1 Introduction
Biodiesel as a biomass-based diesel fuel is a non-fossil fuel alternative to the traditional fuels used in diesel engines. Generally, methyl or ethyl fatty esters synthesized from vegetable oil or animal fats are considered a biodiesel [1,2]. Using biodiesel in diesel engines sustains air quality, since its usage produces 78% less CO2 emissions, which is the major gas causing greenhouse effect, compared to petroleum diesel, while it reduced CO2 emissions by 15% when mixing petroleum with 20% biodiesel [3].
Many studies have been carried out to obtain biodiesel from the transesterification of different vegetable oils, like soybean, sunflower, peanut, rapeseed, palm, olive, cottonseed, linseed, coconut, etc. Transesterification at 60 °C in addition to vigorous stirring at 600 rpm for Jojoba oil-wax with methanol was carried out to yield biodiesel in the presence of 1 wt% of sodium methoxide as a catalyst. A quantitative conversion of the oil was developed after 4 h for a molar methanol/oil ratio of 7.5:1 [4]. Transesterification using alkali-catalysts was widely utilized for biodiesel fuel production [5]. In the same manner, non-edible oil was used to produce biodiesel [6].
EFEs can be used as a major component of biodiesel fuel, which is a considerable alternative energy resource to petroleum [7,8]. DFAU was produced as a by-product. DFAU has attracted much attention because of its activity against bacteria, yeasts, molds, in addition to its industrial applications as a surfactant, lubricant, disinfectant, and in cosmetics, shampoos, detergents and antifoams [9]. Many researchers have studied the synthesis of fatty acyl urea from fatty acids or their esters with various amine compounds, either at elevated temperature and pressure [10] or by enzymatic synthesis [11]. The presence of long-chain fatty acids from soybean oil containing O and N donor sets suggests that DFAU should be very useful as an organic reagent for the extraction and the separation of metal ions from aqueous solutions [12]. Additionally, DFAU offers potential applications as surfactants, modifying natural clays used to produce polymer nanocomposites [13,14].
Clay is hydrophilic in nature because it contains inorganic cations on the basal planar. Its particles can only disperse on the micro-scale in a polymer matrix. Clay modification is required to improve its reinforcing ability [15]. A recent way of enhancing this ability is by making clay an organophilic material via ionic exchange of its interlayer cations with organic cations, such as the amidonium ions of DFAU.
The present study describes the production of EFEs from soybean oil by refluxing it with urea using sodium ethoxide as a catalyst.
2 Experimental
2.1 Materials
Commercial soybean oil (Nacalai Co., Kyoto, Japan) was used, as well as urea, ethanol, and sodium metal (Merck, Germany).
2.2 Synthesis of EFEs and DFAU
In a 250-mL round-bottom flask fitted with a reflux condenser and a magnetic stirrer, finely cut sodium (0.50 g) was dissolved in 100 mL of absolute ethanol. After the reaction of all sodium, 4.00 g of soybean oil were added into the solution, which was followed by the addition of 1.38 g of dried urea (pre-dried at 60 °C for 4 h) dissolved in 50 mL of hot (70 °C) ethanol. After thorough shaking, the mixture was refluxed for 8 h in an oil bath at 70 °C. The mixture was left to cool at room temperature, then was transferred into a separation funnel, and allowed to settle overnight. The thick phase of glycerol was removed from the bottom layer. The top layer, which comprises the products, was poured into a beaker and mixed with 100 mL of hot distilled water (60 °C) and 10 mL of concentrated hydrochloric acid, and stirred for 15 min.
The white mass of ethyl fatty ester was filtered out of the DFAU solution, while the clear solution containing DFAU was cooled in an ice bath. The pale yellow precipitate was collected on a Buchner funnel to be washed with 50 mL of cold water and then dried in a vacuum desiccator over phosphorous pentoxide. The conversion ratio of soybean oil into DFAU was 76%. The preparation reaction is shown in Fig. 1.

Synthesis of ethyl fatty esters and difatty acyl urea from soybean oil and urea.
