1 Introduction
The 1,3-dipolar cycloaddition reaction is one of the most useful reactions for the synthesis of heterocyclic compounds [1]. It has a nearly singular capability of establishing large numbers of stereochemical centers in one synthetic step. 1,3-dipolar reactions of alkenes with nitrile oxides and diazoalkanes have been used to prepare isoxazolines and pyrazolines. Isoxazolines are a class of heterocyclic compounds having a remarkable number of applications and have been demonstrated to be very versatile building blocks in organic synthesis. The wide range of biological activities includes pharmacological properties such as anti-influenza virus activities [2], antifungal properties [3], anti-inflammatory, antibacterial and HIV-inhibitory activity [4]. The key feature of these heterocycles is that they possess the typical properties of an aromatic system but contain a weak nitrogen-oxygen bond which, under certain reaction conditions, particularly in reductive or basic conditions, is a potential site of ring cleavage The ring opening provides difunctionalized compounds, namely γ-amino alcohol, ß-hydroxy ketone, etc., so that isoxazolines can be considered masked forms of these synthetic units [5]. Pyrazolines present an interesting group of compounds, many of them show antibacterial [6], antidepressant [7], anticonvulsant [8], antiparkinsonian [9], and anti-inflammatory activities [10].
Our group has a current interest in the synthesis of pyrazolines derivatives based on 1,3-dipolar cycloaddition of 2-diazopropane (DAP) to C-C double bands [11]. Because of controls exerted by electronic and steric factors [12]. Consequently, pyrazolines have become an important synthetic tool. In this line, an impressive effort has been devoted to the synthetic application of the cycloaddition of arylnitrile oxides and 2-diazopropane to alkenes to give isoxazolines and pyrazolines. In this paper, we present complete regioselectivity and highly stereoselectivity 1,3-dipoar cycloaddition reactions of 5-hydroxy-3-methyl-1,5-dihydropyrrol-2-one derivatives.
2 Results and discussion
The labile arylnitrile oxides generated in situ were allowed to react with pyrrolidinones 1 and 2 in toluene. The reaction of racemic 5-hydroxy-3-methyl-1,5-dihydropyrrol-2-one derivatives 1 [13] and the arylnitrile oxides 2 proceeded with the formation of diastereoisomers 3 and 4, in favour of diastereoisomer 3 (Scheme 1). We now have to determine the addition mode of arylnitrile oxides with 1. Unambiguous proofs for the obtained cycloadducts regiochemistry arised from their spectral data. However, regiochemical assignments of all adduct were deduced from their 13C-NMR spectra. In particular, the chemical shifts of C-6a are in excellent agreement with those usually obtained when this quaternary carbon is attached to oxygen atom [14].
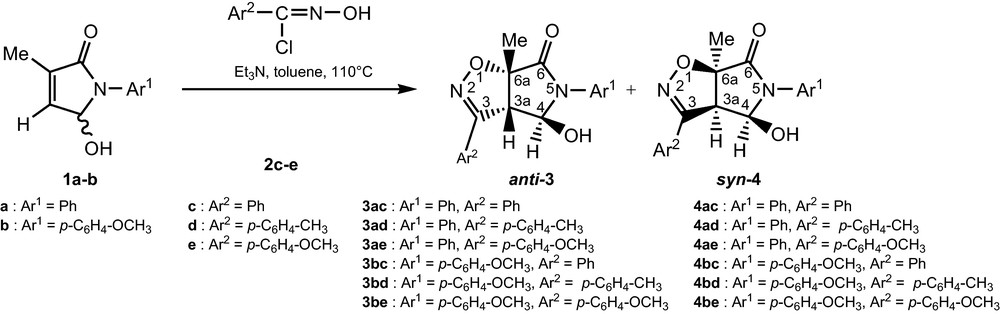
Synthesis of isoxazolines.
The attack of the 1,3-dipole occurred from the less hindered face of the dipolarophile 1 giving the major isomer 3 (Table 1) [15]. The syn or anti stereochemistry1 of the 2-isoxazolines 3 and 4 was deduced from the values observed for J3a,4 (0 and 9.4–9.6 Hz, respectively) [16].
