1 Introduction
Pyrochlore type compounds (A2B2O7) have many potential applications areas, because of their compositional range and a wide variety of interesting physical properties including electrical, magnetic, optical, dielectric, and catalytic behavior [1–5]. These compounds have high photocatalytic activity, piezoelectric behavior, ferro-and ferrimagnetisms, an order/disorder transformation, high thermal expansion coefficient [6–9]. Pyrochlore structures are remarkable due to their ability to form substituted and defective structures, permitting one thus the reach to interesting physical properties. The unit cell of this structure is usually face centered cubic with space group Fd3 m with eight molecules per unit cell (Z = 8) [10]. The A site cation with higher ionic radius is in eight fold coordination, with oxygen anions, while the smaller B cation resides in six fold coordination forming a (BO6) oxygen octahedra. Of the seven oxygen anions, one is separate from the (BO6) octahedra and its only bond is to the A cations, located in the voids between the (BO6) octahedra. The larger A sites can accommodate rare earths and other trivalent cations such as Bi, Sc, In, Bi, while the B sites can be occupied by tetravalent or pentavalent elements i.e. (Ti,Zr) and (Sb,Nb). Divalent cation (Cu+2) can log in the two sites like compounds (Bi1.524−x(Ca,Pb)xCu0.476)(Sb1.524Cu0.476)O7+δ [11]. For the formation of any pyrochlore compound, there are two criteria: (1) the ratio of the ionic radius of the cation at the A site to that at the B site must be between 1.46 and 1.80; and (2) the chemical valences of various ions must make the compound neutral [12]. In this work, we propose to study the impact of the substitution of antimony for niobium on the structural, magnetic and electrical properties of the new pyrochlore compound Bi1.5Sb1.5MnO7. [13].
2 Experimental
The compound were prepared by solid-state reaction of stoichiometric amounts of reagent grade Bi2O3 (99.9%), Sb2O3 (99%), Nb2O5 (99%) and MnO (99%) (Aldrich Chemical Company Ltd.). The mixture was ground for 1 h with an agate mortar and pestle. The resulting powder was calcined at 700 °C for 48 h in an alumina crucible. Then, the powder was again ground and calcined two more times successively at 800 and 900 °C for 72 h each one. The resulting powder were re-milled for 1/2 h and uniaxially pressed into a pellet about 13 mm in diameter and 3 mm in thickness. It was then sintered at 1000 °C for 72 h. The samples color change with x; it passes from brown (x = 0) to green (x = 1.5). The specimen was initially characterized by X-ray powder diffraction using a pan Analytical X’pert Pro X-ray diffraction system. Data were collected using CuKα radiation with 0.02° step size. Polished specimens were examined using conventional Philips SEM XL30 scanning electron microscope. The magnetic susceptibility of a powder sample was measured from 4 to 300 K with SQUID. The electrical conductivity measurements were carried out using an impedance Lock-in EG & G7220-type with 10 mV AC signal amplitude at frequencies between 30 Hz and 120 KHz.
3 Results and discussion
3.1 Structural characterization
The previous Rietveld refinements for Bi1.5Sb1.5MnO7 compound [13] using X-ray powder diffraction data confirmed an overall A2B2O7 cubic pyrochlore structure with a = 10.42749(4) Å and Fd-3 m symmetry. The manganese is distributed between A and B sites with the same proportion (Bi1.5Mn0.5)(Sb1.5Mn0.5)O7, where A site = [(Bi1.5Mn0.5)] and B site = [(Sb1.5Mn0.5)]. The absence of 442-type in the powder diffraction pattern reflections permits one to use as this model the highly symmetrical ideal pyrochlore structure A2B2O6O’ crystallizes in space group Fd-3 m [14] with A and B cations on two special positions (16d, 16c) and oxygen on two sites, 48f (O) and 8b(O’), resulting in a single positional variable (x for 48f site oxygen in the B2O6 octahedral network). Several compounds possess this organization as the zinc pyrochlore compound for example with formula (Bi1.5Zn0.5)(Sb1.5Zn0.5)O7 [15–17]. Antimony oxide can completely be substituted by niobium oxide giving a new solid solution Bi1.5Sb1.5−xNbxMnO7 (Fig. 1). All compositions 0 ≤x ≤ 1.5 have a single phase with a face-centred pyrochlore-type cubic structure (Fig. 2). Table 1 gives positions (2θ[°]), relative intensity (I/Imax) and the miller indexation for Bi1.5Sb1.5MnO7 (x = 0) and Bi1.5Nb1.5MnO7 (x = 1.5) compounds. Fig. 3 shows the variation of the lattice constant (a) with the composition x. There is an almost linear relation between lattice constant and composition x. That is, lattice constant increased linearly with increasing of niobium concentration. In addition, cell parameters of the system were practically on the correlation line, indicating that the difference of the niobium ionic radii RNb(VI) = 0.72 Å [18] large than antimony ionic radii Ǻ RSb(VI) = 0.60 Å [18] is the reason for this observed increase. This result shows any lattice distortion. Fig. 4 shows SEM photographs of the surface morphologies of the disk samples. It is found that the grain size of the samples slightly decreases with the increase of x. For 0 ≤ x ≤ 1.5, we can see a similar dense phase with well-formed grains, the compounds’ shape is not changed. The external area of compound is under shape of connected grains and porous shape. At x = 1.5, the external area of the compound changes. In fact, grains are not bound between them and they take a cubic shape. The difference in the grain growth rate during sintering, which causes the variation in grain size, is attributed to the different diffusion rate of the ionic species (Table 1).
