1 Introduction
Often, the largest contributor to the environmental footprint of a chemical process is the solvent used. Solvents have a significant impact because of the quantity used, e.g. in pharmaceutical productions they typically account for between 80 and 90% of the mass utilization of a batch operation. Consequently, replacing volatile organic solvents with more environmentally benign media, is one of the central tenets of Green Chemistry and a subject of significant academic and commercial interest [1]. The use of task-specific ionic liquids (TSILs) further enhances the versatility of ILs for the cases in which the reagent and medium are coupled [2–4]. Brønsted acidic ionic liquids (BAILs) consist of the useful characteristics of solid acids and mineral liquid acids and are designed to replace traditional mineral liquid acids such as sulfuric acid and hydrochloric acid in chemical procedures. Such BAILs have potential as dual solvent-catalysts in organic reactions [5].
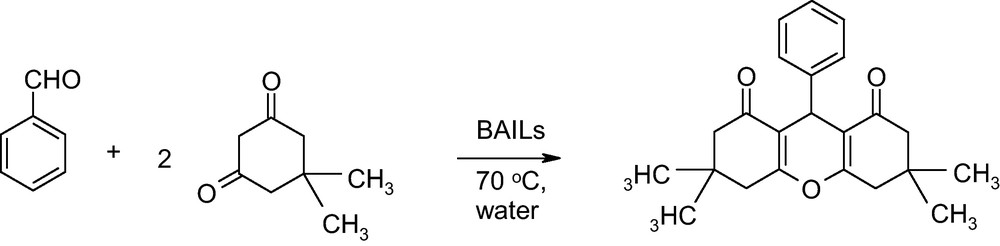
In a continuation of our catalytic investigation study [6], herein we report a new, convenient, mild and efficient route for one-pot multicomponent reaction (MCR) of 1,8-dioxo-octahydroxanthene. The xanthene scaffold is probably the most ubiquitous heterocyclic structure in natural and unnatural compounds that usually have biological activities [7] and have been used as versatile synthon because of the inherent reactivity of the pyran ring [8]. The synthesis of xanthene derivatives has been a major object of research for several years, and a variety of catalysts [9–14] were used for the same. However, some of the reported methods suffer from disadvantages such as prolonged reaction time, low yield of products, toxic and corrosive reagents. Therefore, the discovery of clean procedures and the use of a green and eco-friendly catalyst with high catalytic activity and short reaction time for the production of 1,8-dioxo-octahydroxanthene have gained considerable attention. A slight attempt has been taken in view of green synthesis [15]. There is some evidence which indicates the use of BAILs as a catalyst for the synthesis of 1,8-dioxo-octahydroxanthene [16–19]. Remarkably, the synthesis of 1,8-dioxo-octahydroxanthene using [CMIM][HSO4] as a catalyst has not been reported yet.
In this article, we have reported synthesis of 1,8-dioxo-octahydroxanthene (Scheme 1) in presence of water by developing a conceivable mechanism (Scheme 2) by using BAILs (Scheme 3). The reaction proceeded smoothly and, therefore, the purification of products was fairly simple. Furthermore, BAILs were conveniently separated from the products and easily recycled for another round of reaction. This will significantly reduce the effect of the reaction on the environment and thus pave a way for large scale applications.

2 Results and discussion
2.1 Promoting effect of BAILs
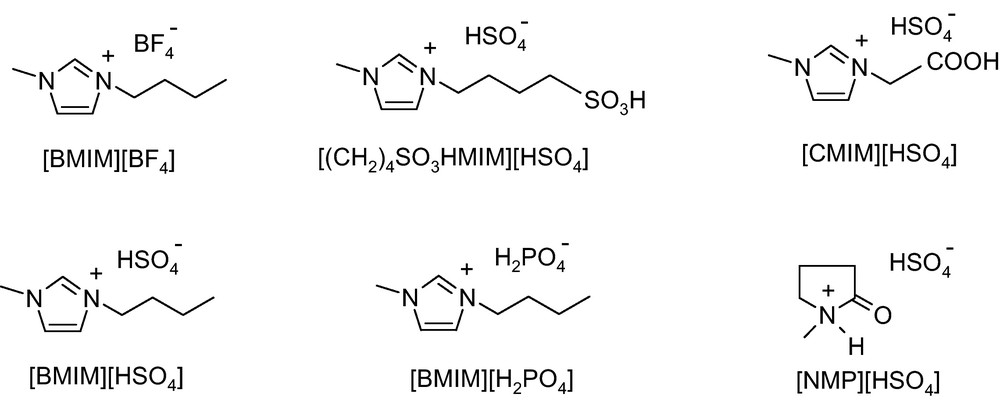
Our efforts were focussed on investigating the synthesis of 1,8-dioxo-octahydroxanthene using BAILs as the new catalyst. The promoting effect of BAILs for the synthesis of 1,8-dioxo-octahydroxanthene is summarized in Table 1. Acidity and the structure of ILs, molar ratio of IL/substrate and reaction time all have significant effects on the reaction. The reaction hardly occurred in the presence of [BMIM][BF4] (Table 1, entry 1) while the reaction conversion was enhanced from 8.9 to 95%, when BAILs with a −SO3H group in the anions were used as promoters. As an exception [(CH2)4SO3HMIM][HSO4], [NMP][HSO4] and [BMIM][H2PO4] showed a relatively weaker promoting effect (Table 1, entries 2–7) than [CMIM][HSO4] and [BMIM][HSO4].These results imply that the performance of BAILs is dependent upon the character of the side chain of the cation and the N-heterocyclic ring. These initial investigational results showed that the change of BAILs concentration would increase the reaction rate, and the change of acid group introduced into the imidazolium cation would affect the acidic activity. In this method, BAILs plays an important role in enhancing the reactivity as well as reducing formation of by-products. The use of these BAILs in this reaction can be considered as new green alternative.