The irradiation of H-3a in the minor isomer 4 shows positive NOE for CH3 and H-4. These observations show that H-3a, CH3 and H-4 are on the same side of the pyrrolidinone ring. The presence of NOE at CH3 and its absence at H-4 on irradiating H-3a confirms the anti stereochemistry of the major isomer 3 (Fig. 1).

Coupling constants and major NOE interactions of adduct 3 and 4.
Also, the stereochemistry syn or anti could be deduced from a NOESY spectrum. The steric interactions between the substituents at nitrile oxide and at C-4 of the pyrrolidinone rings are the main reasons for the observed syn-selectivity [17].
The addition of 2-diazopropane 5 with racemic 5-hydroxy-3-methyl-1,5-dihydropyrrol-2-ones as both a regio- and diastereospecific reaction (Scheme 2).
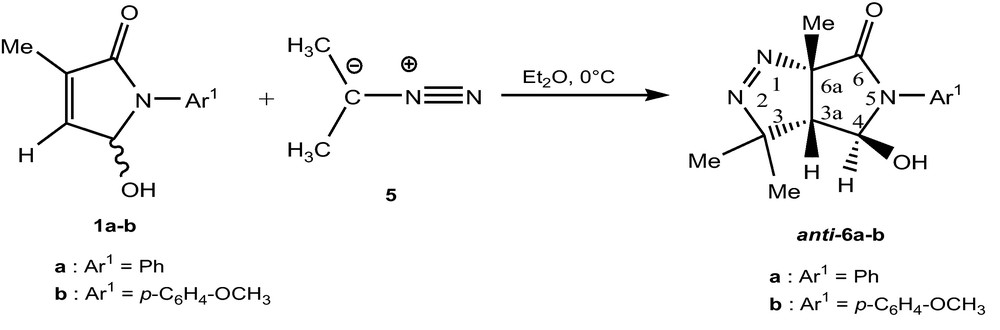
Formation of pyrazolines.
The 1,3-dipolar cycloaddition of DAP is, in each case, regiospecific. 1H-NMR spectra of adduct 6a-b showed a singulet near 2 ppm assigned to H-3a, in accordance with Δ1-pyrazolines structure. Their 13C-NMR spectra showed a quaternary carbon signal (C-6a ∼90 ppm). This indicated that DAP cycloaddition to proceeded via the “direct” way, [18] e.g. bond formation between the nucleophilic carbon of DAP and the C-3a carbon atom of pyrrolidinone. The stereochemistry of this cycloaddition product was determined from a NOESY spectrum. The trans relationship between protons 3a-H and 4-H was deduced from absence of an NOE effect. The complete anti selectivity observed in reactions with 5-hydroxy-3-methyl-1,5-dihydropyrrol-2-ones, steric interactions should account for the observed results [19].
3 Conclusion
We can conclude that the reactions of arylnitrile oxides and 2-diazopropane with racemic 5-hydroxy-3-methyl-1,5-dihydropyrrol-2-one derivatives are quite interesting in asymmetric synthesis because they evolve in high yields affording bicyclic-isoxazolines and pyrazolines with complete regioselectivity and very high π-facial selectivity. The methyl group increases the dipolarophilic reactivity of the pyrrolidinones, as well as their regioselectivity. Finally, the hydroxyl group was able to completely control the π-facial selectivity of all these reactions.
4 Experimental
IR spectra were recorded on a Perkin-Elmer IR-197 spectrometer. NMR spectra were obtained on a Bruker AC 300 spectrometer operating at 300 MHz for 1H and at 75.64 MHz for 13C. Melting points were determined on a Buchi-510 capillary melting point apparatus. All reagents were of commercial quality or purified by standard procedures.
4.1 1,3-dipolar cycloaddition of nitrile oxides with dipolarophiles
A solution of dipolarophiles 1a-b (1 mmol) and chloroximes 2c-e (1.1 mmol) in toluene (10 mL), was stirred at 110 °C. To this solution trimethylamine (0.2 mL), dissolved in toluene (10 mL), was added dropwise. The precipitated triethylammonium chloride was removed by filtration and the filtrate was concentrated in vacuo, and chromatography (SiO2; ethyl acetate/petroleum ether, 2:1) to afford compounds 3ac-be and 4ac-be.
