1 Introduction
Organocatalysis is the acceleration of chemical reactions with a substoichiometric amount of organic compounds which do not contain even a small amount of enzyme or inorganic element [1].
The use of water as a medium for organic reactions has a number of potential advantages: (i) it is the cheapest solvent available on earth; (ii) it is non-hazardous to the environment and non-toxic; (iii) isolation of the organic products can be performed by simple phase separation [2]. Moreover, when a water soluble catalyst is used, the insoluble products can be separated by simple filtration and the catalyst system can be recycled. Therefore, development of a catalyst system that is not only stable in water, but also completely soluble in it is highly desirable.
Indole is widely distributed in nature and provides a basic framework for a number of structural and functional units in plants and animals as well as in many compounds that show pharmacological and biological activities [3]. The bis(indolyl) methanes (BIMs) are found in cruciferous plants and are known to promote beneficial estrogen metabolism [4]. They are also effective in the prevention of cancer due to their ability to modulate certain cancer causing estrogen metabolites [5]. In recent years, syntheses of this class of molecules under mild conditions have been reported [6]. However, most of the existing methods involve toxic metal ions and solvent, high cost, high catalyst loading, corrosive reagents, large amounts of solid supports and cumbersome work-up procedures. Consequently, new procedures that address these drawbacks are desirable.
2 Result and discussion
The calixarenes are a class of cyclooligomers formed via a p-substituted phenols and formaldehyde condensation. The p-sulfonic acid calixarenes have been reported as catalysts in Mannich-type reactions [7], allylic alkylation reactions [8] and esterification reactions [9].
In continuation of our previous study in organic methodology using acidic catalysis [10], herein we describe an efficient and green method for the synthesis of BIMs 2 with a variety of aldehydes and ketones in water and under solvent-free conditions using p-sulfonic acid calix[4]arene as organocatalyst (Scheme 1).
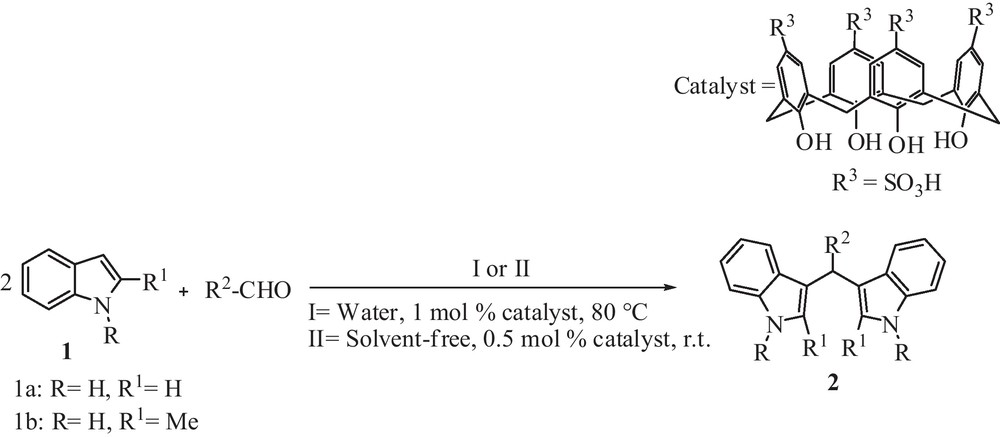
Preparation of BIMs.
We initiated our survey with producing parent calixarene according to Shinkai et al. procedure published in literature using p-tertbutylphenol [11]. The completion of the sulfonation was determined by controlling the complete dissolution of the aliquots in water. After purification of the product according to the procedure described in the literature, the obtained product was used as an acidic organocatalyst (Fig. 1).

Preparation of p-sulfonic acid calix[4]arene.
In order to determine the best reaction conditions, we studied the reaction of indole with benzaldehyde in the presence of different amounts of p-sulfonic acid calix[4]arene under different conditions (Table 1). As could be seen in Table 1, water was found to be a suitable solvent in the presence of 1 mol % of the catalyst (Table 1, entry 10). So for the synthesis of our target compounds, solvent-free condition was also selected and as could be seen in Table 1, good results were obtained (Table 1, entry 5).