Effect of different BAILs as catalyst for 1,8-dioxo-octahydroxanthene synthesis.
| Entry | Ionic liquids | Molar ratio (%) | Time (h) | Yield (%) |
| 1 | [BMIM][BF4] | 20 | 3 | 8.9 |
| 2 | [(CH2)4SO3HMIM][HSO4] | 10 | 3 | 56 |
| 3 | [(CH2)4SO3HMIM][HSO4] | 20 | 2.5 | 63.1 |
| 4 | [NMP][HSO4] | 10 | 3 | 68 |
| 5 | [NMP][HSO4] | 20 | 2.5 | 70 |
| 6 | [BMIM][H2PO4] | 10 | 2.5 | 65.8 |
| 7 | [BMIM][H2PO4] | 20 | 2.5 | 71 |
| 8 | [CMIM][HSO4] | 10 | 2.5 | 95 |
| 9 | [CMIM][HSO4] | 15 | 2 | 90.8 |
| 10 | [CMIM][HSO4] | 20 | 2 | 90.2 |
| 11 | [BMIM][HSO4] | 10 | 2 | 93 |
| 12 | [BMIM][HSO4] | 15 | 2 | 92 |
| 13 | [BMIM][HSO4] | 20 | 2.5 | 89 |
2.2 Effects of different reaction conditions using [CMIM][HSO4] as a catalyst
Initially, to optimize the amount of ionic liquid, the reaction of dimedone (10 mmol) and aldehyde (5 mmol) was performed in 10 ml water at 70 °C in the presence of different quantities of [CMIM][HSO4] (Table 2). As shown in Table 2, the yield of product g using high amounts of [CMIM][HSO4] was comparatively low and the reaction time was long (Table 2, entries f-h). No improvement in the reaction rate was observed by decreasing the amount of ionic liquid from 15 to 5 mol % but the yield of product c in the presence of 10 mol % of [CMIM][HSO4] was higher than the others (Table 2, entries c,d). A conceivable mechanism for formation of 1,8-dioxo-octahydroxanthene is proposed (Scheme 2).
Results of 1,8-dioxo-octahydroxanthene synthesis using [CMIM][HSO4] as catalyst under different conditions.
| Entry | Molar ratio (%) | Time (h) | Yield (%) |
| a | 5 | 2 | 87 |
| b | 5 | 2.5 | 90 |
| c | 10 | 2.5 | 95 |
| d | 10 | 3 | 94 |
| e | 15 | 2.5 | 91 |
| f | 15 | 3 | 91 |
| g | 15 | 4 | 89 |
| h | 20 | 3 | 90 |
2.3 Reusability of [CMIM] [HSO4]
One of the most effective BAIL, [CMIM][HSO4] was selected to investigate the possibility of reusability (Fig. 1). The product is insoluble in water and it was collected by simple filtration, while the ionic liquid is completely soluble in water. The dissolved IL could be recovered easily by rotary evaporator and directly reused for subsequent runs. As shown in Fig. 1, slight decrease was observed with the recovered IL. 1H NMR spectrums of IL before the run and after fifth run exhibits almost same values which confirmed that the IL is structurally stable even after fifth run.

Reusability of [CMIM][HSO4].
3 Conclusion
Several BAILs with different acidic scales were synthesized. Their acidity was influenced by the structures of both the anion and the cation. In particular, the presence of a SO3H group in the anions of BAILs might be responsible for a higher acidity, which brought about a significant promoting effect on the formation of 1,8-dioxo-octahydroxanthene. The product yield attained a plateau when the reaction was promoted with BAILs. Further study of [CMIM][HSO4] with similar reaction shows discrimination in the good to excellent yield. The reusability of ILs has given the green touch to the research.
