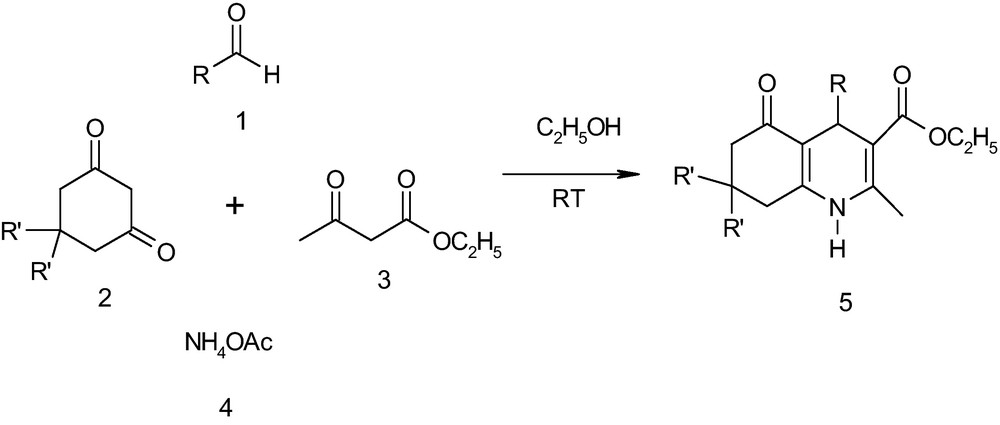
1 Introduction
Heterocycles are of immense importance in the design and discovery of new compounds for pharmaceutical applications [1]. 1,4-dihydropyridine (DHP), which is abitious nucleus of polyhydroquinolines is an important structural motif in the preparation of the drugs for the treatment of cardiovascular diseases, including hypertension [2]. These also exhibit interesting biological properties such as vasodilator, bronchodilator, anti-atherosclerotic, antitumor, geroprotective, hepatoprotective and antidiabetic agent [3] and also behave as neuroprotectants [4a], as well as chemosensitizers [4b] in tumor therapy. Other than their biological importance, DHPs have been meticulously used as reducing agents for the direct reductive amination of aldehydes and ketones [5] as well as their oxidation to pyridines being also extensively studied [6].
In 1882, Hantzsch and Jusius [7] reported the first synthesis of symmetrical substituted 1,4-dihydropyridines via multi-component condensation of aldehyde, ethylacetoacetate and ammonia in acetic acid or refluxing alcohol, while polyhydroquinolines can be synthesized by the utilization of cyclic 1,3-diketone by replacing one molecule of ethylacetoacetate in the Hantzsch reaction [8–18]. A detailed literature survey about the synthesis of polyhydroquinoline compounds revealed that most of the protocols employed for this reaction operate under reflux conditions [8] i.e., under high thermal activation or they need microwave irradiation [9]. There are a few protocols operable at room temperature using triflates of Yb [10] and Sc [11], I2 [12], bakers yeast [13], HY-zeolite [14], CAN [15], ionic liquids [16], organo-catalyst [17a], p-TSA [17b], etc. Recently, Mekheimer et al. [18] reported uncatalyzed synthesis of polyhydroquinolines induced by solar heat. However, many of these methodologies have been associated with several shortcomings such as requirement of long reaction times, use of expensive reagents and harsh reaction conditions. Similarly, the difficulties such as low product yields, occurrence of several by-products and recovery of the catalyst are encountered. Therefore, it was thought that there is room for improvement especially towards developing a green protocol for synthesis of polyhydroquinolines at ambient temperature. In continuation with our research devoted to the development of green organic chemistry [19a–e] we report herein an eco-friendly, one-pot multi-component synthesis of polyhydroquinolines at ambient temperature (Scheme 1).