The proposed mechanism for soybean oil conversion into DFAU and ethyl fatty ester is shown in Fig. 2. Catalysis by sodium ethoxide initiates proton withdrawing from (–NH2) in the urea molecule, as shown in Fig. 2A. This pathway is feasible from a physical organic point of view [10]. While Fig. 2B demonstrates it, the urea ion attacks one of the triglyceride groups and forms an N-carbamoyl fatty amide that attacks the second group of triglyceride after withdrawal of a proton by the basic catalyst. In two consecutive reactions, 2,3-dihydroxypropyl fatty ester, 1,3 dihydroxypropan-2-fatty ester (as intermediates), and DFAU are formed. The final step is displayed in Fig. 2C, which shows the alkoxy group attacking the third group of triglyceride to form ethyl fatty ester and glycerol [12].
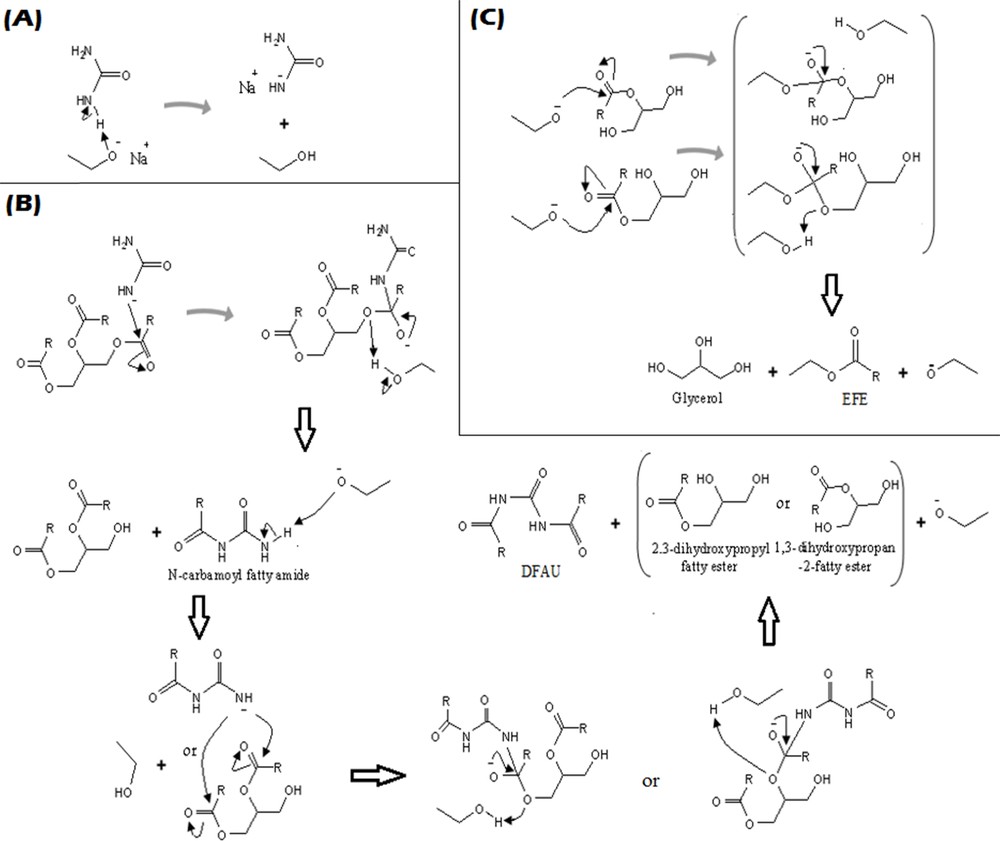
The proposed role of sodium ethoxide catalyzing the amidation and transesterification of palm oil: (A) proton withdrawing from urea, (B) amidation and (C) transesterification.