4 Experimental
4.1 Materials and methods
All the chemicals were purchased from Sigma Aldrich and used as received without further purification. The IR spectra was run on a Perkin–Elmer, FTIR-1600 spectrophotometer and expressed in cm−1 (KBr). 1H and 13C NMR spectra were recorded on Bruker Avance (300 MHz) spectrometer. An LC-MS spectrum was obtained with Shimadzu LC-MS–2010 equipped with electrospray ionization interface.
4.2 Synthesis of ionic liquids
All the six ionic liquids such as [BMIM][BF4], [BMIM][HSO4], [BMIM][H2PO4], [CMIM][HSO4], [NMP][HSO4] and [(CH2)4SO3HMIM][HSO4] were synthesized according to reported methods [20–23].
4.3 General procedure
The round-bottomed flask was charged with benzaldehyde (5 mmol), dimedone (10 mmol) and [CMIM][HSO4] (10 mol %). Unfortunately, as the [CMIM][HSO4] is very viscous, water (10 mL) was used as sequester. The mixture was then stirred at 70 °C for an appropriate time (Table 1). On completion of reaction (monitored by TLC), the precipitated crude product was collected by filtration to get a product in excellent yield (95%). The product was identified by IR, 1H NMR, 13C NMR and LC-MS with those reported in the literature.
3,3,6,6-Tetramethyl-9-phenyl-3,4,5,6,7,9-hexahydro-2H-xanthene-1,8-dione (3):
mp 202-204 °C; IR (KBr): 2960 cm−1 (-CH3 stretching), 2874 cm−1 (Ar C – H stretching), 1665 cm−1 (α, β – unsaturated carbonyl group), 1622 and 1453 cm−1 (Ar C – C stretching); 1H NMR (300 MHz, CDCl3): δ 0.9 (s, 6H), 1.1 (s, 6H), 2.2 (s, 4H), 2.4 (s, 4H), 4.7 (s, 1H), 7.1 – 7.3 (m, 5H); 13C NMR (300 MHz, CDCl3): δ 27.3, 29.2, 31.8, 32.1, 40.8, 50.7, 115.6, 126.3, 128.0, 128.3, 144.0, 162.2, 196.2; M/z = 351 (M + 1); Purity by LC-MS = 100%.
[BMIM][BF4]: 1H NMR (DMSO-d6): δ 0.72 (t, J = 7.2 Hz, 3H), 1.11 (m, 2H), 1.67 (m, 2H), 3.89 (s, 3H), 4.17 (s, 2H), 7.85 (s, 1H), 7.94 (s, 1H), 9.63 (s, 1H).
[(CH2)4SO3HMIM][HSO4]: 1H NMR (DMSO-d6): δ 1.49 – 1.59 (m, 2H), 1.82 – 1.92 (m, 2H), 2.51 – 2.58 (m, 2H), 3.85 (s, 3H), 4.18 (t, J = 7.2 Hz, 2H), 7.59 (s, 2H), 7.72 (s, 1H), 7.78 (s, 1H), 9.16 (s, 1H).
[CMIM][HSO4] (before any run): 1H NMR (DMSO-d6): δ 3.63 (s, 3H), 4.84 (t, J = 3.0 Hz, 2H), 7.20 (s, 2H), 8.49 (bs, 1H).
[CMIM][HSO4] (after fifth run): 1H NMR (DMSO-d6): δ 3.82 (s, 3H), 5.11 (t, J = 2.9, 2H), 7.76 (s, 2H), 9.0 (bs, 1H).
[BMIM][HSO4]: 1H NMR (DMSO-d6): δ 0.86 (t, J = 7.2 Hz, 3H), 1.25 – 1.18 (m, 2H), 1.73 (s, 2H), 3.84 (s, 3H), 4.15 (s, 2H), 7.41 (bs, 1H), 7.72 (s, 1H), 7.80 (s, 1H), 9.31 (s, 1H).
[BMIM][H2PO4]: 1H NMR (DMSO-d6): δ 0.87 (t, J = 7.2, 3H), 1.22 (m, 2H), 1.74 (s, 2H), 3.84 (s, 3H), 4.16 (s, 2H), 7.71 (s, 1H), 7.78 (s, 1H), 9.28 (s, 1H), (H of H2PO4 not seen).
[NMP][HSO4]: 1H NMR (DMSO-d6): δ 1.83 – 1.91 (m, 2H), 2.15 (t, J = 7.8 Hz, 2H), 2.65 (s, 3H), 3.27 (t, J = 6.9 Hz, 2H), 9.78 (bs, NH, HSO4).
Acknowledgments
The authors P.P.S. and S.S.K., thanks to DAE–BRNS, New Delhi (India) for the research fellowship and financial assistance respectively.


