1 Introduction
Multicomponent reactions (MCRs) have developed as a powerful tool for delivering the molecular diversity needed in the combinatorial approaches for the synthesis of interesting heterocyclic scaffolds [1]. The MCRs are highly convergent processes that have been extensively employed in the synthesis of complex molecules that are particularly useful for the creation of diverse chemical libraries of ‘drug-like’ molecules for biological screening [2].
Indolizidines with different degrees of unsaturation constitute the core structural element found in a large family of natural alkaloids that occupy an important and privileged position in modern organic chemistry due to their remarkable and attractive pharmacological properties [3]. The indolizidine ring systems and spirooxindole derivatives containing the indolizidine moiety, found in a large number of compounds, display important biological activities, as exemplified by those shown in Fig. 1 [4,5].
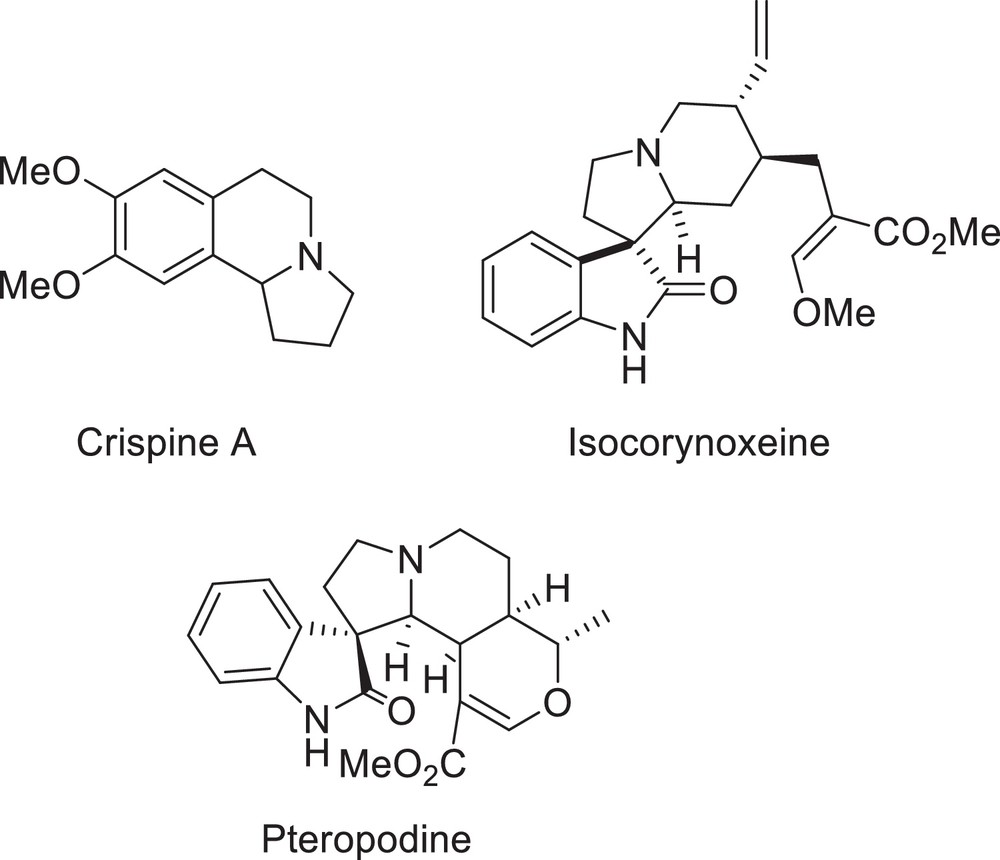
Biologically important molecules containing the indolizidine ring.
In order to understand the structure–activity relationship (SAR) as well as to improve the efficacy of the indolizidine skeleton as an anticancer agent and biologically active molecules, a flexible approach for the synthesis of different derivatives of these classes of molecules is in great demand. Recently, many synthetic methods have been developed for the synthesis of new pyrrolidine and indolizidine alkaloids that were found to exhibit superior biological and pharmacological activities with therapeutic potential [6].