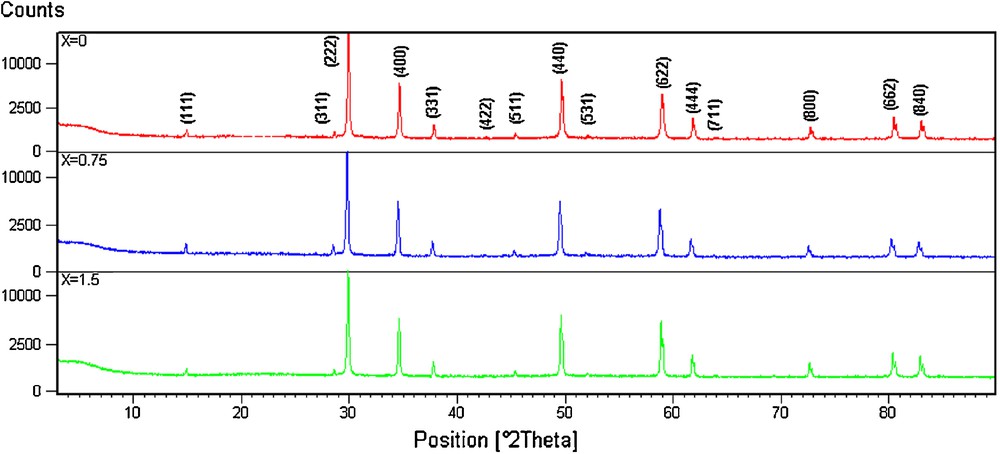
XRD pattern of Bi1.5Sb1.5−xNbxMnO7 solid solution.

Cell parameter variation of Bi1.5Sb1.5−xNbxMnO7 solid solution.
XRD characterisation for Bi1.5Sb1.5MnO7 and Bi1.5Nb1.5MnO7 pyrochlore compounds.
| Bi1.5Sb1.5MnO7 x = 0 | |||||||||||||
| 2θ (°) | 14.96 | 28.63 | 29.93 | 34.65 | 37.85 | 45.41 | 49.67 | 52.12 | 58.94 | 61.83 | 72.69 | 80.43 | 82.96 |
| I/Imax | 1.82 | 1.26 | 100.00 | 31.91 | 3.72 | 1.19 | 35.12 | 0.38 | 28.12 | 6.67 | 3.10 | 7.21 | 5.46 |
| hkl | 111 | 311 | 222 | 400 | 331 | 511 | 440 | 531 | 622 | 444 | 800 | 662 | 840 |
| Bi1.5Nb1.5MnO7 x = 1.5 | |||||||||||||
| 2θ (°) | 14.94 | 28.57 | 29.86 | 34.60 | 37.79 | 45.36 | 49.62 | 52.07 | 58.89 | 61.76 | 72.63 | 80.35 | 82.87 |
| I/Imax | 1.76 | 1.39 | 100.00 | 34.43 | 4.28 | 1.24 | 38.89 | 0.52 | 32.96 | 7.83 | 4.16 | 8.95 | 7.07 |
| hkl | 111 | 311 | 222 | 400 | 331 | 511 | 440 | 531 | 622 | 444 | 800 | 662 | 840 |

SEM micrograph of Bi1.5Sb1.5−xNbxMnO7 compounds (x = 0; x = 0.25; x = 1; x = 1.5).