(3aR*,4S*,6aS*)-4-exo-hydroxy-6a-methyl-3,5-diphenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3ac)
Yield (0.227 g, 73.6%); colourless solid. M.p = 160 °C, Anal. Calcd. For C18H16N2O3: C, 70.12; H, 5.23; N, 9.09%; Found: C, 69.98; H, 5.13; N, 9.20%; IR (KBr) νcm−1; 1638 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.60 (s, 3H, CH3), 4.00 (s, 1H, 3a-H), 5.23 (s, 1H, OH), 5.33 (s, 1H, 4-H), 7.25-7.85 (m, 10H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 19.10 (CH3), 60.94 (C-3a), 89.46 (C-4), 91.00 (C-6a), 123.02–138.01 (Carom), 154.06 (C3), 168.92 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-6a-methyl-3,5-diphenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4ac)
Yield (0.02 g, 6.4%); colourless solid. M.p = 204 °C, Anal. Calcd. For C18H16N2O3: C, 70.12; H, 5.23; N, 9.09%; Found: C, 70.25; H, 5.40; N, 8.89%; IR (KBr) νcm−1; 1637 (C = N); 1745 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.67 (s, 3H, CH3), 4.01 (s, 1H, OH), 4.66 (d, 1H, 3a-H, J = 9.6 Hz), 5.57 (d, 1H, 4-H, J = 9.6 Hz), 7.24–7.86 (m, 10H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 19.08 (CH3), 60.24 (C-3a), 89.25 (C-4), 92.05 (C-6a), 123.88–136.81 (Carom), 154.46 (C3), 168.54 (C-6).
(3aR*,4S*,6aS*)-4-exo-hydroxy-6a-methyl-3-(4-methylphenyl)-5-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3ad)
Yield (0.203 g, 63%); colourless solid. M.p = 159 °C, Anal. Calcd. For C19H18N2O3: C, 70.79; H, 5.63; N, 8.69%; Found: C, 70.75; H, 5.66; N, 8.74%; IR (KBr) νcm−1; 1640 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, DMSO) δ: 1.56 (s, 3H, CH3), 2.54 (s, 3H, CH3), 4.12 (s, 1H, 3a-H), 5.11 (s, 1H, OH), 5.31 (s, 1H, 4-H), 7.29–7.72 (m, 9H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 19.23 (CH3), 20.98 (CH3), 60.06 (C-3a), 89.34 (C-4), 91.03 (C-6a), 123.31–141.09 (Carom), 153.79 (C3), 168.51 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-6a-methyl-3-(4-methylphenyl)-5-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4ad)
Yield (0.039 g, 12%); colourless solid. M.p = 161 °C, Anal. Calcd. For C19H18N2O3: C, 70.79; H, 5.63; N, 8.69%; Found: C, 70.60; H, 5.51; N, 8.50%; IR (KBr) νcm−1; 1640 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.70 (s, 3H, CH3), 2.62 (s, 3H, CH3), 4.26 (s, 1H, OH), 4.64 (d, 1H, 3a-H, J = 9.5 Hz), 5.81 (d, 1H, 4-H, J = 9.5 Hz), 7.47–7.95 (m, 9H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 19.12 (CH3), 21.93 (CH3), 60.38 (C-3a), 89.27 (C-4), 91.66 (C-6a), 123.55–141.46 (Carom), 154.30 (C3), 168.64 (C-6).