1,3-Dipolar cycloadditions are fundamental processes in organic chemistry, and have taken an important place as a synthetic strategy for the synthesis of bioactive compounds [7], natural products, and alkaloids [8]. 1,3-Dipolar cycloaddition of azomethine ylides with various dipolarophiles become extremely interesting when the absolute configuration of the newly created stereocentres can be controlled in an enantio- and stereoselective manner [9,10]. These aspects of azomethine ylides have been successfully applied in the total synthesis of biologically interesting molecules.
Nitropyrrolidines have been synthesized by various methods, starting from nitro olefins [11]. Recently, the synthesis of spiropyrrolidines from the reaction between β-nitrostyrene and the azomethine ylides generated decarboxylatively from isatin/ninhydrin and sarcosine/proline was reported [12]. However, among several methods available for generating azomethine ylides [13], only a few reports on its preparation through 1,5-prototropic shift have so far appeared in the literature [14]. In this regard, and as a part of our program aimed at developing studies in the synthesis of heterocyclic systems by 1,3-dipolar cycloaddition reaction [14,15], we studied the regio- and stereoselective synthesis of novel spiroindolizidines via the one-pot, three-component condensation of trans-β-nitrostyrenes with azomethine ylides generated by a 1,5-prototropic shift route.
2 Results and discussion
In our first attempt, the reaction of ninhydrin 1 with 1,2,3,4-tetrahydroisoquinoline 2 in boiling ethanol led to the formation, by a 1,5-prototropic shift route, of an azomethin ylide that readily underwent a 1,3-dipolar cycloaddition reaction with β-nitrostyrene 3a to give a single cycloadduct 4a (Scheme 1). Encouraged by this success, we extended the scope to the reaction of ninhydrin 1 and 1,2,3,4-tetrahydroisoquinoline 2 with various derivatives of β-nitrostyrene 3b–i with both electron-withdrawing and electron releasing-substituents under similar conditions; the corresponding novel spiroindolizidines 4b–i containing the oxindole ring system were synthesized in high yields (Table 1).
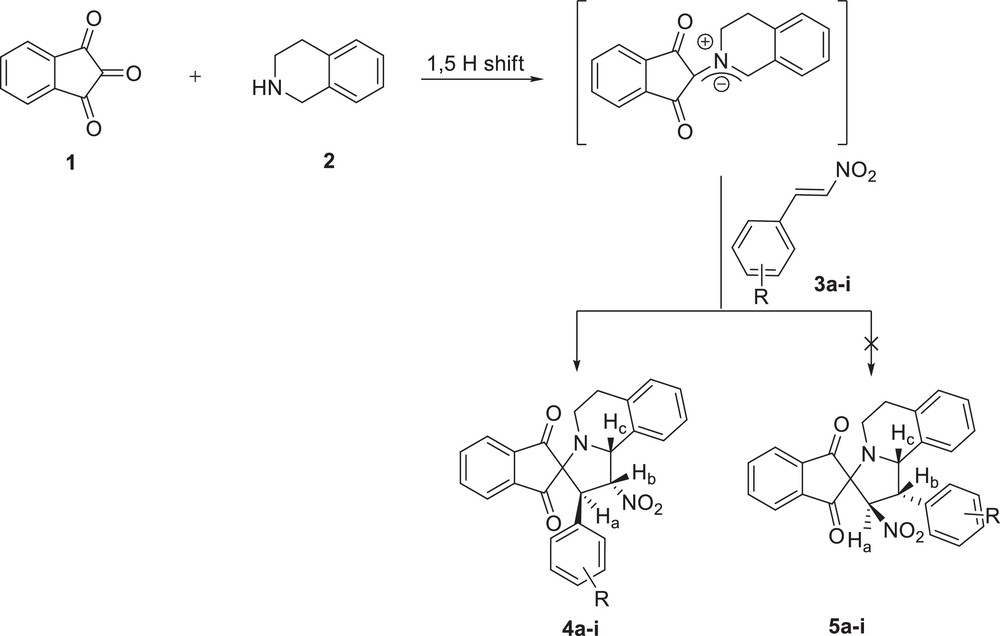
Regioselective synthesis of spiroindolizidines 4a–i.
