1 Introduction
The development of environmentally benign synthesis has attracted much attention in recent years. Therefore, development of polymer-bound catalysts and reagents with high activity and selectivity is of great importance in organic synthesis [1–3]. Since most of metal complexes are often expensive to purchase or prepare, the immobilization of these complexes on a support can provide catalysts which are easier to handle, and may exhibit improved selectivities and activities because of the support environment [4].
From synthetic point of view, polystyrene is one of the most popular polymeric supports used in synthetic organic chemistry because of its low cost, ready availability, mechanical robustness, chemical inertness, and facile functionalization.
The protection of hydroxyl groups is often necessary during the course of various transformations in a synthetic sequence, especially in the synthesis of fine chemicals and natural products. Several methods such as acetylation, tetrahydropyranylation, methoxymethylation and trimethylacetylation have been reported for protection of hydroxyl groups [5,6]. A variety of procedures using homogeneous or heterogeneous catalysts such as iodine [7], p-toluenesulfonic acid [8], alumina [9], zinc chloride [10], cobalt chloride [11], montmorillonite K-10 and KSF [12], zeolite HSZ-360 [13], zirconium sulfophenyl phosphonate [14], Sc(OTf)3 [15], TaCl5 [16], TMSOTf [17], Cu(OTf)2 [18], In(OTf)3 [19], magnesium bromide [20], bismuth(III) salts [21], ferric perchlorate adsorbed on silica-gel [22], RuCl3 [23], InCl3 [24], Ce(OTf)3 [25], Mg(ClO4)2 [26], ZrCl4 [27], Cp2ZrCl2 [28], ZrOCl2·8H2O [29], Al(OTf)3 [30], NaHSO4·SiO2 [31], La(NO3)3·6H2O [32], NbCl5 [33], Gd(OTf)3 [34], Alumina supported MoO3 [35], cerium polyoxometalate [36], Zn(ClO4)2·6H2O [37], Mg(NTf2)2 [38], Cu(BF4)2 [39], BiO(ClO4)2 [40], HClO4-SiO2 [41], HBF4-SiO2 [42], electron-deficient tin [IV] porphyrins [43–45] and ZrO(OTf)2 [46] have been routinely reported for acetylation of alcohols and phenols with Ac2O. Although these procedures provide an improvement, many of these catalysts or activators need long reaction times, drastic reaction conditions or tedious work-up and are moisture-sensitive or expensive in terms of the catalyst. Hence, the introduction of new procedures to circumvent these problems is still in demand.
Electron-deficient metalloporphyrins have been used as mild Lewis acid catalysts [47–50]. The Suda group has reported the use of chromium and iron porphyrins in organic synthesis. They used Cr(tpp)Cl for regioselective [3,3] rearrangement of aliphatic allyl vinyl ethers and for Claisen rearrangement of simple aliphatic allyl vinyl ethers, Fe(tpp)OTf for rearrangement of α,β-epoxy ketones into 1,2-diketones and Cr(tpp)OTf for highly regio- and stereoselective rearrangement of epoxides to aldehydes [51–54].
Recently, we have reported the use of tetraphenylporphyrinatotin(IV) perchlorate [43,55], tetraphenylporphyrinatotin(IV) trifluoromethanesulfonate [44,56–59], and tetraphenylporphyrinato- tin(IV) tetrafluoroborate [45,59–61] in organic transformations.
In the present work, we report the preparation, characterization and investigation of catalytic activity of tetrakis(p-aminophenyl)porphyrinatotin(IV) trifluoromethanesulfonate, [SnIV(TNH2PP)(OTf)2], supported on chloromethylated polystyrene in the acetylation of alcohols and phenols with acetic anhydride (Scheme 1).
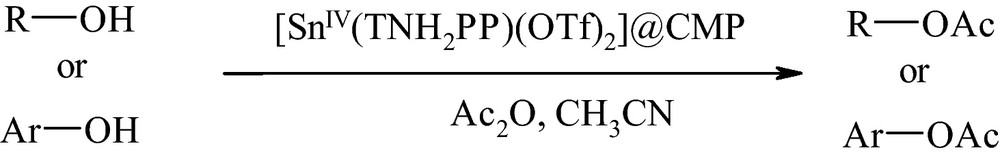
Acetylation of alcohols and phenols with Ac2O catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP.