(3aR*,4S*,6aS*)-4-exo-hydroxy-3-(4-methoxyphenyl)-6a-methyl-5-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3ae)
Yield (0.265 g, 78.3%); colourless solid. M.p = 232 °C, Anal. Calcd. For C19H18N2O4: C, 67.44; H, 5.36; N, 8.28%; Found: C, 67.50; H, 5.41; N, 8.24%; IR (KBr) νcm−1; 1630 (C = N); 1735 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.48 (s, 3H, CH3), 3.76 (s, 3H, OCH3), 4.27 (s, 1H, 3a-H), 5.05 (s, 1H, OH), 5.48 (s, 1H, 4-H), 6.84–8.03 (m, 9H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 18.86 (CH3), 55.52 (OCH3), 59.86 (C-3a), 89.04 (C-4), 91.69 (C-6a), 114.44–158.46 (Carom), 154.46 (C3), 167.94 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-3-(4-methoxyphenyl)-6a-methyl-5-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4ae)
Yield (0.04 g, 11.7%); colourless solid. M.p = 222 °C, Anal. Calcd. For C19H18N2O4: C, 67.44; H, 5.36; N, 8.28%; Found: C, 67.55; H, 5.39; N, 8.26%; IR (KBr) νcm−1; 1645 (C = N); 1735 (C = O); 3300 (OH), 1H-NMR (300 MHz, C3D6O) δ: 1.69 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 4.52 (s, 1H, OH), 5.12 (d, 1H, 3a-H, J = 9.6 Hz), 5.67 (d, 1H, 4-H, J = 9.6 Hz), 6.99 (d, 2H) and 7.99 (d, 2H): AA’BB’ part. J = 9 Hz, 7.21–7.58 (m, 5H, Harom); 13C{1H}NMR (75 MHz, C3D6O) δ: 19.21 (CH3), 56.01 (OCH3), 61.98 (C-3a), 85.37 (C-4), 90.45 (C-6a), 115.05–162.06 (Carom), 155.03 (C3), 167.60 (C-6).
(3aR*,4S*,6aS*)-4-exo-hydroxy-5-(4-methoxyphenyl)-6a-methyl-3-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3bc)
Yield (0.259 g, 76.5%); colourless solid. M.p = 139 °C, Anal. Calcd. For C19H18N2O4: C, 67.44; H, 5.36; N, 8.28%; Found: C, 67.37; H, 5.25; N, 8.20%; IR (KBr) νcm−1; 1640 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.74 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 3.98 (s, 1H, 3a-H), 5.10 (s, 1H, OH), 5.46 (s, 1H, 4-H), 6.75 (d, 2H) and 7.16 (d, 2H): AA’BB’ part. J = 8.7 Hz, 7.43–7.74 (m, 5H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 20.31 (CH3), 55.47 (OCH3), 60.18 (C-3a), 85.43 (C-4), 89.24 (C-6a), 114.48–158.68 (Carom), 156.00 (C3), 171.24 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-5-(4-methoxyphenyl)-6a-methyl-3-phenyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4bc)
Yield (0.046 g, 13.5%); colourless solid. M.p = 189 °C, Anal. Calcd. For C19H18N2O4: C, 67.44; H, 5.36; N, 8.28%; Found: C, 67.60; H, 5.45; N, 8.19%; IR (KBr) νcm−1; 1638 (C = N); 1735 (C = O); 3300 (OH), 1H-NMR (300 MHz, C3D6O) δ: 1.55 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 4.53 (s, 1H, OH), 5.69 (d, 1H, 3a-H, J = 9.4 Hz), 6.03 (d, 1H, 4-H, J = 9.4 Hz), 6.98 (d, 2H) and 7.94 (d, 2H): AA’BB’ part. J = 8.7 Hz, 7.20–7.63 (m, 5H, Harom); 13C{1H}NMR (75 MHz, C3D6O) δ: 19.24 (CH3), 55.58 (OCH3), 60.41 (C-3a), 86.07 (C-4), 90.95 (C-6a), 114.65–160.85 (Carom), 154.77 (C3), 168.10 (C-6).
