1 Introduction
The adsorption of UO22+ ions is a process that has recently found many applications in the treatment of the effluents generated in the nuclear industry. The toxicity of these radioactive wastes has been recognized ever since the dawn of nuclear industry. The issue of the waste management represents a great challenge, as the negative impact on both the human health and on the environment is very serious. The lowest standard radioactive waste is fixed to 5 ppm [1,2]. In this respect, many adsorbents have been developed and proposed to deal with these wastes, such as zeolites, porous silica, activated carbon, clay, and resins [3–7]. Besides their application in the nuclear industry, these adsorbents play an important role in the industrial field and primarily those related to the environment; they have also been widely used in the petrochemical synthesis and oil refining [8,9], other diverse applications have significantly increased lately.
Among all these porous materials, the aluminosilicate- and aluminophosphate-based catalysts and their derivatives have, by far, the best technological impact due to their variety, their catalytic activity, and their behaviour as ion exchangers as well as their selective adsorption property. A-Type zeolites are commonly used as dryers in the toxic gases, purification processes [10], while those of faujasite Y and ZSM-5 types are preferred in the petrochemical field and residual oil selective catalytic cracking. However, both of these adsorbents are used in the depollution processes by adsorption/separation [11].
The zeolites are generally prepared under a hydrothermal procedure [12,13] starting from a gel whose basic constituents are silicon, aluminium, a mineralizing agent (hydroxide ion or fluoride media), and an organic template. The first artificial zeolite was synthesized according to the sequences observed in nature, and since then, this synthesis has been constantly improved in order to obtain new structures and a new shape and size-selective properties [14].
This work aims at using two aluminosilicate-types, namely 4A and P1, in the adsorption of UO22+ ions from synthetic and genuine solutions. 4A zeolite has a cubic structure with LTA-type (Na12Al12Si12O48) and having an aperture of 4 × 4 Ǻ, while P1 has a monoclinic structure with GIS type (Na4Al8Si8O32) and an aperture of 4.5 × 3.1 Ǻ [15].
We have carried out a treatment process based on both exchange reactions and adsorption. Many parameters related to these unit operations have been studied, such as temperature, solid–liquid ratio, pH, and uranyl ions concentration. Besides the kinetics, the equilibrium and the thermodynamics aspects, and as originality, we have dealt with the UO22+ ion diffusion onto the 4A and P1 zeolites. The results obtained confirmed the effectiveness of both zeolites we synthesized in nuclear waste disposal.
2 Material and method
2.1 4A and P1 zeolites synthesis and characterization
The process used in this work for the preparation of our zeolites was hydrothermal crystallization as reported in [1,2]. We used aluminium isopropoxide (Al[OCH(CH3)2]3 from Fluka) as a new alumina source, fumed silica (from Fluka) and sodium hydroxide (from Prolabo). The mixture was kept under mixing until the formation of a homogenous gel. The overall synthesis mixture was then placed into a stainless steel Teflon lined autoclave. The crystallization occurred on the one hand at a temperature of 90 °C during 72 h without maturation for the preparation of zeolite 4A and on the other hand at temperature of 100 °C during 24 h with maturation for the preparation of zeolite P1. After cooling, the products were filtered, washed many times with distilled water and eventually dried at 100 °C for 6 h.
The identification of the products was carried out by X-ray diffraction (Philips PW 1800, using Cu Kα radiation, with a 2θ scan range from 5 to 50°). The XRD patterns obtained were compared with those of reference [16] and identified as the 4A Fig. 1a and P1 Fig. 2a zeolite phases.

XRD patterns of zeolites (a) 4A and (b) exchanged UO2 4A at pH = 2.

XRD patterns of zeolites (a) P1, (b) exchanged UO2P1 at pH = 2.5.
Surface morphology was observed by using scanning electronic microscopy (Philips XL 30) equipped with energy-dispersive spectrometry for chemical analysis. The zeolites were coated with a thin film of carbon. It was found that the crystallites of 4A and P1 form as fine cubic particles with an average size of 3.5 and 1.5 μm, respectively. The anhydrous chemical compositions were also determined and the formulas obtained for both zeolites are respectively Na0.1804Si0.4118Al0.4007O2 and Na0.1192Si0.5424Al0.3383O2. The zeolite surface area was determined by the BET method and the results found ranged between 260 to 350 m2·g−1 and porous volumes of 0.16 to 0.17 mL·g−1 for P1 and 4A, respectively. An XRD study was carried out on samples after UO22+ ion exchange; it was shown that the crystallinity of the zeolites remained stable even at low pH as it is illustrated in Fig. 1b and Fig. 2b. Comparing the relative intensities of the planes corresponding to 4A and P1 zeolites before and after UO22+ ion exchange, no significant change has been observed either in the position of the most intense peaks nor in their crystallinity. In another study [1] about UO22+ removal onto synthetic NaA zeolite, a shift of inter reticular distance (d222) of about 0.04 Å was observed and diffraction planes (640) and (840) had completely disappeared due to the presence of uranium metal.