2 Experimental
Chemicals were purchased from Merck or Fluka chemical companies. Chloromethylated polystyrene (cross-linked with 2% divinylbenzene, 4–5% Cl content, 1.14–1.40 mmol/g Cl) was purchased from Fluka. FT IR spectra were obtained with potassium bromide pellets in the range 400–4000 cm−1 with a Nicolet Impact 400D spectrometer. Gas chromatography experiments (GC) were performed with a Shimadzu GC-16A instrument using a 2-m column packed with silicon DC-200 or Carbowax 20 m. In the GC experiments, n-decane was used as an internal standard. 1H NMR spectra were recorded on a Bruker-Avance AQS 400 MHz spectrometer. Tetra(4-aminophenyl)porphyrin was prepared according to the literature [62]. The [SnIV(TNH2PP)Cl2] catalyst was prepared according to the procedure reported for metallation of porphyrins [63]. In this manner, tetra(4-aminophenyl)porphyrin was reacted with SnCl2·2H2O in refluxing DMF.
2.1 Supporting of [SnIV(TNH2PP)Cl2] on chloromethylated polystyrene, [SnIV(TNH2PP)Cl2]@CMP
To a solution of [SnIV(TNH2PP)Cl2] (0.5 g) in DMF (50 mL), were added chloromethylated polystyrene (2.5 g) and triethylamine (3 mL). The mixture was heated at 100 °C for 72 h. After cooling to room temperature, the green solids were filtered, washed with Et2O and acetone and dried.
2.2 Conversion of [SnIV(TNH2PP)Cl2]@CMP to [SnIV(TNH2PP)(OTf)2]@CMP
To a suspension of [SnIV(TNH2PP)Cl2]@CMP (2 g) in THF (50 mL) was added NaOTf (1 g) and stirred at 60 °C for 8 h, after which the catalyst was filtered and washed with THF.
2.3 General procedure for acetylation of alcohol and phenols with Ac2O catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP
To a solution of alcohol or phenol (1 mmol) and Ac2O (3 mmol per OH group) in CH3CN (0.5 mL) was added [SnIV(TNH2PP)Cl2]@CMP (70 mg, 0.01 mmol) and stirred at room temperature for appropriate time. The progress of the reaction was monitored by GC. After completion of the reaction, Et2O (10 mL) was added and the catalyst was filtered. The filtrates were washed with brine, dried over Na2SO4 and concentrated under reduced pressure to afford the crude product.
2.4 Catalyst reusability
At the end of each reaction, the catalyst was filtered, washed thoroughly with Et2O, dried and reused.
3 Results and discussion
3.1 Preparation and characterization of [SnIV(TNH2PP)(OTf)2]@CMP
Scheme 2 shows the preparation route for [SnIV(TNH2PP)(OTf)2]@CMP. First, chloromethylated polystyrene (cross-linked with 2% divinylbenzene, 4–5% Cl content, 1.14–1.40 mmol/g Cl) was reacted with [SnIV(TNH2PP)Cl2] to afford the [SnIV(TNH2PP)Cl2]@CMP. Then, the chlorines were substituted by OTf− by the reaction of [SnIV(TNH2PP)Cl2]@CMP with NaOTf. This substitution increases the electron-deficiency of tin(IV).