Determining the best reaction conditions for treatment of indole with benzaldehyde and different amounts p-sulfonic acid calix[4]arenea.
| Entry | Catalyst | Solvent | Time (min) | Yield (%)b |
| 1 | 0.5 mol % | CH3CN | 30 | 80 |
| 2 | 0.5 mol % | EtOH | 60 | 70 |
| 3 | 0.5 mol % | CH2Cl2 | 45 | 60 |
| 4 | 0.3 mol % | None | 10 | 80 |
| 5 | 0.5 mol % | None | 10 | 95 |
| 6 | 1 mol | None | 10 | 95 |
| 7 | 0.5 mol % | H2O/r.t. | 6 (h) | 5 |
| 8 | 0.5 mol % | H2O/60 °C | 30 | 50 |
| 9 | 0.5 mol % | H2O/80 °C | 30 | 85 |
| 10 | 1 mol % | H2O/80 °C | 20 | 85 |
| 11 | 0.5 mol % | H2O – 5 mol % CTABc | 6 (h) | 5 |
a Reaction condition: indole (1 mmol), PhCHO (0.5 mmol), catalyst, r.t.
b Isolated yield.
c CTAB (N-acetyl-N,N,N-trimethylammonium bromide).
These results prompted us to investigate the scope and the generality of this new protocol for various aldehydes and ketones under optimized conditions (Table 2). A series of aromatic, aliphatic and heterocyclic aldehyde underwent smooth transformation to afford a wide range of substituted BIMs in high to excellent yield.
Synthesis of BIMs by the reaction of indole with aldehydes and ketones in the presence of p-sulfonic acid calix[4]arene under aqueous or solvent-free conditions.
| Entry | Carbonyl compound | Indole | Solvent-free | Water | Ref. | ||
| Time (min) | Yield (%) | Time (min) | Yield (%) | ||||
| 1 | 1a | 10 | 95 | 20 | 85 | [6e] | |
| 1b | 9 | 94 | 18 | 90 | [6v] | ||
| 2 | 1a | 10 | 95 | 25 | 88 | [6e] | |
| 1b | 8 | 95 | 20 | 85 | [6v] | ||
| 3 | 1a | 12 | 93 | 25 | 85 | [6e] | |
| 1b | 10 | 95 | 18 | 88 | [6y] | ||
| 4 | 1a | 14 | 93 | 30 | 80 | [6e] | |
| 1b | 12 | 90 | 25 | 82 | [6c] | ||
| 5 | 1a | 12 | 97 | 25 | 85 | [6e] | |
| 1b | 10 | 95 | 18 | 84 | [6y] | ||
| 6 | 1a | 10 | 95 | 28 | 86 | [6z] | |
| 1b | 9 | 92 | 20 | 85 | [6z] | ||
| 7 | 1a | 7 | 98 | 18 | 90 | [6e] | |
| 1b | 5 | 97 | 15 | 92 | [6l] | ||
| 8 | 1a | 8 | 98 | 17 | 95 | [6y] | |
| 1b | 6 | 98 | 14 | 95 | [6y] | ||
| 9 | 1a | 13 | 90 | 18 | 85 | [6e] | |
| 10 | 1a | 12 | 90 | 20 | 85 | [11] | |
| 1b | 10 | 95 | 15 | 80 | [12] | ||
| 11 | 1a | 15 | 95 | 23 | 90 | [6z] | |
| 1b | 12 | 90 | 18 | 85 | [6z] | ||
| 12 | 1a | 12 | 85 | 30 | 85 | [6l] | |
| 13 | 1a | 12 | 75 | 20 | 90 | [6e] | |
| 14 | 1a | – | – | 25 | 85 | [6e] | |
| 15 | 1a | – | – | 30 | 80 | [6e] | |
| 16 | 1a | – | – | 35 | 80 | [6e] |
The reactions of aromatic aldehydes having electron withdrawing groups (Table 2, entries 7–8) were somewhat faster than electron donating groups (Table 2, entries 2–6). The aliphatic aldehydes underwent the reaction cleanly in excellent yield (Table 2, entry 13). Unsaturated aldehydes afforded corresponding BIMs without by-products (Table 2, entry 9). A rapid condensation of various carbonyl compounds with active 2-methyl indole under the same reaction condition was observed (Table 2, entries 1–8 and 10–11). The reactions of ketones with indole under solvent-free conditions were not formed corresponding BIMs, but in water at 80 °C afforded in good yield (Table 2, entries 14–16).