Synthesis of spiroindolizidines 4a–i.
| Entry | R1 | Product | Yielda (%) |
| 1 | H | 4a | 92 |
| 2 | 2-Cl, 5-NO2 | 4b | 94 |
| 3 | m-OCH3 | 4c | 92 |
| 4 | p-OCH3 | 4d | 88 |
| 5 | p-CH3 | 4e | 82 |
| 6 | p-NO2 | 4f | 84 |
| 7 | p-F | 4g | 87 |
| 8 | p-Cl | 4h | 95 |
| 9 | p-CN | 4i | 92 |
a Isolated yield.
The structures and regiochemistry of the cycloadducts were established by spectral analysis. The 1H NMR spectrum of 4a evidenced two doublets at δ 4.47 (J = 5.2 Hz) and δ 5.61 (J = 6.8 Hz) for Ha and Hc protons, respectively. The CHNO2 proton Hb resonated at δ 6.56 (J = 5.2, 6.8 Hz), as a doublet of doublet (not a doublet, as expected for 5a), which clearly confirmed the correct regiochemistry of the product 4a (Scheme 1). The 13C NMR showed a signal at δ 64.8 ppm due to the spiro carbon and two peaks at δ 198.3 and δ 201.1 ppm for the ninhydrin carbonyl carbons.
In contrast with our previous report [13], in which we subjected (E)-1-phenyl-2-nitropropene 6 to these reaction conditions, no regioselectivity inversion was observed and the corresponding spiroindolizidine 7 was obtained as a sole product in 84% yield (Scheme 2). In the 1HNMR spectrum of 7, the appearance of two singlets attributed to the Ha and Hb protons instead of two doublets expected for regioisomer 8 clearly confirms the correct regiochemistry of the cycloaddition reaction.
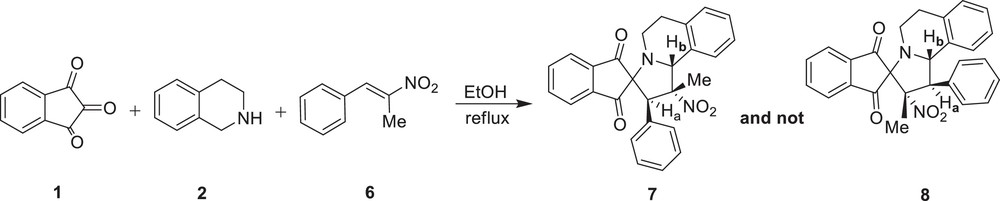
Regioselective synthesis of spiroindolizidine 7.
The structure and regiochemistry of the spirocycloadduct 4d was further confirmed through X-ray diffraction studies (Fig. 2). The ORTEP diagram of 4d indicates that the cycloaddition proceeded via an endo-transition state. The proposed mechanism is shown in Scheme 3.
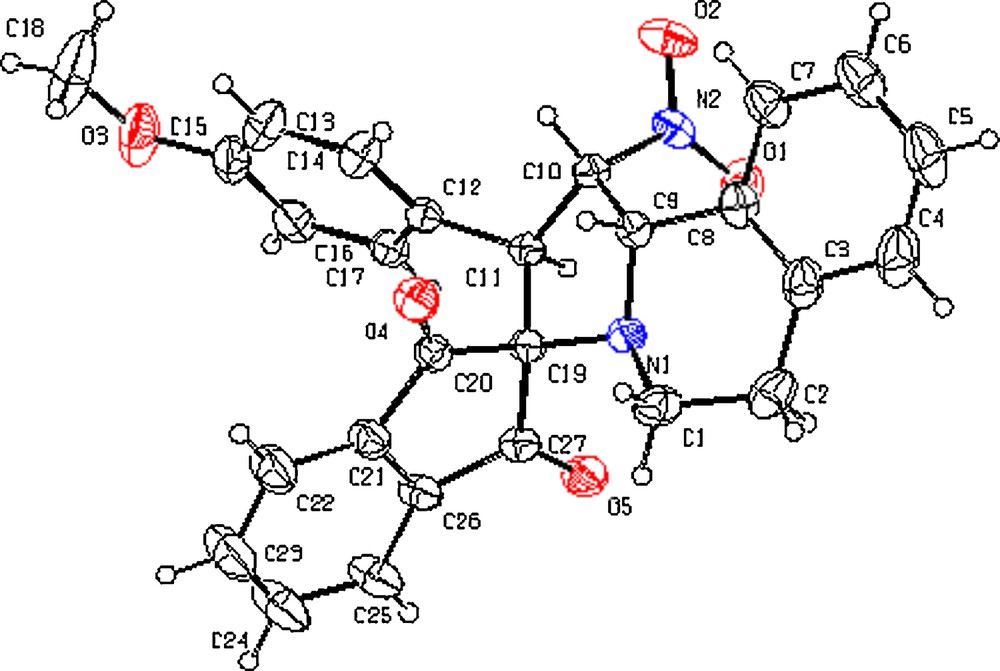
ORTEP diagram of 4d. Color available online.