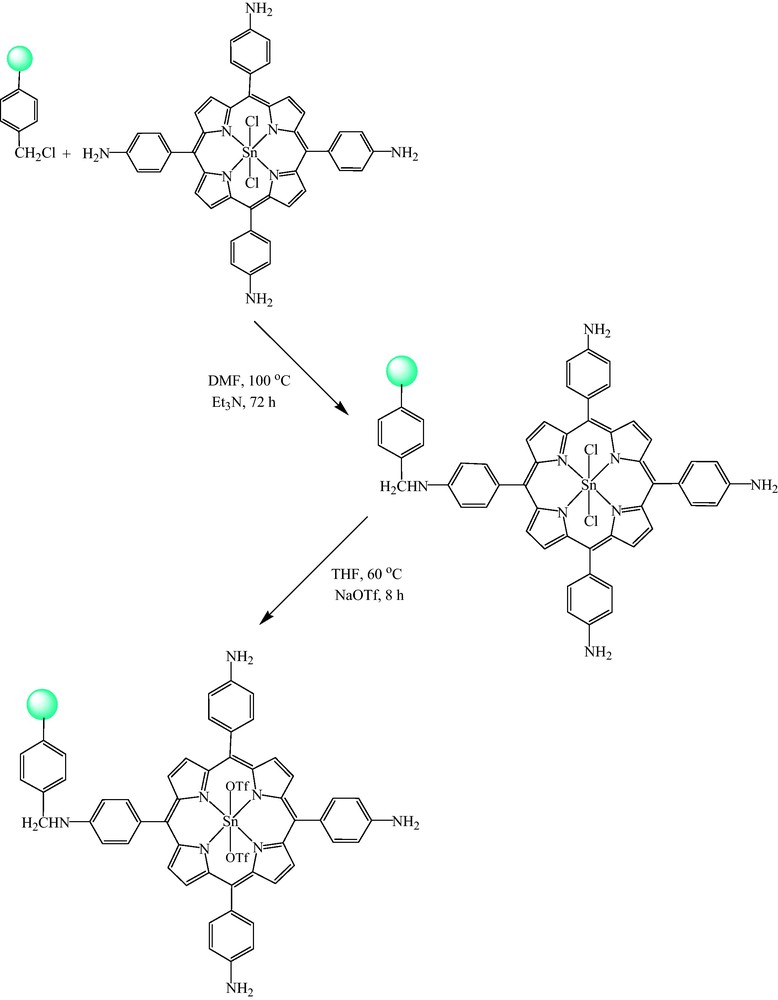
The preparation route for [SnIV(TNH2PP)(OTf)2]@CMP.
The prepared catalyst was characterized by elemental analysis, FT IR and UV-Vis spectroscopic methods. The nitrogen content of the catalyst, measured by CHNS analysis, was obtained as 1.64% (1.17 mmol/g). According to this value, the amount of porphyrin introduced to polystyrene was calculated as 0.146 mmol/g. The Sn content of the catalyst was also determined by ICP (0.143 mmol/g) which was in accordance with the data obtained by CHNS analysis.
The evidence for attachment of tin(IV) porphyrin on the polystyrene was obtained from FT IR spectra of chloromethylated polystyrene and [SnIV(TNH2PP)(OTf)2]@CMP. The sharp C-Cl peak (due to -CH2Cl groups) at 1264 cm−1 in the starting polymer (Fig. 1A) was practically absent or seen as a weak band after introducing of tin(IV) porphyrin on the polymer (Fig. 1B). A peak at 1622 cm−1 assigned to C = N and a peak at 1175 cm−1 assigned to C-N stretching modes also appeared, which confirmed the supporting of tin(IV) porphyrin on the support.

The FT IR spectrum of (A) chloromethylated polystyrene and (B) [SnIV(TNH2PP)(OTf)2]@CMP.
The reflectance spectrum of the polymer-bound porphyrin resembles solution counterpart spectrum with only a slight red shift, and the Soret band was appeared at 445 nm and the Q bands observed at 558 and 619 nm. These observations clearly proved the presence of metalloporphyrin on the polystyrene (Fig. 2).