2 Results and discussion
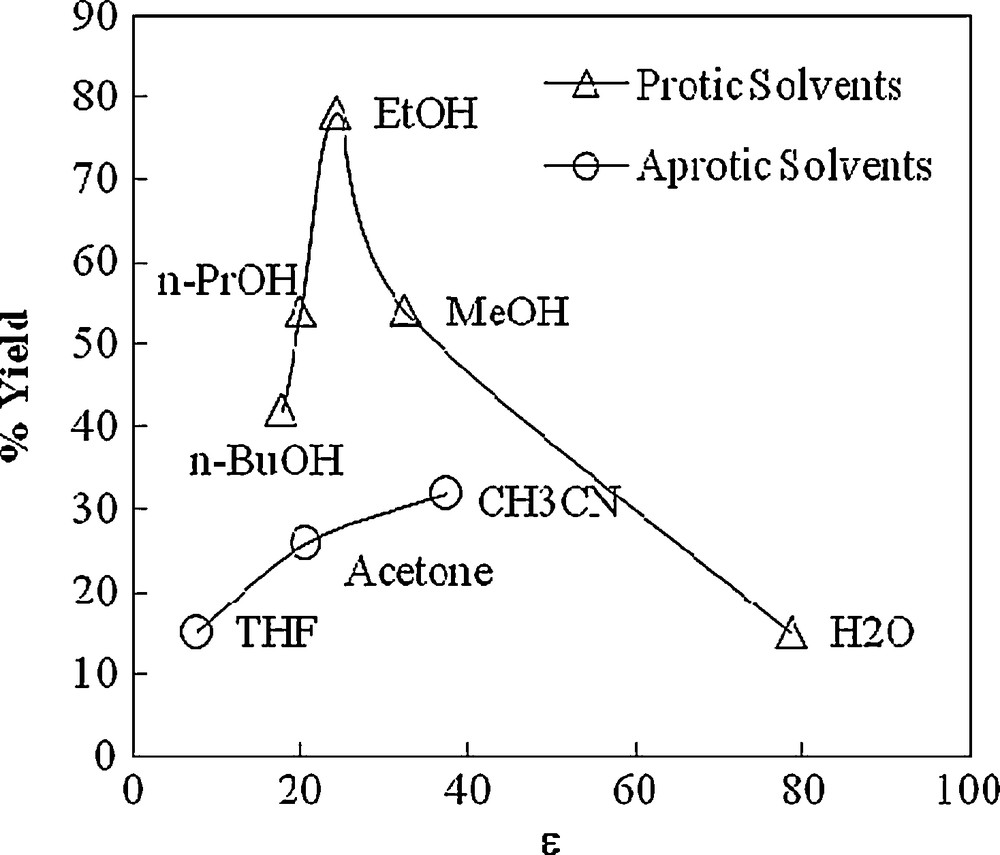
Considering the view points of green chemistry, initially the reaction of anisaldehyde, dimedone, ethylacetoacetate and ammonium acetate was carried out without using any external catalyst in aqueous medium at room temperature. However, the reaction was sluggish and corresponding polyhydroqinoline 5 was obtained in 15% (5 h). Since solvent properties play a crucial role in organic synthesis, the effect of solvent was studied for synthesis of polyhydroquinolines. The comparative results for polyhydroquinolines obtained using different solvents are summarized in Table 1 and it was found that ethanol was a more economical and efficient solvent for the present transformation. The % yield as a function of ɛ (dielectric constant) of solvent is exhibited in graphical form in Fig. 1.
Effect of solvent on the yield of polyhydroquinolines.
| Entry | Solvent | Yield (%)a |
| 1 | H2O | 15 |
| 2 | CH3OH | 54 |
| 3 | CH3CH2OH | 78 |
| 4 | CH3CH2CH2OH | 54 |
| 5 | CH3CH2CH2CH2OH | 42 |
| 6 | CH3CN | 32 |
| 7 | THF | 15 |
| 8 | CH3COCH3 | 26 |
a Reaction conditions: anisaldehyde (1 mmol), ethyl acetoacetate (1 mmol), dimedone (1 mmol), ammonium acetate (1 mmol), solvent (5 mL), time = 8 h, room temp.
Effect of dielectric constant on the yield of 5.