3 Results and discussion
3.1 Characterizations of EFEs and DFAU
3.1.1 FTIR spectra
EFEs and DFAU were characterized using FTIR spectroscopy (APS-1650 Automated Prep Station, PerkinElmer, USA). The characteristic FTIR peaks of soybean oil were observed at 3010, 2922, 2855, 1742, 1461, 1161, and 720 cm−1; they result from CH stretching of CHCH, CH asymmetric stretching of CH2, CH symmetric stretching of CH2, CO stretching of ester (glyceride), CH2 scissoring, OCC stretching, and CH2 rocking, respectively [13], while DFAU spectra show new bands at 3348, 1623 and 1045 cm−1 attributed to NH stretching, CO stretching, and CN stretching of amide, respectively. The fading of peaks at 1742 and 1161 cm−1 and the appearance of peaks at 3345, 1623 and 1043 cm−1 indicate the formation of fatty amides [10]. The FTIR analysis of EFEs shows major absorption bands of soybean oil, with no peaks corresponding to the amide (Fig. 3).

(Color online.) Fourier transform infrared spectra of soybean oil, ethyl fatty esters and difatty acyl urea.
3.1.2 1H NMR spectra of EFEs
The analysis of EFEs by 1H NMR technique gives the following spectral data: (400 MHz) (CDCl3): δ 0.88 (t, 3H CH3), 1.28 (m, H, CH2), 1.29 (t, 3H CH3), 1.60 (2H, CH2CH2 CO), 2.19 (4H, 2 x CH2 CHCH), 2.31 (t, 2H, CH2 CO), 4.12 (q, 2H, CH2–O), 5.81 (2H, CHCH).
3.1.3 1H NMR spectra of DFAU
The analysis of DFAU by 1H NMR technique shows a peak at 10.7 ppm related to the proton in the N–H group. The following 1H NMR data were obtained: (400 MHz) (CDCl3): δ 0.88 (t, J = 8.5 Hz, 6H, 2 × CH3), 1.20–1.27 (m, H, CH2), 1.51 (4H, 2 × CH2CH2 CO NH), 2.15 (4H, 2 × CH2 CHCH), 2.32 (t, J = 10.5 Hz, 2H, 2 × CH2CO NH), 5.83 (2H, CHCH), 10.7 (b, s, 2H, CO NH).
3.1.4 Reaction time optimization
The optimal reaction time for the synthesis of EFEs was determined by performing the reaction for different times between 2 and 12 h. Fig. 4 shows that the reaction proceeded rapidly during the first 8 h, while the maximum conversion ratio was obtained after 10 h.

(Color online.) Reaction time vs. conversion percentage of palm oil into ethyl fatty esters.
3.1.5 Effect of urea/soybean oil ratio
One of the important parameters that affect the conversion to ethyl fatty esters is the urea-to-soybean oil molar ratio. Fig. 5 shows the change in the conversion under reaction conditions as a function of the urea/soybean oil ratio (mmol/mmol). Conversion increases considerably with the increase in the urea-feeding amount. The highest conversion ratio of urea/soybean oil into EFEs was 76%, which was obtained when the molar urea/soybean oil ratio was from 6.3 mmol to 1 mmol. Further increase in urea concentration decreases reaction conversion, due to an inhibitory effect.