(3aR*,4S*,6aS*)-4-exo-hydroxy-5-(4-methoxyphenyl)-6a-methyl-3-(4-methylphenyl)-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3bd)
Yield (0.273 g, 77.4%); colourless solid. M.p = 151 °C, Anal. Calcd. For C20H20N2O4: C, 68.17; H, 5.72; N, 7.95%; Found: C, 68.00; H, 5.80; N, 8.00%; IR (KBr) νcm−1; 1635 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, DMSO) δ: 1.55 (s, 3H, CH3), 2.37 (s, 3H, CH3), 3.77 (s, 3H, OCH3), 4.17 (s, 1H, 3a-H), 5.63 (s, 1H, 4-H), 6.25 (s, 1H, OH), 6.89 (d, 2H) and 7.40 (d, 2H): AA’BB’ part. J = 8.7 Hz, 7.30 (d, 2H) and 7.66 (d, 2H): AA’BB’ part. J = 7.8 Hz; 13C{1H}NMR (75 MHz, DMSO) δ: 19.17 (CH3), 20.86 (CH3), 55.58 (OCH3), 60.10 (C-3a), 86.05 (C-4), 89.34 (C-6a), 114.17–156.04 (Carom), 155.88 (C3), 170.19 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-5-(4-methoxyphenyl)-6a-methyl-3-(4-methylphenyl)-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4bd)
Yield (0.034 g, 9.6%); colourless solid. M.p = 215 °C, Anal. Calcd. For C20H20N2O4: C, 68.17; H, 5.72; N, 7.95%; Found: C, 68.40; H, 5.60; N, 7.70%; IR (KBr) νcm−1; 1640 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.83 (s, 3H, CH3), 2.30 (s, 3H, CH3), 3.78 (s, 3H, OCH3), 4.30 (s, 1H, OH), 4.54 (d, 1H, 3a-H, J = 9.6 Hz), 5.73 (d, 1H, 4-H, J = 9.6 Hz), 6.99–7.75 (m, 8H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 19.17 (CH3), 21.84 (CH3), 55.69 (OCH3), 60.31 (C-3a), 86.17 (C-4), 91.00 (C-6a), 117.81–159.75 (Carom), 153.97 (C3), 168.15 (C-6).
(3aR*,4S*,6aS*)-4-exo-hydroxy-3,5-di(4-methoxyphenyl)-6a-methyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (3be)
Yield (0.247 g, 67.15%); colourless solid. M.p = 229 °C, Anal. Calcd. For C20H20N2O5: C, 65.21; H, 5.47; N, 7.60%; Found: C, 65.11; H, 5.39; N, 7.46%; IR (KBr) νcm−1; 1640 (C = N); 1740 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.47 (s, 3H, CH3), 3,76 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.09 (s, 1H, 3a-H), 4.67 (s, 1H, OH), 5.44 (s, 1H, 4-H), 6.82 (d, 2H) and 7.47 (d, 2H): AA’BB’ part. J = 9 Hz, 6.89 (d, 2H) and 7.80 (d, 2H): AA’BB’ part. J = 8.7 Hz; 13C{1H}NMR (75 MHz, CDCl3) δ: 19.20 (CH3), 55.78 (OCH3), 55.83 (OCH3), 60.41 (C-3a), 89.37 (C-4), 91.75 (C-6a), 114.49–161.80 (Carom), 154.18 (C3), 168.37 (C-6).
(3aS*,4S*,6aR*)-4-endo-hydroxy-3,5-di(4-methoxyphenyl)-6a-methyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]isoxazole-6-one (4be)
Yield (0.044 g, 11.85%); colourless solid. M.p = 191 °C, Anal. Calcd. For C20H20N2O5: C, 65.21; H, 5.47; N, 7.60%; Found: C, 65.32; H, 5.65; N, 7.73%; IR (KBr) νcm−1; 1635 (C = N); 1735 (C = O); 3300 (OH), 1H-NMR (300 MHz, DMSO) δ: 1.53 (s, 3H, CH3), 3,75 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 4.56 (s, 1H, OH), 5.41 (d, 1H, 3a-H, J = 9.6 Hz), 6.36 (d, 1H, 4-H, J = 9.6 Hz), 6.93 (d, 2H) and 7.30 (d, 2H): AA’BB’ part. J = 9 Hz, 7.02 (d, 2H) and 7.86 (d, 2H): AA’BB’ part. J = 8.7 Hz; 13C{1H}NMR (75 MHz, DMSO) δ: 21.58 (CH3), 55.60 (OCH3), 55.67 (OCH3), 60.33 (C-3a), 87.89 (C-4), 88.56 (C-6a), 114.11–161.06 (Carom), 154.14 (C3), 166.57 (C-6).