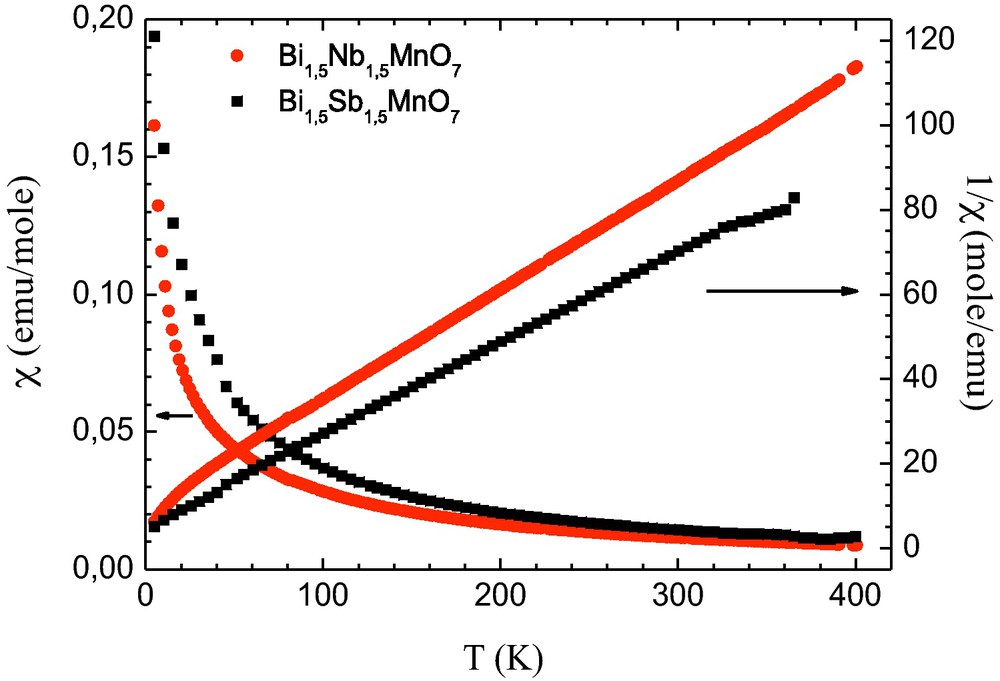
Susceptibility and inverse susceptibility versus temperature of Bi1.5Sb1.5MnO7 (x = 0) and Bi1.5Nb1.5MnO7 (x = 1.5) compounds.
3.2 Magnetic characterization
Magnetic data obtained for Bi1.5Sb1.5MnO7 (x = 0) and Bi1.5Nb1.5MnO7 (x = 1.5) compound indicate qualitatively similar properties. The magnetization variation confirmed that the samples were overall paramagnetic in nature with an effective moment of 5.84 μβ (x = 0) and 5.61 μB (x = 1.48). The observed effective magnetic moments (μeff/molMn), calculated from the slopes of the Curie-Weiss fits indicate “ + 2” oxidation state of the manganese. The temperature dependence of the magnetic susceptibility and its inverse are shown in Fig. 4. Above 100 K, the inverse magnetic susceptibility was linear and that range was fitted to the Curie-Weiss law. The extrapolation of the linear fits indicated small negative temperature intercepts −18.1 (x = 0) and −38.4 (x = 1.5) (θ (K)), suggesting the presence of weak antiferromagnetic cooperative interactions [14]. The observed effective magnetic moments (μeff/molMn), calculated from the slopes of the Curie-Weiss fits are assigned to the spin-only state (Mn2+, high spin S = 5/2, 5.92 μβ). Values found for the two compounds Bi1.5Sb1.5MnO7 (5.84 μβ) and Bi1.5Nb1.5MnO7 (5.61 μβ) studied here are similar for other Mn-containing pyrochlore Mn2Sb2O7 [19] with Mn2+ filling the A-sites. The complex magnetic behaviour exhibited by the Bi-Mn-(SbNb)-O pyrochlores is not unexpected which results in geometric magnetic frustration [20,21].