2.2 Used reagents
An amount of 1 g·L−1 uranyl nitrate stock solution was prepared by dissolving an appropriate amount of uranyl nitrate hexa-hydrated salt (UO2(NO3)2·6 H2O) in distilled water with 1 mL of concentrated nitric acid. The experiments were carried out in batch, in a set of 200-cm3 screw-cap conical flasks containing zeolites. A fixed volume of uranyl nitrate solution with different initial uranium concentrations ranging from 5 to 100 mg·L−1 is introduced into each flask. The mixture was carefully stirred by means of a rotary shaker at a rate of 350 rpm. Both solid and liquid phases were separated by centrifugal action and the filtrates collected were analyzed by UV–visible spectroscopy. Arsenazo-III was used to complex uranium species [17].
2.3 Parametric study
This part was aimed at studying of uranium adsorption onto 4A and P1 zeolites. It consists in investigating the influence of the following parameters: the contact time, the pH, the uranyl initial concentration and the temperature on the adsorption process yield. The instantaneous adsorbed uranium quantity in mg·g−1 is determined by the equation:
| qt = (C0 – Ct) = V/m | (1) |
| R(%) = [(C0 – Ct)/C0] × 100 | (2) |
Adsorption capacity of UO22+onto zeolites 4A and P1 at time t.
| UO22+ initial concentration (mg·L−1) | 5 | 10 | 20 | 30 | 50 | 60 | 80 | 100 |
| Adsorption capacity (mg·g−1) 4A | 8.5 | 12.4 | 15.2 | 18 | 21 | 21.6 | 25.6 | 32 |
| Adsorption capacity (mg·g−1) P1 | 8.3 | 16 | 31.2 | 35.4 | 53 | 62.4 | 80 | 100 |
2.4 Kinetic studies
In order to understand the mechanism of the uranium adsorption process onto 4A and P1 zeolites, three main kinetic models were tested as reported by Nibou et al. [1], the pseudo first-order and pseudo second-order ones and the intraparticle diffusion one.
2.5 Equilibrium isotherms
The Freundlich model is a purely empirical formula. The adsorption takes place in multilayer mode where the amount of adsorbed solute at equilibrium is related to the uranyl ion concentration at equilibrium by the equation:
| qe = Kf Ce1/n | (3) |
According to the Langmuir isotherm, the adsorption process occurs on the active sites of the material, and once the uranyl ions fill these sites, the adsorption stops after proceeding in monolayer mode, and can be modeled by the following equation:
| qe = Q0 b Ce/(1 + b Ce) | (4) |
The last investigated isotherm is the so-called Dubinin–Raduchkevitch (D–R) isotherm; it is more general than the Langmuir one, as in the former, we do not assume the homogeneity of the material surface or a constant adsorption potential. The D–R isotherm is represented by the following relation:
| ln qe = ln qmax – Kads ɛ2 | (5) |
| ɛ = RT log (1 + 1/Ce) | (6) |
where R is the gas constant (8.314 J·mol−1·K−1) and T is the absolute temperature. On the other hand, the value of adsorption energy (Ea) indicating the nature of adsorption has been determined through the equation:
| Ea = (2 Kads)−1/2 | (7) |
2.6 Thermodynamic study
The thermodynamic study of the uranyl adsorption onto the 4A and P1 aluminosilicates consisted primarily of determining the Gibbs free energy ΔG°ads of the process by means of the following well-known equation:
| ΔG°ads = ΔH°ads – T ΔS°ads | (8) |
The thermodynamic properties, the enthalpy ΔH°ads and the entropy ΔS°ads were determined through the plot and the linearization of ln Kd against 1/T using the combined Van’t Hoff equation:
| ln Kd = ΔS°ads/R – ΔH°ads/RT | (9) |
The distribution coefficient Kd (ml/g) is obtained as:
| Kd = (Ci – Ceq)V/m Ceq (mL/g) | (10) |
2.7 UO22+ ion diffusion study
The UO22+ ion diffusion onto 4A and P1 zeolites was studied and the diffusion coefficients during the adsorption process were determined. The Fick's second law: ∂C/∂t = ΔC, written originally in Cartesian coordinates, was converted into polar spherical coordinates and finally resolved with the use of Fick's first law. The final equation, which deals with our experimental data, can be written as follows:
| (11) |
The application of the equation (11) to the short-time-occurred processes and considering the zeolite grains as spherical isotropic particle with radius r0, Eq. (11) becomes then:
| (12) |
The adsorption capacity to infinity, q∞, is replaced by a more practical equilibrium adsorption capacity qe.