Proposed mechanism for the synthesis of spiroindolizidines.
Furthermore, the possibility of the formation of other stereoisomer via an exo-transition state was ruled out with a differential NOE experiment carried out on 7. The irradiation of CH3 enhances the signal of Hb, while no correlation was observed between CH3 and Ha. This observation reveals the cis disposition of the methyl group with Hb and a trans geometry with Ha. Therefore, the correct stereochemistry of compound 7 is shown in Fig. 3.

NOE correlations of cycloadduct 7.
In addition, to explore the potential of this protocol in spiroheterocycle synthesis, the reaction of 1,2,3,4-tetrahydroisoquinoline 2 and isatin derivatives 9a–d with β-nitrostyrene 3a and β-methyl-β-nitrostyrene 6 was investigated in a one-pot three-component process (Scheme 4). The reaction afforded a series of novel spiroindolizidines 10a–h as major products in a similar regio- and stereocontrolled manner (Table 2).
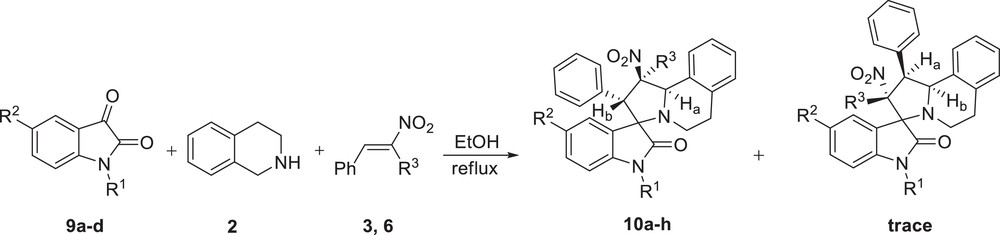
Regioselective synthesis of spiroindolizidines 10a–h.
Synthesis of spiroindolizidines 10a–h.
| Entry | R1 | R2 | R3 | Product | Yielda (%) |
| 1 | H | H | H | 10a | 82 |
| 2 | H | Br | H | 10b | 84 |
| 3 | Me | H | H | 10c | 85 |
| 4 | PhCH2 | H | H | 10d | 89 |
| 5 | H | H | Me | 10e | 82 |
| 6 | H | Br | Me | 10f | 91 |
| 7 | Me | H | Me | 10g | 92 |
| 8 | PhCH2 | H | Me | 10h | 84 |
a Isolated yield.
The structure of the isolated products, fully characterized by the spectroscopic data, was found consistent with the assigned structure. The stereochemistry of cycloadduct 10h was unequivocally determined by single-crystal X-ray crystallography (Fig. 4). The crystal structures analysis data are summarized in Table 3. Moreover, observation of a clear NOE between the CHNO2 proton and Ha of 10a in the differential NOE experiment further confirmed this stereochemistry.