The observed yield of polyhydroquinoline 5 is maximum in ethanol as a solvent having dielectric constant (ɛ) of 24.3 (Fig. 1). This is an important observation indicating that this transformation requires the protic polar solvents of medium dielectric constant for the enolization of active methylene compounds. In a solvent with high dielectric constant viz H2O (ɛ = 78), the enol form of β-ketoester may be strongly H-bonded in aqueous medium becoming comparatively less reactive for Knoevenagel condensation. A literature survey revealed that there was no formation of Knoevenagel product in water when dimedone and aldehyde were taken in 1:1 proportion [20]. Hence, there is no likelihood of formation of product 5 in water. In the case of a low dielectric constant medium, the enolization is energetically unfavoured, leading to the low yield of the product 5. However, these difficulties are easily surmounted by using EtOH as a reaction medium due to its medium dielectric constant as well as the comparatively less ability to form H-bonding with the enol form of the β-keto ester. The requirement of protic solvents for this reaction is also supported by observing very low yield of the product in aprotic solvents where the small increase in % yield with dielectric constant of the medium is observed in the studied solvents (Fig. 1).
The formation of polyhydroquinolines without use of any external catalyst in ethanol medium at ambient temperature is likely due to in situ formation of acetic acid by hydrolysis of ammonium acetate, which acts as a catalyst for the present transformation. This clearly implies that there is no need of any external catalyst for the synthesis of polyhydroquinolines. To improve the yield of the product 5, an excess amount of ammonium acetate could be used. The reason is twofold; firstly excess use of ammonium acetate results in a large amount of in situ generation of acetic acid that acts as a catalyst and secondly unreacted ammonium acetate can be easily removed from the reaction. This prompted us to use a slightly excess amount of ammonium acetate rather than using any external catalyst and led us to explore this economical protocol. The present transformation was also carried out in 100, 95, 90, 85 and 80% ethanol using reaction conditions as specified in Table 1 with the exception that amount of ammonium acetate (1.5 mmol) and obtained 76, 91, 73, 68, 63% yield of polyhydroquinoline, respectively.
Gratifyingly, polyhydroquinoline 5 of anisaldehyde, dimedone, ethylacetoacetate and ammonium acetate were obtained in 91% yield using 95% ethanol as a solvent at ambient temperature. The scope and efficiency of this approach was explored for the synthesis of a wide variety of polyhydroquinolines by treating a diverse range of aldehydes possessing electron-donating as well as electron-withdrawing groups, with dimedone, ethyl acetoacetate, ammonium acetate and results are summarized in Table 2. Therefore, it is amply clear that in all the cases irrespective of the presence of electron-donating or electron-withdrawing groups in an aldehyde moiety, the desired products were obtained in good yields. These reaction conditions were almost equally efficient for heterocyclic aldehydes (Table 2, entries 5j, 5k). Although the heterocyclic and aromatic aldehydes reacted efficiently under the similar conditions, aliphatic aldehydes resulted the lower yield of the products. (Table 2, entries 5m and 5t). Furthermore, the variation of 1,3-diketone compound from dimedone to cyclohexane-1,3-dione, which has been rarely used for present transformation, worked equally well, proving the broad scope of the present protocol.
Synthesis of polyhydroquinolines in ethanol medium at ambient temperature.