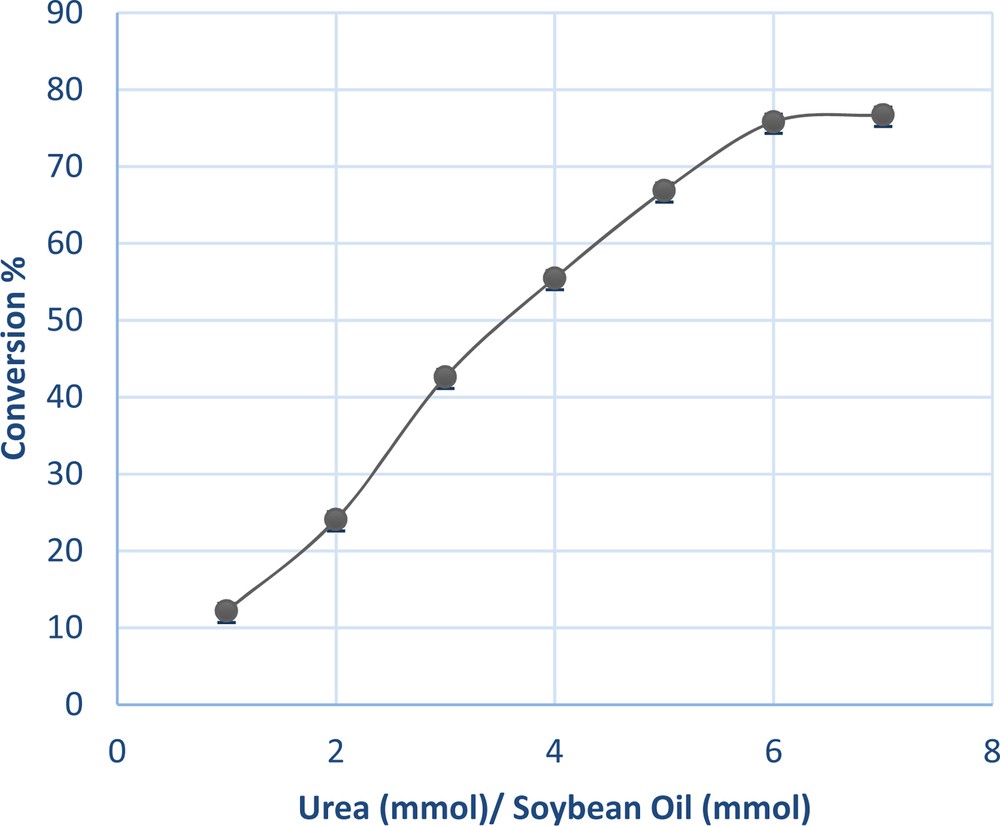
(Color online.) Effect of urea-to-soybean oil molar ratio on the conversion percentage.
3.1.6 Catalytic activity of sodium ethoxide
Ethoxide ion catalyzes amidation reactions [12]. This reaction was carried out in the presence of homogeneous sodium ethoxide to produce EFEs. The reaction proceeded rapidly at the beginning; then conversion leveled off after further increase in the amount of catalyst. The highest production of DFAU was obtained when the catalyst-to-soybean oil ratio was from 6.4 mmol to 1 mmol (Fig. 6).

(Color online.) Effect of catalyst loading on the conversion of soybean oil. Reaction conditions: urea/soybean oil molar ratio = 6.3:1.0 and reaction time = 8 h.
3.1.7 Effect of organic solvents
An organic solvent is a reaction medium where substrate and product can be soluble, facilitating the recovery of products; it may shift reaction equilibria. The effects of different organic solvents on the yield of EFEs converted from palm oil were investigated. Hexane, cyclohexane, chloroform, and heptane were individually used as organic solvents. It was found that hexane is the most effective solvent for EFEs, as shown in Fig. 7, due to the higher solubility of the substrate and to its ability to separate the product from the substrate into two unmixed phases. The effectiveness of hexane is due to its high hydrophobicity compared to that of other organic solvents (log P = 3.5) [12]. Fig. 7 displays conversion ratios versus the organic solvents implemented in the conversion of soybean oil into EFEs.

(Color online.) Conversion of soybean oil into ethyl fatty esters as a function of organic solvents.
4 Conclusion
The studied synthesis method of EFEs and DFAU allows one to obtain both biodiesel and compounds with biological activities from abundant raw materials, i.e. urea and soybean oil, using a simple and environmentally friendly process. The optimal experimental conditions that yield 76% of EFEs were as follows: catalyst-to-soybean oil ratio (mmol:mmol), 6.4:1; urea-to-soybean oil ratio (mmol:mmol), 6.2:1; reaction time, 8 h; hexane was used as an organic solvent.
Acknowledgements
The authors would like to thank Universiti Putra Malaysia (UPM) for supporting this work. All technical staffs in the Department of Chemistry, Faculty of Science, Universiti Putra Malaysia are also acknowledged for their assistance.


