodness of their fit was compared. Based on the results, it can be concluded that the adsorption of Cu(II) from the wastewater on the adsorbent was multilayer and that of Zn(II) was monolayer.
Acknowledgements
Author J.U.K. Oubagaranadin thanks Sophisticated Test and Instrumentation Centre (STIC) of Cochin University of Science and Technology, Kochi, Kerala, India, for extending analytical services for this work, and Dr. A. Venkataramana, Professor, Materials Science Department, Gulbarga University, for his expert advise.