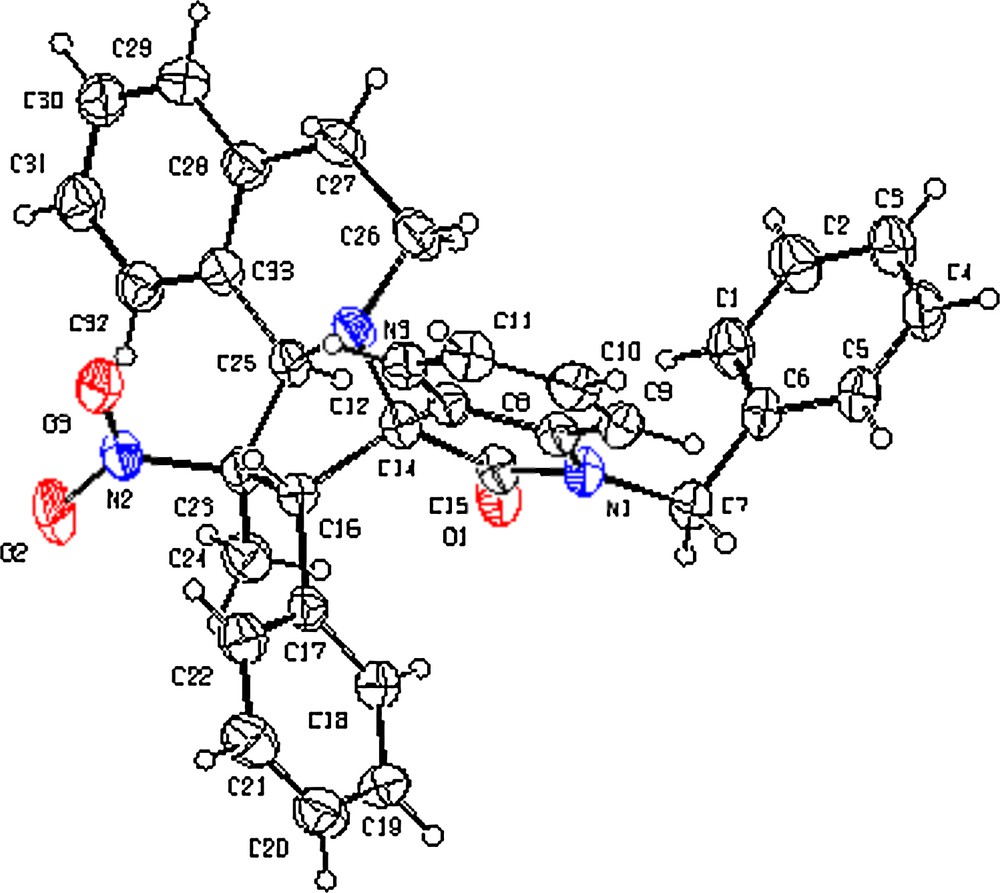
ORTEP diagram of 10h. Color available online.
Crystallographic data.
| 4d | 10h | |
| Formula | C27H22N2O5 | C33H29N3O3 |
| M | 454.47 | 515.59 |
| Crystal size (mm) | 0.40 × 0.15 × 0.10 | 0.40 × 0.25 × 0.15 |
| Color | Yellow | Cream |
| a (Å) | 9.4522 (7) | 9.2205 (10) |
| b (Å) | 11.2223 (10) | 23.370 (3) |
| c (Å) | 11.8315 (10) | 12.7620 (13) |
| α (°) | 108.037 (7) | 90 |
| β (°) | 92.376 (7) | 102.478 (8) |
| γ (°) | 102.972 (7) | 90 |
| V (A3) | 2685.0 (5) | 2685.0 (5) |
| λ (Å) | 0.71073 | 0.71073 |
| ρcalc (g cm−3) | 1.307 | 1.276 |
| μ (mm−1) | 0.091 | 0.083 |
| Z | 2 | 4 |
| Crystal system/space group | Monoclinic/P21a | |
| Reflections collected | 13014 | 19841 |
| Reflections unique/Rmerge | 6165/0.0434 | 7238/0.1251 |
| Reflection observed [I ≥ 2 σ(I)] | 3843 | 3327 |
| Refined parameter | 307 | 352 |
| R1 (observed data) | 0.1203 | 0.1931 |
| wR2 (all data) | 0.1617 | 0.2707 |
| Flack parameter | – | – |
| Max./min. residual electron density | 0.23/–0.23 | 0.25/–0.32 |
| CCDC | 836 574 | 784 682 |
Notably, when 5-nitroisatin 11 and 1,2,3,4-tetrahydroisoquinoline 2 was treated with 3 or 6, the expected spiro compounds were not formed and only 67:33 mixtures of inseparable iminium salts (Z)-12 and (E)-12 were collected, respectively (Scheme 5). The presence of a molecular ion peak at m/z 325 (M+) in the mass spectrum of 12 confirmed the formation of the iminium salt. The geometries of the Z- and E-isomers of 12 were determined based on 1H NMR chemical shift. A singlet at δ 4.96 ppm, attributed to the benzylic proton of (Z)-12, appeared at lower field compared to (E)-12 (δ 4.92 ppm), due to a deshielding effect of the carbonyl group of the oxindole ring.