| Entry | Product (5) | Time (h) | Yield (%)a,b | MP Obs. (lit. °C) |
| a | R = C6H5; R’ = CH3 | 6.5 | 89 | 199-201(202-204) [8a] |
| b | R = 4-CH3-C6H4; R’ = CH3 | 7 | 87 | 258-260(260-261) [8a] |
| c | R = 4-CH3 O - C6H4; R’ = CH3 | 8 | 91 | 256-258(257-259) [8a] |
| d | R = 4-Cl- C6H4; R’ = CH3 | 6 | 84 | 244-246(245-246) [8a] |
| e | R = 2-NO2 - C6H4; R’ = CH3 | 7 | 87 | 205-207(206-208) [8a] |
| f | R = 4-isopropyl-C6H4; R’ = CH3 | 6 | 88 | 180-182 (--) |
| g | R = 2,5-(CH3)2- C6H3; R’ = CH3 | 6 | 89 | 244-246 (--) |
| h | R = 4-OH- C6H4; R’ = CH3 | 6.5 | 87 | 233-234(232-234) [8a] |
| i | R = 4-OH-3-CH3O- C6H3; R’ = CH3 | 7 | 84 | 210-212(210-212) [8a] |
| j | R = 2-furyl; R’ = CH3 | 6 | 77 | 242-245(246-248) [8a] |
| k | R = 2-thienyl; R’ = CH3 | 6 | 78 | 236-239(238-240) [8a] |
| l | R = 4-N(CH3)2- C6H4; R’ = CH3 | 7 | 87 | 261-263(263-264) [8a] |
| m | R = CH3-CH2 ; R’ = CH3 | 7 | 44 | 145-146(145-146) [8a] |
| n | R = 4-CH3 O – C6H4; R’ = H | 7 | 82 | 193-195(194-195) [9a] |
| o | R = 4-CH3- C6H4; R’ = H | 7 | 87 | 241-242(241-242) [9a] |
| p | R = 4-OH - C6H4; R’ = H | 7 | 82 | 233-235(234-235) [9a] |
| q | R = Piperinyl - C6H3; R’ = H | 6 | 85 | 242-244(---) |
| r | R = C6H5; R’ = H | 6.5 | 86 | 239-241(240-241) [12] |
| s | R = 3-NO2 - C6H4; R’ = H | 6.5 | 87 | 196-199(198-200) [12] |
| t | R = (CH3)2- CH ; R’ = H | 7 | 46 | 179-181(180-182) [12] |
| u | R = 3,4-(CH3 O)2- C6H3; R’ = H | 11 | 85 | 192-194(---) |
| v | R = 3,4,5-(CH3 O)3- C6H2; R’ = H | 11 | 85 | 180-182(---) |
a All products showed satisfactory spectroscopic data (IR, 1H and 13C NMR, MS, elemental analysis).
b Yields refer to pure, isolated products.
Prediction of the mechanism of multi-component reactions is a skillful task. In the mechanism of polyhydroquinoline there is ambiguity amongst the reactivity of active methylene compounds viz 1,3-diketones and β-keto ester towards Knoevenagel condensation. To clarify this (Table 3), Knoevenagel condensation was carried out using 1+2, 1+3 without any catalyst and yielded 6, 8 in 13 and 0%, respectively. The influence of ammonium acetate on the same reactions i.e. 1+2+4 yielded 6 in 82% while for 1+3+4 yielded mixture of 7 and 10 in 28 and 17%, respectively. Even though, addition of acetic acid for reaction of 1+3 failed to form 8. The reaction between 3+4 resulted in exclusive formation of product 7.
Results of Knoevenagel condensation.
| Entry | Reactants | Catalyst | Product | Yield (%)a |
| 1 | 1+2 | ---- | 6 | 13 |
| 2 | 1+3 | ---- | 8 | ---- |
| 3 | 1+2+4 | ---- | 6 | 82 |
| 4 | 1+3+4 | ---- | 7 10 | 28 17 |
| 5 | 1+3 | CH3COOH | 8 | ---- |
| 6 | 3+4 | ---- | 7 | 96 |
a Reaction conditions: anisaldehyde(1 mmol), ethyl acetoacetate/dimedone (1 mmol), ethanol (5 mL), ammonium acetate (1.5 mmol), acetic acid (1 mmol), time = 6 h, room temp.
The above observations allow us to conclude that Knoevenagel product 6 is formed by reaction of aldehyde with 1,3-diketone and subsequent Michael addition of 7 yielding corresponding polyhydroquinoline 5 (Scheme 2). The formation of acridinedione [21] 11 is possible when 9 reacts with 6, since this possibility is discarded due to non-formation of intermediates 8 and 9. A literature survey also complies with non-formation of acridinediones [8–18] as a by-product during present transformation justifying the clear mechanism for synthesis of polyhydroquinolines 5.