Interestingly, when we used terephthaldialdehyde 3, p-bis-indolylmethane benzaldehyde 4 [12] was produced in excellent yield. When four molar equivalents of indole were used, p-di(bis-indolylmethane)benzene 5 [12] was obtained in high yield (Scheme 2).

Preparation of di(bis-indolyl)methane.
Both methods are also highly chemoselective for aromatic aldehydes in the presence of ketones. When a 1:1 mixture of benzaldehyde and acetophenone was allowed to react with indole in the presence of catalyst, it was found that the benzaldehyde was chemoselectively converted to the corresponding bis(indolyl)methane, while acetophenone did not give the corresponding product in water and under solvent-free conditions (Scheme 3).

Chemoselectively in preparation of BIMs.
The reaction was clean and the products were obtained in high yields without the formation of any by-products. All the BIMs prepared were known compounds and their structures were confirmed by their physical properties and 1H and 13C NMR spectra and comparison with authentic samples. The catalyst was simply recovered from the reaction mixture by treating the precipitate with deionized water to dissolve the catalyst. Finally, on filtration, water was evaporated and the organocatalyst dried and reused in successive reactions. The recycled catalyst was found to be highly efficient even after four times without significant loss of catalytic activity.
3 General procedure
Method І: A mixture of indole (1.0 mmol), aldehyde or ketone (0.5 mmol) and p-sulfonic acid calix[4]arene (1 mol %) were added to 3 ml of water and the mixture was stirred in around bottomed flask at 80 °C for the appropriate time (Table 2). The progress of the reaction was monitored by TLC (n-hexane/acetone 4:1). After completion of the reaction, the resulting solid (crude product) was filtered and then recrystallized from ethanol–water to afford pure product.
Method ІІ: A mixture of indole (1.0 mmol), aldehyde or ketone (0.5 mmol) and p-sulfonic acid calix[4]arene (0.5 mol %) under solvent-free conditions was stirred at room temperature for the appropriate time (Table 2). The progress of the reaction was monitored by TLC (n-hexane/acetone 4:1). After completion of the reaction, the mixture reaction was added water (1 ml) and the solid obtained was filtered and recrystallized from a mixture of diethyl ether-ethyl acetate to obtain pure product.
The physical data (mp, NMR, IR) of these known compounds were found to be identical with those reported in the literature. Spectroscopic data for selected examples are shown below.
3,3′-(phenyl methylene)bis(1H-indole) (Table 2, entry 1) Pink solid, m.p.: 123–126 °C. IR (KBr): 3412, 3150, 2966, 1610, 1425 and 1112 cm−1. 1H NMR (400 MHz, CDCl3): δH (ppm) 5.48 (s, 1H, CH), 6.65 (s, 2H, NHCH), 7.11 (dd, 2H, J = 7.9 Hz, CH), 7.17 (dd, 2H, J = 7.9 Hz, CH), 7.21 (m, 1H, CH), 7.27 (m, 2H, CH), 7.33 (d, 4H, J = 8.0 Hz, CH), 7.48 (d, 2H, J = 8.0 Hz, CH), 7.79 (br s, 2H, NH). 13C NMR (100.6 MHz, CDCl3): δC (ppm) 36.6 (CH), 110.1 (CH), 111.7 (CH), 118.5 (C), 119.4 (CH), 121.1 (CH), 125.1(C), 127.3 (CH), 128.5 (CH), 129.6 (C), 129.8 (C), 138.0 (C), 145.8 (C). MS (EI): m/z 322 [M]+. Anal. calcd. for C23H18N2: C, 85.68; H, 5.63; N, 8.69. Found: C, 85.75; H, 5.83; N, 8.54.