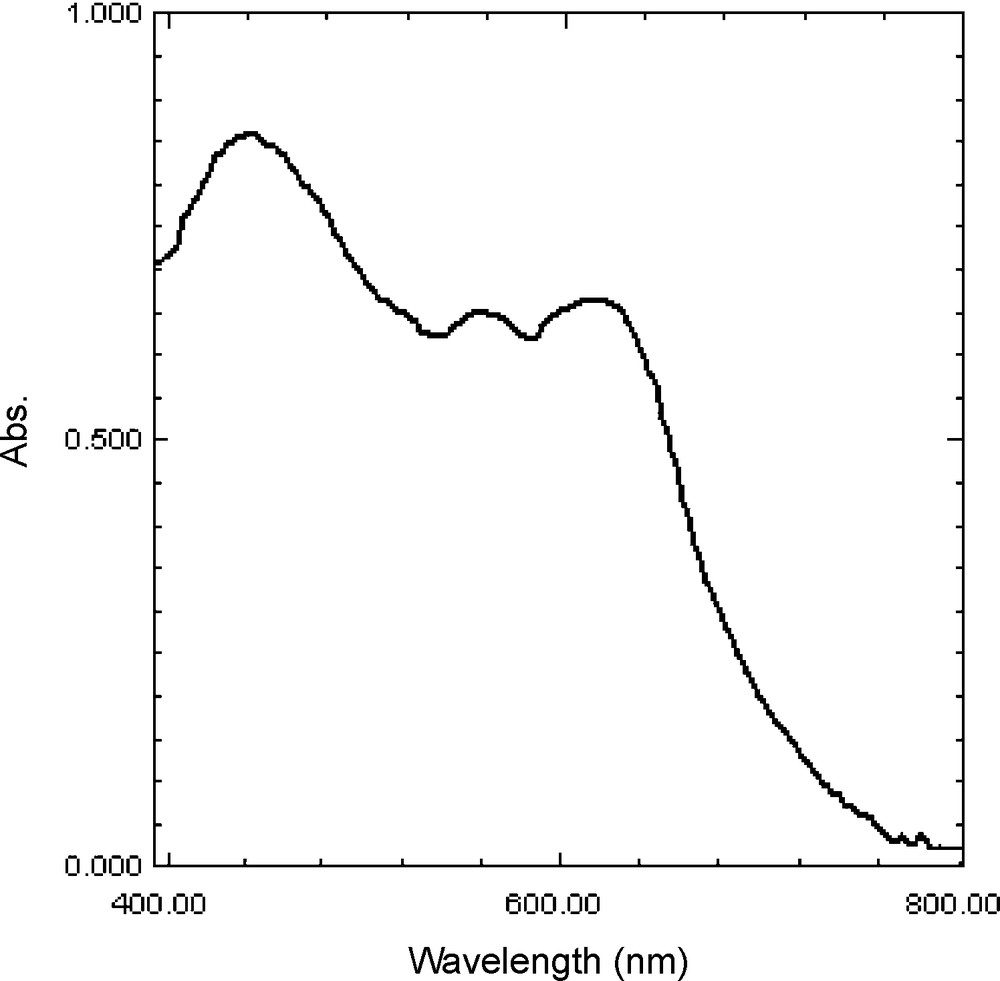
The DR UV-Vis spectrum of [SnIV(TNH2PP)(OTf)2]@CMP.
3.2 Acetylation of alcohols and phenols with Ac2O catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP
First, the amount of catalyst was optimized in the acetylation of 4-chlorobenzyl alcohol with Ac2O. The results, which are summarized in Table 1, showed that in the presence of 0.01 mmol (70 mg) of [SnIV(TNH2PP)(OTf)2]@CMP the highest yield was obtained. Then, the amount of Ac2O was also optimized. The results showed that in the presence of 3 mmol of Ac2O, the reaction was completed (Table 2).
Optimization of catalyst amount in the acetylation of 4-chlorobenzyl alcohol with Ac2Oa.
| Entry | Time (min) | Catalyst amounts (mmol, mg) | Yield (%)b |
| 1 | 6 | 40 mg (0.006 mmol) | 31 |
| 2 | 6 | 50 mg (0.0071 mmol) | 54 |
| 3 | 6 | 60 mg (0.0086 mmol) | 88 |
| 4 | 6 | 70 mg (0.010 mmol) | 100 |
| 5 | 6 | 80 mg (0.011 mmol) | 100 |
a Reaction conditions: alcohol (1 mmol), Ac2O (3 mmol), catalyst, CH3CN (1 mL).
b GC yield.
