1 Introduction
Recently, multicomponent reactions (MCRs) have been considered as a superior synthetic strategy [1]. The MCRs are very flexible, atom economic in nature, and proceed through a sequence of reaction equilibria, yielding the target product [2]. Moreover, these transformations combine classical concerns such as efficiency, selectivity, molecular complexity and diversity [3,4]. The acridine derivatives have been known first to be used as pigments and dyes since the 19th century [5]. A range of acridines continue to be used today for the treatment of actuelekaemia (amsacrine) [6], as anticancer agents (ledakrin) [7]. To date, ranges of acridine have been reported with a range of chemical and physical properties. Their utility in the pharmaceutical industry has also been reported [8]. A number of methods have been developed for the synthesis of acridine compounds containing 1,4-dihydropyridines, from dimedone, aldehyde and different anilines or ammonium acetate via traditional heating in organic solvents [9], in water catalyzed by TEBAC [10], under microwave irradiation [11], and using ionic liquids are emerging as effective solvents for green processes. In recent years l-Proline has drawn much interest in different organic reactions due to its experimental simpilicity in water and organic solvents, l-Proline has shown considerable catalytic efficiency in different transformations such as enamine based direct catalytic asymmetric aldol condensation [12], α-amination reaction [13], Mannich reaction [14], Diels-Alder reaction [15], Knoevenagel reaction [16], Micheal condensation [17], and as excellent promoter for the copper-catalyzed coupling reaction [18], as well as in solvent-free Biginelli reaction [19], in unsymmetric Hantzsch reaction [20], for the selective synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles from wide range of substituted o-phenylenediamines and aldehydes [21], proline catalysis has emerged recently as an efficient means of generating functionalized cyclohexanes ad spirane products via Barbas three-component cycloaddition [22]; proline has also been used as catalyst for two-carbon homologation and in various MCRs in one-pot [23]. The developing of new MCRs and improving known MCRs are an area of considerable current interest. Herein, we report a simple and facile multicomponent one-pot synthesis of substituted tetrahydrobenzo[c]acridin-8(7H)-ones in high yields, using l-Proline (15 mol%) as an organocatalyst. Most of the above reported synthetic methods for the synthesis of substituted tetrahydrobenzo[c]acridin-8(7H)-ones suffer from one or more drawbacks, such as a hazardous reaction condition, complex work-up and purification, strong acidic condition [24], high temperature [25,26], use of toxic metal catalyst [27], poor yields, occurrence of side reactions and expensive reagents. Therefore, the development of a mild generalized method to overcome these shortcomings still remains an ongoing challenge for the synthesis of highly substituted tetrahydrobenzo[c]acridin-8(7H)-ones for organic chemists.
2 Results and discussion
We report herein, for the first time, a simple, mild, and expeditious synthesis of highly substituted tetrahydrobenzo[c]acridin-8(7H)-ones in excellent yields employing l-Proline (15 mol%) as an organocatalyst at room temperature (Scheme 1).
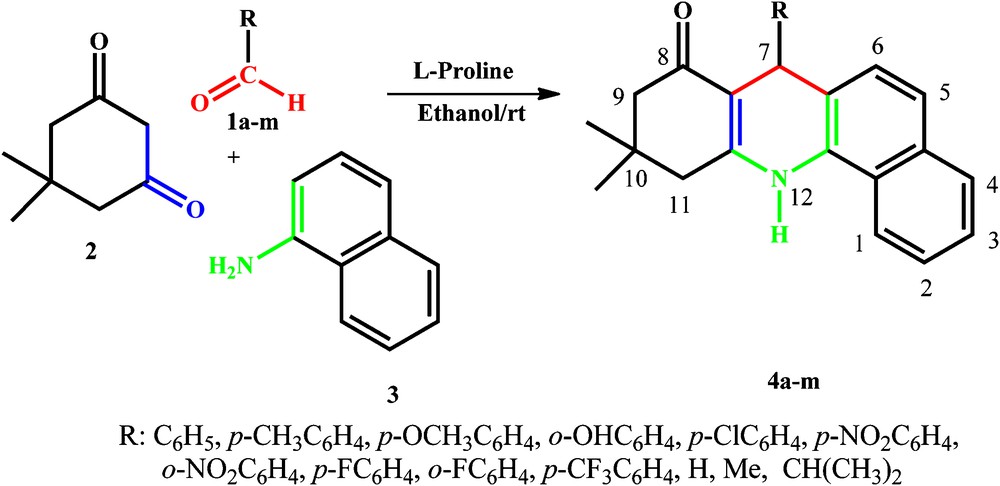
l-Proline catalysed synthesis of substituted tetrahydrobenzo[c]acridin-8(7H)-ones.