3,3′-((4-chlorophenyl)methylene)bis(2-methyl-1H-indole) (Table 2, entry 6) Pink solid, m.p.: 130–137 °C. IR (KBr): 3408, 3103, 2958, 1625, 1459 and 1156 cm−1. 1H NMR (400 MHz, CDCl3): δH (ppm) 2.09 (s, 6H), 5.97 (s, 1H), 6.87–7.28 (m, ArH, 12H), 7.78 (br s, 2H, NH). 13C NMR (100.6 MHz, CDCl3): δC (ppm) 12.5 (CH3), 38.7 (CH), 110.0 (CH), 112.8 (C), 119.2 (CH), 120.7 (CH), 128.2 (C), 128.7 (CH), 130.0 (CH), 131.6 (CH), 131.9 (C), 135.0 (C), 142.3 (C). MS (EI): m/z 384 [M]+. Anal. calcd. for C25H21ClN2: C, 78.01; H, 5.50; N, 7.28. Found: C, 77.87; H, 5.45; N, 7.13.
1,4-bis(di(1H-indol-3-yl)methyl)benzene (Scheme 2, 5) Pink solid, m.p.: 197–198 °C. IR (KBr): 3405, 3052, 2949, 1612, 1452 and 1259 cm−1. 1H NMR (400 MHz, DMSO-d6): δH (ppm) 5.84 (s, 2H), 6.95 (s, 4H), 7.05 (t, 4H, J = 7.5 Hz), 7.16 (t, 4H, J = 7.6 Hz), 7.24–7.40 (m, 12H), 7.31 (br s, 4H, NH). 13C NMR (100.6 MHz, DMSO-d6): δC (ppm) 30.1 (2 CH), 111.5 (CH), 118.5 (C), 118.9 (CH), 119.8 (CH), 121.3 (CH), 123.8 (CH), 127.2 (C), 128.5 (CH), 138.1 (C), 143.9 (C). MS (EI): m/z 566 [M]+. Anal. calcd. for C40H30N4: C, 84.78; H, 5.34; N, 9.89. Found: 84.55; H, 5.44; N, 9.78.
3,3′-(cyclohexane-1,1-diyl)bis(1H-indole) (Table 2, entry 18) Pink solid, m.p.: 116–118 °C. IR (KBr): 3452, 3035, 2925, 1608, 1495 and 1229 cm−1. 1H NMR (400 MHz, CDCl3): δH (ppm) 1.38–1.95 (m, 6H), 2.48–2.55 (m, 4H), 6.81 (s, 2H), 7.10–7.61 (m, 8H), 7.75 (br s, NH, 2H). 13C NMR (100.6 MHz, CDCl3): δC (ppm) 25.9 (2 CH2), 28.4 (CH2), 37.2 (2 CH2), 39.3 (C), 110.1(CH), 117.5 (CH), 119.2 (CH), 120.4 (CH), 121.3 (C), 123.8 (C), 134.2 (CH), 127.6 (C), 138.9 (C). MS (EI): m/z 314 [M]+. Anal. calcd. for C22H22N2: C, 84.04; H, 7.05; N, 8.91. Found: C, 83.98; H, 7.10; N, 8.83.
4 Conclusions
In conclusion, in this study, a simple, efficient, green, and eco-friendly procedure is described for the chemoselectivity synthesis of bis(indolyl)methanes in water and under solvent-free conditions using p-sulfonic acid calix[4]arene as a reusable organocatalyst. Also, good to high yields and high chemoselectivity of this protocol provide a low cost procedure for the synthesis of these compounds.
Acknowledgment
This research is supported by the Islamic Azad University, Ayatollah Amoli Branch.