4.2 1,3-dipolar cycloaddition of 2-diazopropane with dipolarophiles
To a solution of dipolarophiles 1a-b (1.0 mmol) in diethyl ether, cooled at 0 °C, was added portionwise 2.6 M ethereal solution of 2-diazopropane. The reaction was kept at the same temperature during 1 h. The solvent was removed and chromatography (SiO2; ethyl acetate/petroleum ether, 2:1) to afford compounds 6a-b.
(3aR*,4S*,6aS*)-4-endo-hydroxy-5-phenyl-3,3,6a-trimethyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]pyrazole-6-one (6a)
Yield (0.194 g, 75%); colourless solid. M.p = 139 °C, Anal. Calcd. For C14H17N3O2: C, 64.85; H, 6.61; N, 16.20%; Found: C, 64.88; H, 6.54; N, 16.17%; IR (KBr) νcm−1; 1540 (N = N); 1730 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.39 (s, 3H, CH3), 1.42 (s, 3H, CH3), 1.94 (s, 1H, 3a-H), 4.03 (s, 1H, OH), 5.34 (s, 1H, 4-H), 7.13–7.33 (m, 5H, Harom); 13C{1H}NMR (75 MHz, CDCl3) δ: 22.19 (CH3), 22.48 (CH3), 29.30 (CH3), 54.12 (C-3a), 83.90 (C-4), 93.97 (C-6a), 101.38 (C3), 124.33–136.62 (Carom), 170.17 (C-6).
(3aR*,4S*,6aS*)-4-endo-hydroxy-5-(4-methoxyphenyl)-3,3,6a-trimethyl-3a,5,6,6a-tetrahydro-4H-pyrrolo[3,4-d]pyrazole-6-one (6b)
Yield (0.231 g, 80%); colourless solid. M.p = 129 °C, Anal. Calcd. For C15H19N3O3: C, 62.27; H, 6.62; N, 14.52%; Found: C, 62.23; H, 6.69; N, 14.59%; IR (KBr) νcm−1; 1545 (N = N); 1730 (C = O); 3300 (OH), 1H-NMR (300 MHz, CDCl3) δ: 1.33 (s, 3H, CH3), 1.40 (s, 3H, CH3), 2.04 (s, 1H, 3a-H), 3.82 (s, 3H, OCH3), 4.13 (s, 1H, OH), 5.31 (s, 1H, 4-H), 6.77 (d, 2H) and 7.18 (d, 2H): AA’BB’ part. J = 8.7 Hz; 13C{1H}NMR (75 MHz, CDCl3) δ: 22.17 (CH3), 22.39 (CH3), 29.33 (CH3), 54.12 (C-3a), 56.02 (OCH3), 83.89 (C-4), 93.89 (C-6a), 100.96 (C3), 114.23–160.52 (Carom), 171.07 (C-6).
1 The terminology syn/anti indicates the spatial arrangement of the hydroxy group at C-4 and the isoxazoline ring at the pyrrolidinone moiety. It also indicates that the approach of the dipole has taken place either to the face containing the hydroxy group (syn-approach) or to the opposite one (anti-approach).
| Entry | Ar1 | Ar2 | Ratio anti-3: syn-4a | Rdt %b |
| 1 | Ph | Ph | 92/8 | 80 |
| 2 | Ph | p-C6H4-CH3 | 84/16 | 75 |
| 3 | Ph | p-C6H4-OCH3 | 87/13 | 90 |
| 4 | p-C6H4-OCH3 | Ph | 90/10 | 85 |
| 5 | p-C6H4-OCH3 | p-C6H4-CH3 | 89/11 | 87 |
| 6 | p-C6H4-OCH3 | p-C6H4-OCH3 | 85/15 | 79 |
b Combined yield after column chromatography.