In order to standardize the reaction, salicylaldehyde (1mmol), α-naphthylamine (1 mmol), 5,5-dimethylcyclohexane-1,3-dione(dimedone) (1 mmol) were dissolved in ethanol and stirred at room temperature for 30 min. in the absence of the catalyst which led to very poor yields (only 5–6%, as obtained in crude 1H NMR) of the substituted tetrahydrobenzo[c]acridin-8(7H)-ones. We also tried different solvents under similar reaction conditions but no appreciable increment in product yield was observed. Then it was thought worthwhile to study the reaction I in the presence of an organocatalyst like l-Proline. Use of 15 mol% of the catalyst produced maximum yield (95%). A further increase of the catalyst concentration does not increase the yield. On the contrary, the reaction slows down on adding more than 15 mol% of catalyst. The standard reaction was also studied in the presence of glycine (15 mol %), when the desired product was obtained after 72 h in only 10% isolated yield. This lower yield could be attributed firstly to the comparatively poor solubility of glycine in ethanol and secondly to the reaction passing via imine with lower reactivity rather than iminium ion with l-Proline with much higher reactivity (via mechanism). The detailed results of changing catalyst (l-Proline) concentration and solvents are given in Table 1.
Multicomponent reaction of salicylaldehyde, α-naphthylamine and 5,5-dimethylcyclohexane-1,3-dione(dimedone) in the presence of different solvents and different catalyst percentagesa.
| Entry | Catalyst (mol %) | Solvent | Time (h) | Yieldb (%) |
| 1 | – | EtOH | 72 | 5–6 |
| 2 | – | MeOH | 72 | 5–6 |
| 3 | – | CHCl3 | 72 | Trace |
| 4 | – | CH3CN | 72 | Trace |
| 5 | – | CH2Cl2 | 72 | Trace |
| 6 | – | 1,4-Dioxane | 72 | Trace |
| 7 | – | H2O | 72 | N.R.c |
| 8 | 5 | EtOH | 8 | 55 |
| 9 | 10 | EtOH | 4 | 75 |
| 10 | 15 | EtOH | 1 | 95 |
| 11 | 20 | EtOH | 1 | 85 |
| 12 | 15 | MeOH | 12 | 75 |
| 13 | 15 | CHCl3 | 12 | 35 |
| 14 | 15 | CH3CN | 12 | 25 |
| 15 | 15 | CH2Cl2 | 12 | 20 |
| 16 | 15 | 1,4-Dioxane | 12 | 30 |
| 17 | 15 | H2O | 48 | N.Rc |
a 5,5-dimethylcyclohexane-1,3-dione(dimedone)/aldehyde/α-naphthylamine(1:1:1).
b Isolated yield.
c No reaction.
We next examined a wide variety of aldehydes (both aromatic and aliphatic) with various substituents to establish the catalytic importance of l-Proline for this reaction. A wide range of ortho-, meta- and para-substituted aromatic aldehydes undergo this one-pot multicomponent synthesis with dimedone and α-naphthylamine to afford substituted tetrahydrobenzo[c]acridin-8(7H)-ones in good yields. In all cases, we observed the almost same performance towards this cyclocondensation to give the desired product (4a–m) (Table 2). Aliphatic aldehydes gave the corresponding tetrahydrobenzo[c]acridin-8(7H)-ones in lower yield (25–35%) than aromatic aldehydes (90–98%). The reaction profile is very clean and no side products are formed. All the synthesized tetrahydrobenzo[c]acridin-8(7H)-ones have been characterized on the basis of elemental and spectral studies.