Preparation of iminium salt 12.
Given the large number of commercially available isatins and β-nitrostyrenes, the present method should be applicable to the synthesis of libraries with high diversity. We expect this method will find extensive application in the field of drug discovery, combinatorial chemistry, and diversity-oriented synthesis.
3 Conclusion
In summary, we have developed a simple and convenient synthetic route for the construction of functionalized spiroindolizidines via a 1,3-dipolar cycloaddition reaction in which the 1,3-dipoles generated by 1,5-prototropic shift is reacted with β-nitrostyrenes as dipolaraphile. This afforded the anticipated cycloadducts in excellent yields with high regio- and stereoselectivity. The major adduct formed with isatin derivatives and the only adduct formed with ninhydrin are all in good agreement with the regio- and stereochemistry of the 1,3-dipolar cycloaddition.
4 Experimental
4.1 General procedure
A mixture of ninhydrin (0.178 g, 1 mmol) or isatin (0.147 g, 1 mmol), 1,2,3,4-tetrahydroisoquinoline (0.133 g, 1 mmol), and trans-β-nitrostyrene (0.149 g, 1 mmol) in ethanol (10 mL) was stirred at reflux for 2–3 h. After completion of the reaction, as indicated by TLC, the solid was separated by filtration, washed with ethanol (3 × 5), and the pure cycloadducts were obtained by recrystallization from ethanol.
4.1.1 1′-Nitro-2′-phenyl-2′,5′,6′,10b′-tetrahydro-1′H-spiro[indene-2,3′-pyrrolo[2,1-a]isoquinoline]-1,3-dione (4a, C26H20N2O4)
Yellow solid; yield: 0.390 g (92%); M.p.: 187–189 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.64–2.75 (m, 2H, indolizine), 2.94–3.00 (m, 2H, indolizine), 4.47 (d, 1H, benzylic, J = 5.2 Hz), 5.61 (d, 1H, N–CH, J = 6.8 Hz), 6.56 (dd, 1H, CHNO2, J = 7.2 Hz, 5.2 Hz), 7.16–8.05 (m, 13H, Ar–H); 13C NMR (100 MHz, DMSO-d6): δ = 29.6, 43.5, 57.9, 64.8, 77.8, 92.3, 123.1, 123.8, 125.3, 126.2, 127.4, 128.6, 129.0, 129.2, 129.4, 132.8, 133.8, 134.9, 137.6, 138.0, 141.1, 141.6, 198.3, 201.1; IR (KBr):
4.1.2 1′-Nitro-2′-phenyl-2′,5′,6′,10b′-tetrahydro-1′H-spiro[indoline-3,3′-pyrrolo[2,1-a]isoquinolin]-2-one (10a, C25H21N3O3)
Cream solid; yield: 0.337 g (82%); M.p.: 203–204 °C; 1H NMR (400 MHz, CDCl3): δ = 2.66–2.81 (m, 3H, indolizine), 3.14 (m, 1H, indolizine), 4.52 (d, 1H, J = 4.8 Hz, benzylic), 5.86 (d, 1H, J = 6.8 Hz, N–CH), 6.14 (dd, 1H, J = 7.2, 4.8 Hz, CHNO2), 6.72 (d, 1H, J = 8 Hz, Ar–H), 7.06–7.34 (m, 11H, Ar–H), 7.72 (d, 1H, J = 7.2 Hz, Ar–H), 10.18 (s, 1H, NH); 13C NMR (100 MHz, CDCl3): δ = 29.9, 42.5, 60.3, 63.6, 75.6, 91.7, 109.7, 123.6, 124.5, 124.9, 126.1, 127.1, 127.3, 128.1, 128.4, 128.6, 129.3, 130.0, 132.7, 133.7, 135.1, 141.4, 176.8; IR (KBr):
Acknowledgements
The authors acknowledge the University of Mazandaran for financial support of this research.