Optimization of Ac2O amount in the acetylation of 4-chlorobenzyl alcohola.
| Entry | Time (min) | Ac2O (mmol) | Yield (%)b |
| 1 | 6 | 0.5 | 18 |
| 2 | 6 | 1.0 | 38 |
| 3 | 6 | 1.5 | 55 |
| 4 | 6 | 2.0 | 81 |
| 5 | 6 | 2.5 | 92 |
| 6 | 6 | 3.0 | 100 |
a Reaction conditions: alcohol (1 mmol), Ac2O, catalyst (1 mol%), CH3CN (1 mL).
b GC yield.
In order to show the effect of OTf groups on the catalytic activity of tin (IV) porphyrin, the catalytic activity of [SnIV(TNH2PP)Cl2]@CMP (1 mol%) was also investigated in the acetylation of 4-chlorobenzyl alcohol with Ac2O. In this case, only 23% of the corresponding acetate was obtained while in the presence of [SnIV(TNH2PP)(OTf)2]@CMP, the reaction was completed after 6 min. These observations indicated that the presence of OTf groups is necessary for catalytic activity of tin(IV) porphyrin. The OTf groups increases the electron-deficiency of tin(IV) porphyrin which in turn increases the activity of the catalyst.
The optimized conditions, which were obtained for acetylation of 4-chlorobenzyl alcohol, were alcohol, Ac2O and catalyst in a molar ratio of 100: 300: 1. Under these conditions, a wide variety of alcohols were subjected to acetylation with Ac2O. The obtained results showed that different primary, secondary (including aliphatic and aromatic alcohols) and tertiary alcohols were acetylated successfully at room temperature (Table 3). As can be seen, in benzylic alcohols the nature of substituent (electron-withdrawing or electron-releasing) has no significant effect on the product yield. In the absence of catalyst only small amounts of the corresponding acetates were produced. In the case of tertiary alcohols such as 1-adamantol and 2-methyl-1-phenyl-2-propanol (entries 17 and 18) no elimination product was observed.
Acetylation of alcohols with Ac2O catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP at room temperaturea.
| Entry | Hydroxy compound | Acetate | Time (min) | Yield (%)b,c |
| 1 | 6 | 100 (95) | ||
| 2 | 6 | 100 (96) | ||
| 3 | 6 | 100 (95) | ||
| 4 | 12 | 100 (93) | ||
| 5 | 6 | 100 | ||
| 6 | 6 | 100 | ||
| 7 | 6 | 100 (96) | ||
| 8 | 6 | 100 | ||
| 9 | 6 | 100 (94) | ||
| 10 | 6 | 100 | ||
| 11 | 6 | 100 | ||
| 12 | 6 | 100 | ||
| 13 | 6 | 100 | ||
| 14 | 6 | 100 (95) | ||
| 15 | 6 | 100 (93) | ||
| 16 | 6 | 99 | ||
| 17 | 8 | 96 | ||
| 18 | 8 | 98 (90) |
a Reaction conditions: alcohol (1 mmol), Ac2O (3 mmol), catalyst (1 mol%), CH3CN (1 mL).
b GC yield.
c Yields in the parentheses refer to isolated products.
In order to show the advantage of the presented method in the acetylation reactions, we have compared the obtained results in the acetylation of benzyl alcohol with acetic anhydride catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP with some of those reported in the literature (Table 4). It is clear that the method presented is superior in terms of reaction time, catalyst amount, or product yield. Recently, we reported the use of homogeneous [SnIV(TPP)(OTf)2] and [SnIV(TPP)(BF4)2] in the acetylation of alcohols and phenols [44,45]. The reaction times in the presence of 1 mol% of homogeneous catalysts were 1–15 min and the product yields were 87–99%. In comparison with its homogeneous counterparts [44,45], in the presence of 1 mol% of [SnIV(TNH2PP)(OTf)2]@CMP, the corresponding acetates were obtained in 96–100% in 8–12 min. These observations showed that [SnIV(TNH2PP)(OTf)2]@CMP, which is a heterogeneous catalyst, in some cases the reaction times are longer for [SnIV(TNH2PP)(OTf)2]@CMP. On the other hand, the reusability of this catalyst was higher and can be separated by simple filtration in which does not contaminate the reaction product.