Synthesis of highly tetrahydrobenzo[c]acridin-8(7H)-ones (4a–m) at room temperature using l-Proline (15 mol %) as an organocatalysta.
| Entry | R | Time (h) | Product | Yieldb (%) | Mp (oC) | |
| Found | Reported | |||||
| 1 | C6H5 | 1 | 4a | 95 | 257–258 | 258–259 [28,29] |
| 2 | 4-CH3C6H4 | 2 | 4b | 91 | 212–214 | – |
| 3 | 4-CH3OC6H4 | 2 | 4c | 92 | 206–207 | – |
| 4 | 2-OHC6H4 | 2 | 4d | 92 | 221–223 | 220–222 [30] |
| 5 | 4-ClC6H4 | 1 | 4e | 97 | 289–290 | 290–292 [28,29] |
| 6 | 4-NO2C6H4 | 0.5 | 4f | 95 | 281–283 | 281–283 [31] |
| 7 | 2-NO2C6H4 | 0.5 | 4g | 93 | 228–229 | – |
| 8 | 4-FC6H4 | 0.5 | 4h | 95 | 218–219 | – |
| 9 | 2-FC6H4 | 0.5 | 4i | 96 | 231–233 | – |
| 10 | 4-CF3C6H4 | 0.5 | 4j | 97 | 276–277 | – |
| 11 | H | 4 | 4k | 88 | 187–189 | – |
| 12 | Me | 4 | 4l | 87 | 191–192 | – |
| 13 | CH(CH3)2 | 5 | 4m | 89 | 195–197 | – |
b Isolated yield.
A plausible mechanism for the l-Proline catalyzed synthesis of highly substituted tetrahydrobenzo[c]acridin-8(7H)-ones has been proposed (Scheme 2) in which the reaction proceeds through two different pathways (Path-I and Path-II). Path-I involves the activation of aldehydic carbonyl oxygen by the acidic part of l-Proline through intermolecular H-bonding and subsequent condensation with 5,5-dimethylcyclohexane-1,3-dione(dimedone) to form the ene dione intermediate B. Path-II gives the same intermediate B via iminum catalysis which condenses with the condensation product of amine and 5,5-dimethylcyclohexane-1,3-dione(dimedone) to form the intermediate C which, on dehydration, gives the tetrahydrobenzo[c]acridin-8(7H)-ones (4a–m).
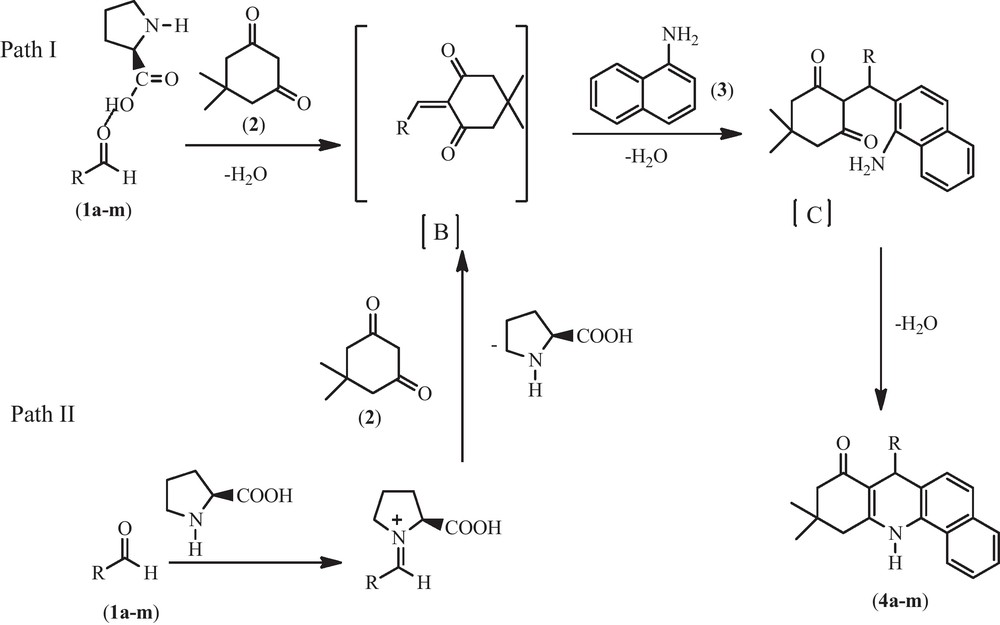
A plausible mechanism for the formation of tetrahydrobenzo[c]acridin-8(7H)-ones, Pathway I-acid catalysis through intermolecular hydrogen bonding. Pathway II-iminium catalysis.