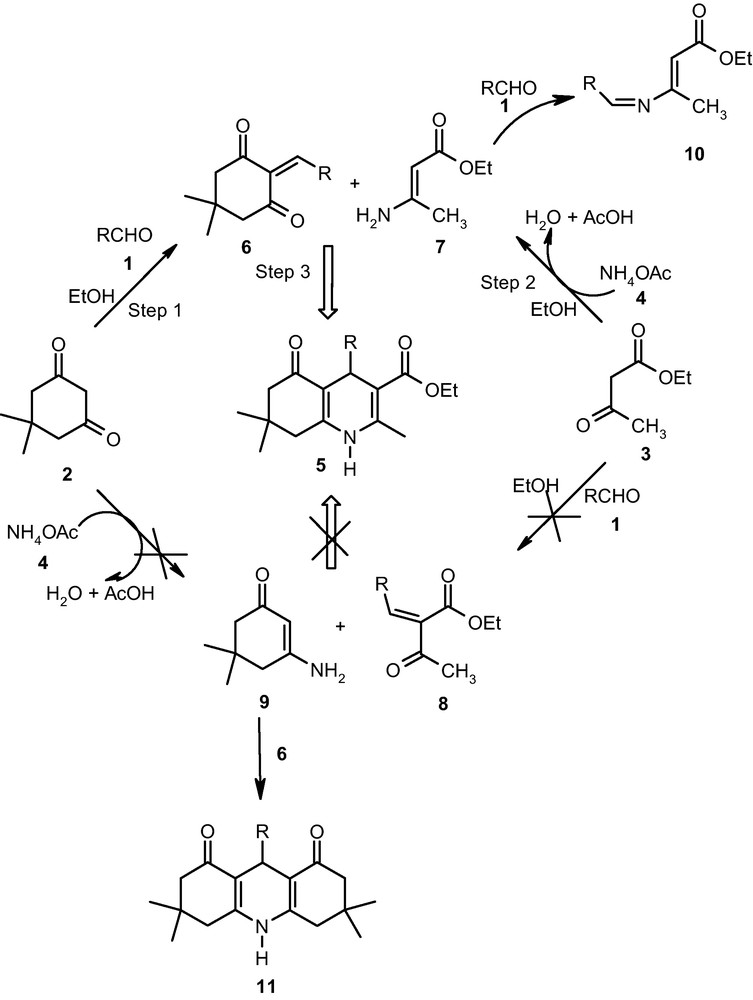
Proposed mechanism for the formation of 5.
3 Conclusion
The developed one-pot procedure for multi-component synthesis of polyhydroquinolines was found to be quite general for a variety of aryl and heterocyclic aldehydes as well as for 1,3-diketones viz dimedone and cyclohexane-1,3-dione. The attractive features of this method are simple experimental procedure worked well at ambient temperature and easy isolation of product. The beauty of protocol lies in mechanistic and green approach in terms of absence of external catalyst, which makes the process economical.
4 Experimental: Typical procedure
One-pot synthesis of polyhydroquinolines: A mixture of an aldehyde (1 mmol), ethyl acetoacetate (1 mmol), dimedone (1 mmol) and ammonium acetate (1.5 mmol) in ethanol (5 mL) was stirred at room temperature, for an appropriate time (Table 2). On completion of reaction (TLC), the reaction mixture was poured into crushed ice and the solid product separated was filtered and recrystallized from ethanol to afford pure polyhydroquinoline, which was characterized by physical constant and spectroscopic methods.
5 Spectral data of unknown compounds
Entry 5f: mp. 180–182 °C; IR (KBr):3280, 3210,1649, 1605 cm−1; 1H NMR (400 MHz, CDCl3): δ 0.96 (s, 3H), 1.08(s, 3H), 1.17-1.2(m, 9H), 1.68-1.70(m, 2H), 2.19-2.34(m, 5H), 2.79-2.82(m, 1H), 4.07 (q, J = 7.2 Hz, 2H), 5.02(s, 1H), 6.02(s, 1H), 7.03(d, J = 8 Hz, 2H), 7.19(d, J = 8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 14.1, 19.1, 27.3, 29.3, 32.7, 33.6, 36.1, 41.1, 50.9, 59.6, 106.3, 112.1, 125.9, 127.5, 143.2, 144.5, 146.2, 148.5, 167.6, 195.5; EIMS: m/z 381 (M+); anal. calcd. for C24H31O3N: C, 75.56; H, 8.19; N, 3.67; found: C, 75.61; H, 8.19; N, 3.69.