Comparison of the results obtained for the acetylation of benzyl alcohol catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP with those obtained by the recently reported catalysts.
| Entry | Catalyst | Catalyst (mol%) | Temperature | Time (min) | Yield (%) | Ref. |
| 1 | [SnIV(TNH2PP)(OTf)2]@CMP | 1 | RT | 6 | 100 | - |
| 2 | I2 | 10 | RT | 1 | 99 | [7] |
| 3 | CoCl2 | 0.5 | RT | 240 | 98 | [11] |
| 4 | Montmorillonite KSF | 20 mg | RT | 60 | 90 | [12] |
| 5 | Zeolite HSZ-360 | 20 mg | 60 °C | 60 | 84 | [13] |
| 6 | TaCl5 | 10 mg | RT | - | 77 | [16] |
| 7 | Cu(OTf)2 | 2 | RT | 30 | 97 | [18] |
| 8 | In(OTf)3 | 0.1 | RT | 15 | 97 | [19] |
| 9 | BiCl3 | 10 | RT | 35 | 98 | [21] |
| 10 | Bi(TFA)3 | 5 | RT | 60 | 96 | [21] |
| 11 | Bi(OTf)3 | 1 | RT | 5 | 99 | [21] |
| 12 | RuCl3 | 5 | RT | 10 | 95 | [23] |
| 13 | InCl3 | 0.1 | RT | 30 | 85 | [24] |
| 14 | Ce(OTf)3 | 1 | RT | 12 | 98 | [25] |
| 15 | Mg(ClO4)2 | 1 | RT | 15 | 100 | [26] |
| 16 | Cp2ZrCl2 | 1 | RT | 600 | 93 | [28] |
| 17 | [SnIV(TPP)(OTf)2] | 1 | RT | 1 | 99 | [44] |
| 18 | [SnIV(TPP)(BF4)2] | 1 | RT | 1 | 99 | [45] |
Under the optimized conditions, which were described for acetylation of alcohols, the acetylation of phenols with Ac2O was also investigated in the presence of [SnIV(TNH2PP)(OTf)2]@CMP. The results showed that all reactions were completed after 8 min at room temperature (Table 5). The acetylation of polyhydroxybenzenes such as hydroquinone, pyrocatechol, resorcinol and pyrogallol was also performed. The results showed that all hydroxyl groups were acetylated and the desired polyacetates were obtained in excellent yields (Table 5, entries 5–8).
Acetylation of phenols with Ac2O catalyzed by [SnIV(TNH2PP)(OTf)2]@CMP at room temperaturea.
| Entry | Phenol | Acetate | Time (min) | Yield (%)b,c |
| 1 | 8 | 100 (96) | ||
| 2 | 8 | 100 (95) | ||
| 3 | 8 | 100 (93) | ||
| 4 | 8 | 100 (95) | ||
| 5d | 8 | 100 (94) | ||
| 6d | 8 | 100 | ||
| 7d | 8 | 100 | ||
| 8d | 8 | 100 | ||
| 9 | 8 | 100 (94) |
a Reaction conditions: phenol (1 mmol), Ac2O (3 mmol), catalyst (1 mol%), CH3CN (1 mL).
b GC yield.
c Yields in the parentheses refer to isolated products.
d Reaction was performed with 2 mmol of Ac2O per OH group.
The actual mechanism is not clear at present. However, a plausible explanation is that acetic anhydride is first activated by catalyst to afford 1. Alcohol or phenol attacks 1 which in turn converts to the final product and releases the catalyst for the next catalytic cycle (Scheme 3).