3 Conclusion
In conclusion, we have developed a simple and efficient one-pot multicomponent methodology for the synthesis of substituted tetrahydrobenzo[c]acridin-8(7H)-ones (4a–m) catalyzed by 15 mol% l-Proline at room temperature. Simplicity of operation, high yields, easy work-up, purification of compounds by non-chromatography method (crystallization only) and wide range of substrate applicability are the key advantages of this methodology.
4 Experimental
4.1 General
All reagents were purchased from Merck. Aldehydes were distilled before use. Melting points were determined using a Linkman HF591 heating stage, used in conjunction with a TC92 controller, and re-uncorrected. NMR spectra were recorded using either a Brucker DRX500 machine at room temperature. 1H, 13C NMR and 19FNMR spectra were measured using DMSO-d6 as solvent. CHN analyses were performed on Exeter Analytical Inc. ‘Model C-400 CHN Analyzer’. Mass spectra were obtained using a Micro Mass LCT machine in ES or EI mode. Infrared spectra were measured on a Perkin Elmer Paragon 100 FT-IR spectrometer. All the reactions were monitored by TLC using 0.25 mm silica gel plates (Merck 60F254) UV indicator.
4.2 General procedure for the synthesis of substituted tetrahydrobenzo[c]acridin-8(7H)-ones (4a–m)
In a 50 mL round bottom flask 5,5-dimethylcyclohexane-1,3-dione (1 mmol), aldehyde (1 mmol) and α-naphthylamine (1 mmol) were stirred in the presence of 15 mol% of l-Proline in ethanol (2 mL) at room temperature for the stipulated time (Table 2). The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was diluted with water (5 mL) and extracted with ethyl acetate (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4, concentrated, and recrystallized from hot ethanol to afford the pure product.
4.3 Characterization data of some representative compounds
4.3.1 10,10-Dimethyl-7-(p-tolyl)-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4b)
0.334 g (91%); pale yellow solid; mp 212–214 °C. IR (KBr): 3323, 3077, 2985, 1655, 1589 (C = O), 1523, 1446, 804 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.11 (s, 3H, CH3), 1.26 (s, 3H, CH3), 2.07–2.27 (dd, 2H, C9-H), 2.51–2.71 (dd, 2H, C11-H), 2.89 (s, 3H, CH3), 5.42 (s, 1H, C7-H), 7.11–8.10 (m, 9H, Ar-H), 8.52 (d, 1H, J = 7.3, C6-H), 9.40 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 27.23, 29.67, 32.64, 36.87, 41.00, 50.56, 67.76, 87.98, 106.84, 119.85, 121.86, 122.72, 123.59, 124.05, 126.53, 128.19, 128.75, 128.92, 131.33, 133.05, 146.14, 152.87, 156.07, 194.01. MS (EI), m/z (%) = 367 (M+, 70), 276 (95). HRMS (EI) Found: M+, 367.1904. C26H25NO requires M+, 367.1908. Anal Calcd for C26H25NO: C, 84.98; H, 6.86; N, 3.81. Found: C, 90.05; H, 6.91; N, 3.65.