Entry 5g: mp. 244–246 °C; IR (KBr):3284, 3237, 1699, 1602 cm−1; 1H NMR (400 MHz, CDCl3): δ 0.94 (s, 3H), 1.08(s, 3H), 1.19 (t, J = 7.2 Hz, 3H), 1.61-1.64(m, 2H), 2.11-2.31(m, 5H), 2.38(s, 3H), 2.65(s, 3H), 4.06 (m, 2H), 5.11(s, 1H), 5.67(s, 1H), 6.79(d, J = 8 Hz, 1H), 6.90(d, J = 8 Hz, 1H), 6.96(s, 1H); 13C NMR (100 MHz, CDCl3): δ 14.2, 19.1, 19.6, 21.1, 27.2, 28.4, 29.2, 32.7, 33.1, 41.6, 50.9, 59.7, 107.7, 113.7, 126.8, 129.5, 129.8, 133, 134.7, 142.3, 146.3, 147.1, 195.1; EIMS: m/z 367 (M+); anal. calcd. for C23H29O3N: C, 75.17; H, 7.95; N, 3.81; found: C, 75.14; H, 7.94; N, 3.79.
Entry 5q: mp. 242–244 °C; IR (KBr): 3281, 1692, 1646, 1607 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.21 (t, J = 7.2 Hz, 3H), 1.65-1.69(m, 3H), 1.91-2.02(m, 1H), 2.27-2.46(m, 5H), 4.08 (q, J = 7.2 Hz, 2H), 5.02(s, 1H), 5.87(s, 2H), 5.88(s, 1H), 6.65(d, J = 8 Hz, 1H), 6.78-6.80(m,2H); 13C NMR (100 MHz, CDCl3): δ 14.2, 19.1, 21.1, 27.4, 36.2, 37.1, 59.8, 100.6, 106.4, 107.7, 108.7, 113.5, 121, 141.5, 143, 145.7, 147.3, 149.6, 167.4, 195.6; EIMS: m/z 355 (M+); anal. calcd. for C20H21O5N: C, 67.59; H, 5.96; N, 3.94; found: C, 67.63; H, 5.97; N, 3.95.
Entry 5u: mp. 192–194 °C; IR (KBr):3307, 1697, 1647, 1607 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.20 (t, J = 7.2 Hz, 3H), 1.68-1.71(m, 3H), 1.94-2.02 (m, 1H), 2.28-2.45(m, 5H), 3.80(s, 3H), 3.85(s, 3H), 4.07(q, J = 7.2 Hz, 2H), 5.05(s, 1H), 6.07(s, 1H), 6.71(d, J = 8.2 Hz, 1H), 6.76(d, J = 8.2 Hz, 1H), 6.95(s, 1H); 13C NMR (100 MHz, CDCl3): δ 14.2, 19, 21.1, 27.3, 36, 37.1, 56, 59.7, 106.2, 111.5, 112.5, 113.3, 119.8, 140.4, 143.2, 147.5, 148.5, 150, 167.5, 196; EIMS: m/z 371 (M+); anal. calcd. for C21H25O5N: C, 67.91; H, 6.78; N, 3.77 found: C, 67.86; H, 6.75; N, 3.79.
Entry 5v: mp. 180–182 °C; IR (KBr): 3275, 3192, 1695, 1607 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.21 (t, J = 7.2 Hz, 3H), 1.95-2.01(m, 2H), 2.25-2.40(m, 7H), 3.78(s, 3H), 3.80(s, 6H), 4.09 (q, J = 7.2 Hz, 2H), 5.07(s, 1H), 6.30(s, 1H), 6.5(s, 2H); 13C NMR (100 MHz, CDCl3): δ 14.2, 19.3, 20.3, 21.2, 27.7, 33, 36.5, 37.2, 56.3, 59.7, 60.8, 105.1, 106, 106.4, 113.5, 142.6, 142.8, 149, 153, 167.5, 196; EIMS: m/z 401 (M+); anal. calcd. for C22H27O6N: C, 65.82; H, 6.78; N, 3.49 found: C, 65.85; H, 6.78; N, 3.52.
Acknowledgments
Authors DMP and KAU thank UGC, New Delhi for financial assistance [F.2-3/2007(Policy/SR)] and for the research fellowship, respectively.