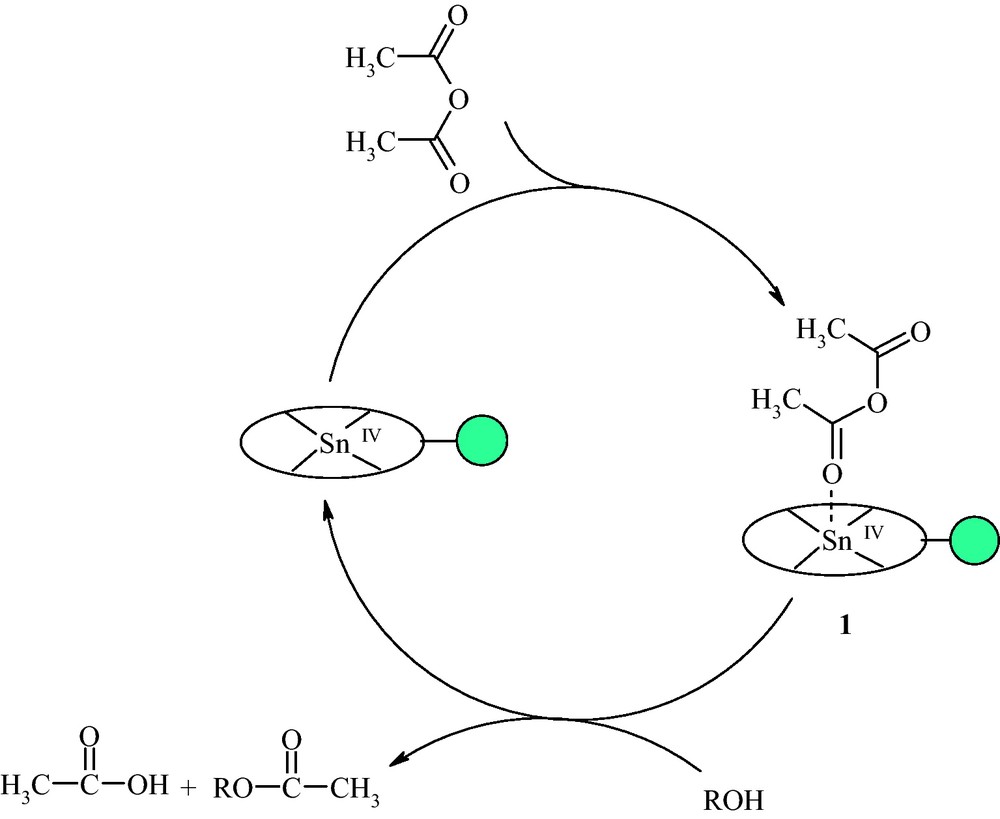
The proposed mechanism.
3.3 Catalyst reuse and stability
The stability of the [SnIV(TNH2PP)(OTf)2]@CMP catalyst was monitored using multiple sequential acetylation of 4-chlorobenzyl alcohol with Ac2O. For each of the repeated reactions, the catalyst was filtered, washed exhaustively with water, acetonitrile and diethyl ether, respectively, and dried before using with fresh 4-chlorobenzyl alcohol and Ac2O. The catalyst was consecutively reused seven times without a detectable catalyst leaching or a significant loss of its activity. After each run the filtrates were used for determination of catalyst leaching. No Sn was detected in the filtrates by ICP. This is not surprising because the reaction times are short. When the same reaction was continued for 2 h, about 2% of initial Sn was leached. Also, the catalytic behavior of the separated liquid was tested by addition of fresh 4-chlorobenzyl alcohol and Ac2O to the filtrates after each run. Execution of the acetylation reaction under the same reaction conditions, as with catalyst, showed that the obtained results were as same as blank experiments. The nature of recovered catalyst was monitored by diffuse reflectance UV-Vis spectrophotometery. No change was observed in the DR UV-Vis spectrum of the catalyst which indicates the stability and robustness of the catalyst (Fig. 3).

The DR UV-Vis spectrum of recovered [SnIV(TNH2PP)(OTf)2]@CMP.
4 Conclusion
In conclusion, a new, robust, stable and heterogeneous tin(IV) catalyst was prepared, characterized for the first time. This new electron-deficient tin(IV) porphyrin, [SnIV(TNH2PP)(OTf)2]@CMP, was used for efficient and chemoselective acetylation of primary, secondary and tertiary alcohols and phenols with Ac2O. Short reaction times, excellent yields, easy work-up and reusability and stability of the catalyst are noteworthy advantages of this method.
Acknowledgement
We are thankful to the Center of Excellence of Chemistry of University of Isfahan (CECUI) for financial support of this work.