4.3.2 7-(4-Methoxyphenyl)-10,10-dimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4c)
0.352 g (92%); white solid; mp 206–207 °C. IR (KBr): 3318, 3098, 2921, 1651, 1532 (C = O), 1505, 1434, 1154,832 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.13 (s, 3H, CH3), 1.31 (s, 3H, CH3), 2.03–2.25 (dd, 2H, C9-H), 2.45–2.74 (dd, 2H, C11-H), 4.32 (s, 3H, OCH3), 6.06 (s, 1H, C7-H), 7.14–8.06 (m, 9H, Ar-H), 8.62 (d, 1H, J = 7.5, C6-H), 9.54 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 26.31, 28.56, 30.54, 35.53, 40.09, 51.43, 64.87, 76.98, 81.21, 89.76, 104.65, 118.76, 120.65, 123.65, 123.97, 125.03, 127.43, 128.20, 129.43, 129.54, 130.43, 132.43, 144.32, 150.43, 155.43, 195.54. MS (EI), m/z (%) = 383 (M+, 70), 276 (92). HRMS (EI) Found: M+, 383.2109. C26H25NO2 requires M+, 383.1921. Anal Calcd for C26H25NO2: C, 81.43; H, 6.57; N, 3.65. Found: C, 81.21; H, 6.65; N, 3.86.
4.3.3 10,10-Dimethyl-7-(4-nitrophenyl)-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4f)
0.378 g (95%); yellow solid; mp 241–242 °C. IR (KBr): 3306, 3065, 2897, 1643, 1567 (C = O), 1512, 1421, 1142, 819 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.18 (s, 3H, CH3), 1.41 (s, 3H, CH3), 2.08–2.34 (dd, 2H, C9-H), 2.56–2.83 (dd, 2H, C11-H), 5.89 (s, 1H, C7-H), 7.18–7.98 (m, 9H, Ar-H), 8.74 (d, 1H, J = 8.0, C6-H), 9.67 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 27.21, 29.33, 31.43, 33.43, 42.49, 52.55, 66.54, 74.88, 88.76, 106.88, 117.43, 121.33, 122.21, 123.30, 124.21, 126.10, 129.11, 129.43, 129.42, 131.34, 133.54, 147.21, 153.65, 157.87, 196.33. MS (EI), m/z (%) = 398 (M+, 70), 196 (94). HRMS (EI) Found: M+, 398.2002. C25H22N2O3: requires M+, 398.1611. Anal Calcd for C25H22N2O3: C, 75.36; H, 5.57; N, 7.03. Found: C, 75.30; H, 5.87; N, 7.21.
4.3.4 10,10-Dimethyl-7-(2-nitrophenyl)-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4g)
0.378 g (93%); yellow solid; mp 228–229 °C. IR (KBr): 3318, 3098, 2921, 1651, 1532 (C = O), 1505, 1434, 1154, 832 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.16 (s, 3H, CH3), 1.38 (s, 3H, CH3), 2.10–2.41 (dd, 2H, C9-H), 2.58–2.89 (dd, 2H, C11-H), 5.91 (s, 1H, C7-H), 7.14–8.03 (m, 9H, Ar-H), 8.82 (d, 1H, J = 8.0, C6-H), 9.83 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ δ 25.43, 30.23, 32.54, 36.42, 40.87, 53.33, 67.34, 77.41, 88.76, 119.55, 120.22, 121.66, 122.41, 124.09, 125.31, 125.87, 129.09, 129.65, 130.42, 132.76, 135.31, 148.43, 156.72, 158.34, 197.67. MS (EI), m/z (%) = 398 (M+, 70), 196 (95). HRMS (EI) Found: M+, 398.1342. C25H22N2O3 requires M+, 398.1611. Anal Calcd for C25H22N2O3: C, 75.36; H, 5.57; N, 7.03. Found: C, 75.16; H, 5.65; N, 7.09.