3.3 Electrical characterisation
Fig. 5 shows Arrhenius plots of the bulk conductivity for the as-prepared Bi1.5Nb1.5MnO7. Typical complex impedance diagrams Z′′ = f(Z′) at 452, 472, 492, 512, 532 K are presented also in Fig. 5 (inset). The electrical conductivity obeys to the Arrhenius Relation: σ = σ0exp(−Ea/KBT) where σ0 represents a pre-exponential factor, KB the Boltzmann constant, Ea was estimated from the slope of log(σ) versus T−1. The activation energy (Ea) of the compound is 0.58(1) eV. At 675 K the bulk conductivity is as high as 10−2 S cm−1 for the compound. This is close to the value of 5 × 10−2 S cm−1 found for Bi1.5Sb1.5MnO7 [13] with an activation energy (Ea) 0.49(9) eV. Results indicate a semiconducting behaviour of these materials. The structure of (Bi1.5Mn0.5)(Sb1.5Mn0.5)O7 is built by forming infinite corner-sharing BO6 octahedral formed [BO3]∞ chains along [110]. This arrangement can allow the move of charge carriers in this direction. The complex impedance results indicate the Bi1.5Sb1.5MnO7 compound is slightly conductor than the Bi1.5Nb1.5MnO7 compound. This behavior is in relation with the cell parameter increases. With cell parameter increases the distance between atoms increases and the electrical charge find difficulties to move. In fact the activation energy and cell parameter (a = 10.427(2)Å; Ea = 0.49(9) eV) of Bi1.5Sb1.5MnO7 compound are lower than Bi1.5Nb1.5MnO7 compound (a = 10.478(2)Å; Ea = 0.58(1)eV). The DC-measurement achieved under the two compounds put in evidence an electronic conductivity.
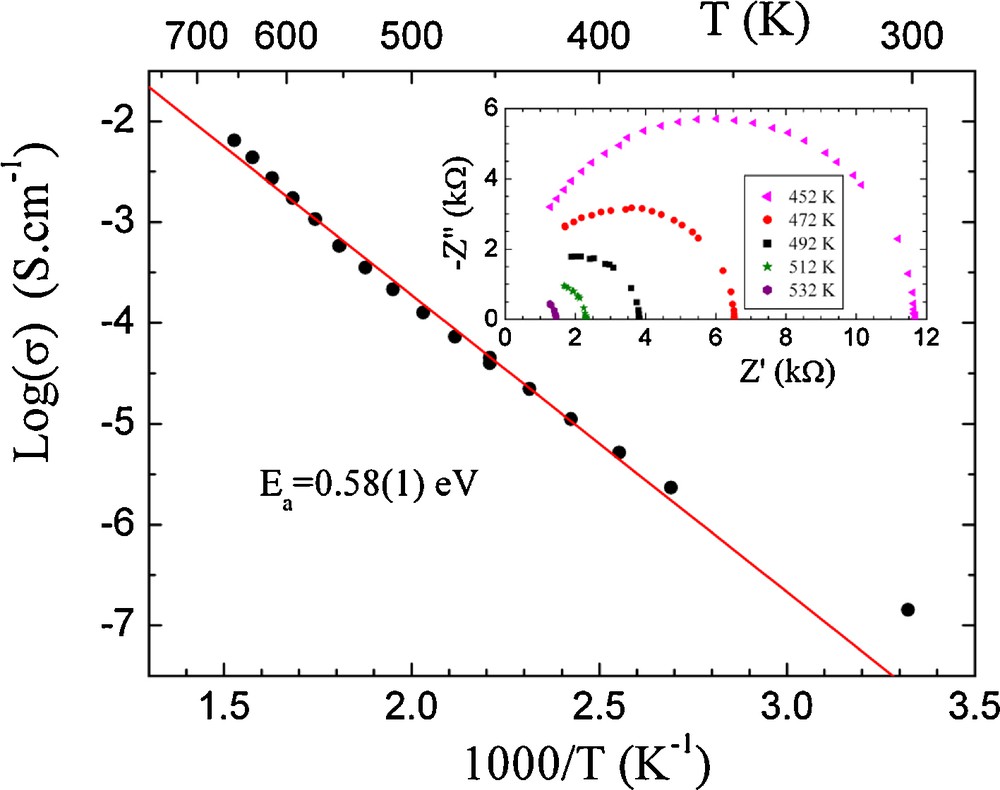
Electrical conductivity variation with inverse temperature of Bi1.5Nb1.5MnO7 compound and typical impedance complex diagrams (inset).
4 Conclusion
A new pyrochlore solid solution with formula Bi1.5Sb1.5−xNbxMnO7 was synthesized by solid state reaction at 1000 °C. X-ray powder diffraction pattern for these compositions is consistent with both the cubic pyrochlore unit cell increasing with x and Fd-3 m symmetry. Magnetic characterization confirmed a “+2” oxidation state of the manganese cation. The electric conductivity indicates semi-conducting behaviour of compounds. This solid solution is characterised by electronic conductivity.