4.3.5 7-(4-Fluorophenyl)-10,10-dimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4h)
0.352 g (95%); white solid; mp 218–219 °C. IR (KBr): 3315, 3069, 2973, 1640, 1531 (C = O), 1514, 1439, 1125, 836 m−1. 1H NMR (DMSO-d6, 500 MHz): 1.10 (s, 3H, CH3), 1.29 (s, 3H, CH3), 2.08–2.34 (dd, 2H, C9-H), 2.45–2.81 (dd, 2H, C11-H), 5.92 (s, 1H, C7-H), 7.11–7.89 (m, 9H, Ar-H), 8.62 (d, 1H, J = 7.5, C6-H), 9.61 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ δ 27.32, 31.03, 33.04, 37.23, 44.92, 58.41, 69.29, 78.44, 89.70, 119.02, 120.44, 120.89, 123.07, 124.26, 125.04, 125.89, 129.12, 129.54, 131.07, 133.52, 139.54, 149.43, 157.56, 159.42, 198.98. 19F NMR (DMSO-d6, 470 MHz): −60.09. MS (EI), m/z (%) = 371 (M+, 70), 276 (92). HRMS (EI) Found: M+, 371.2101. C25H22FNO requires M+, 371.1703. Anal Calcd for C25H22FNO: C, 80.84; H, 5.97; N, 3.77. Found: C, 80.42; H, 6.05; N, 3.73.
4.3.6 7-(2-Fluorophenyl)-10,10-dimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4i)
0.356 g (96%); pale yellow solid; mp231–233 °C. IR (KBr): 3373, 3053, 2983, 1674, 1530 (C = O), 1520, 1423, 1145,823 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.12 (s, 3H, CH3), 1.42 (s, 3H, CH3), 2.05–2.35 (dd, 2H, C9-H), 2.42–2.65 (dd, 2H, C11-H), 6.05 (s, 1H, C7-H), 7.21–8.11 (m, 9H, Ar-H), 8.74 (d, 1H, J = 8.0, C6-H), 9.65 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 29.43, 32.43, 35.04, 39.54, 44.56, 59.54, 68.65, 75.87, 89.43, 118.54, 121.44, 121.99, 123.65, 124.54, 125.43, 125.65, 129.43, 129.84, 131.37, 133.62, 141.54, 147.43, 154.58, 159.54, 197.87. 19F NMR (DMSO-d6, 470 MHz): −61.03. MS (EI), m/z (%) = 371 (M+, 70), 276 (95). HRMS (EI) Found: M+, 371.165709. C25H22FNO requires M+, 371.1708. Anal Calcd for C25H22FNO: C, 80.84; H, 5.97; N, 3.77. Found: C, 80.61; H, 5.65; N, 3.83.
4.3.7 10,10-Dimethyl-7-(4-(trifluoromethyl)phenyl)-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4j)
0.408 g (97%); white solid; mp 276–277 °C. IR (KBr): 3332, 3076, 2971, 1651, 1522 (C = O), 1525, 1474, 1124, 822 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.11 (s, 3H, CH3), 1.23 (s, 3H, CH3), 2.05–2.32 (dd, 2H, C9-H), 2.43–2.84 (dd, 2H, C11-H), 5.97 (s, 1H, C7-H), 7.17–8.12 (m, 9H, Ar-H), 8.73 (d, 1H, J = 7.5, C6-H), 9.45 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 26.34, 29.42, 31.54, 33.65, 42.76, 55.65, 68.65, 76.54, 85.43, 88.65, 105.43, 119.54, 121.43, 123.98, 124.54, 126.32, 127.08, 128.21, 129.21, 129.76, 131.32, 133.83, 137.96 (q, 1JCF = 255.76 Hz), 147.34, 156.43, 158.09, 198.56. 19F NMR (DMSO-d6, 470 MHz): −111.2. MS (EI), m/z (%) = 421 (M+, 70), 276 (98). HRMS (EI) Found: M+, 421.2106. C26H22F3NO requires M+, 421.1709. Anal Calcd for C26H22F3NO: C, 74.10; H, 13.52; N, 3.32. Found: C, 74.54, 13.21; H, 3.45.
4.3.8 10,10 -Dimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4k)
0.243 g (88%); yellow solid; mp 187–189 °C. IR (KBr): 3323, 3065, 2937, 1665, 1548 (C = O), 1512, 1423, 1129, 823 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.09 (s, 3H, CH3), 1.23 (s, 3H, CH3), 2.15–2.43 (dd, 2H, C9-H), 2.48–2.87 (dd, 2H, C11-H), 6.18 (s, 1H, C7-H), 7.08–7.78 (m, 6H, Ar-H), 8.65 (d, 1H, J = 8.0, C6-H), 9.76 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 28.31, 29.50, 33.74, 38.03, 44.87, 76.98, 81.21, 89.76, 125.03, 127.43, 128.20, 129.43, 129.54, 130.43, 132.43, 144.32, 150.43, 155.43, 195.54. MS (EI), m/z (%) = 277 (M+, 70), 151 (76). HRMS (EI) Found: M+, 277.1509. C19H19NO requires M+, 277.1501. Anal Calcd for C19H19NO: C, 82.28; H, 6.90; N, 5.05. Found: C, 82.34; H, 6.65; N, 5.36.
4.3.9 7,10,10-Trimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4l)
0.253 g (87%); yellow solid; mp 191–192 °C. IR (KBr): 3336, 3087, 2987, 1643, 1543 (C = O), 1532, 1465, 1132, 818 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.12 (s, 3H, CH3), 1.28 (s, 3H, CH3), 2.12–2.45 (dd, 2H, C9-H), 2.55–2.60 (dd, 2H, C11-H), 3.56 (s, 3H, CH3), 6.32 (s, 1H, C7-H), 7.09–7.78 (m, 5H, Ar-H), 8.56 (d, 1H, J = 8.0, C6-H), 9.65 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 28.08, 29.23, 33.34, 37.76, 46.65, 57.88, 68.98, 79.07, 80.54, 88.76, 110.65, 125.43, 129.28, 129.74, 131.43, 138.43, 149.33, 154.43, 156.76, 196.87. MS (EI), m/z (%) = 291 (M+, 70), 166 (79). HRMS (EI) Found: M+, 291.180309. C20H21NO requires M+, 291.1608. Anal Calcd for C20H21NO: C, 82.44; H, 7.26; N, 4.81. Found: C, 82.06; H, 7.32; N, 4.43.
4.3.10 7-Isopropyl-10,10-dimethyl-9,10,11,12-tetrahydrobenzo[c]acridin-8(7H)-one (4m)
0.283 g (89%); pale yellow solid; mp 195–197 °C. IR (KBr): 3318, 3098, 2921, 1651, 1532 (C = O), 1505, 1434, 1154,832 cm−1. 1H NMR (DMSO-d6, 500 MHz): 1.13 (s, 3H, CH3), 1.31 (s, 3H, CH3), 2.03–2.25 (dd, 2H, C9-H), 2.45–2.74 (dd, 2H, C11-H), 3.45 (hep., 1H, CH), 4.56 (d, 6H, 2 × CH3), 6.21 (s, 1H, C7-H), 7.16–8.02 (m, 5H, Ar-H), 8.72 (d, 1H, J = 7.5, C6-H), 9.65 (s, 1H, NH). 13C NMR (DMSO-d6, 125 MHz): δ 26.31, 28.56, 30.54, 32.43, 33.27, 34.65, 35.53, 40.09, 51.43, 64.87, 76.98, 81.21, 89.76, 128.20, 129.43, 129.54, 131.04, 131.23, 143.32, 152.65, 153.43, 194.60. MS (EI), m/z (%) = 319 (M+, 70), 276 (90), 193 (45). HRMS (EI) Found: M+, 319.1908. C22H25NO requires M+, 319.1932. Anal Calcd for C22H25NO: C, 82.72; H, 7.89; N, 4.38. Found: C, 82.56; H, 7.65; N, 4.66.
Acknowledgements
We thank the Payame Noor University (PNU), Tehran, Iran, for financial support. Also, I express special thanks to my wife (Dr. A. Hashemi) and my daughters (Niloofar and Niki).