The activation energies of the diffusion, Edif (kJ·mol−1), of uranyl ions onto 4A and P1 zeolites were determined by means of Arrhenius equation applied to the adsorption process:
| (13) |
Similarly, the activation entropy was determined through Arrhenius equation applied to the diffusion:
| (14) |
3 Results and discussion
3.1 Determination of the adsorption equilibrium time
Uranium (VI) kinetic adsorption onto 4A and P1 zeolites was studied in the following conditions: [UO22+]: 10 mg·L−1, S/L ratio: 1/100, T: 293 K, and pH: 2 (4A) and 2.5 (P1). The results are shown in Fig. 3, where the representative curve of the phenomenon consists of two distinct phases. The uranium adsorbed reached a maximal value at a stirring time t = 60 min. However, the optimal equilibrium time of 120 min was retained and used in the subsequent experiments.

Effect of contact time on uranium (VI) adsorption onto 4A and P1 zeolites (T = 293 K, [UO22+] = 10 mg·L−1, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100).
3.2 Effect of pH
The influence of the pH on the adsorption of the uranyl ions onto 4A and P1 zeolites was studied in the following conditions: [UO22+]: 10 mg·L−1, S/L ratio = 1/100, T: 293 K, and contact time: 120 min, in a pH range from 2 to 11 (Fig. 4). The pH of the solution was adjusted using KOH and HCl solutions. The aqueous solution pH seems to be an important factor for controlling the process of uranium adsorption on aluminosilicates [1,3,20]. The adsorption percentage has been found maximal at pH = 2 onto 4A and at pH = 2.5 onto P1; this is mainly due to the hydrolysis of the uranyl ion and to its freedom, which means that there was no precipitated uranyl species present, and hence that adsorption was improved. However, with increasing pH, the complexes form with the prevalent uranium species (UO2(OH)+, (UO2)2(OH)22+, (UO2)3(OH)53+ and (UO2)2(OH)2), preventing adsorption [19].
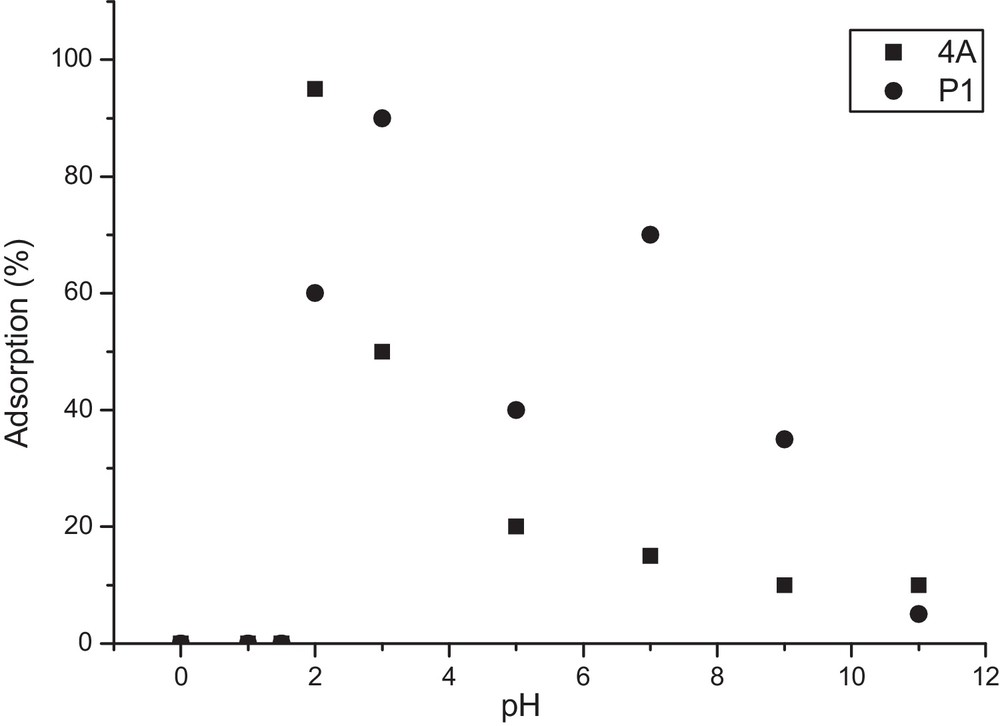
Effect of pH on uranium (VI) adsorption onto 4A and P1 zeolites. ([UO22+] = 10 mg·L−1, t = 2 h, S/L = 1/100 and T = 293 K).
3.3 Effect of uranium (VI) initial concentration
Uranium (VI) adsorption onto 4A and P1 zeolites was studied as a function of the initial uranyl concentration, which was varied in the range from 5 to 100 mg·L−1. The prevailing experimental conditions were: the ambient temperature, solid–liquid ratio = 1/100, contact time = 120 min, pH = 2 and 2.5 for 4A and P1 zeolites, respectively. The results are shown in Fig. 5. It can be seen from this figure that the adsorbed uranium percentage decreases with increasing the initial concentration; this is mainly due to the higher mobility of the UO22+ ion in diluted solutions and therefore to its higher interaction with the adsorbent.
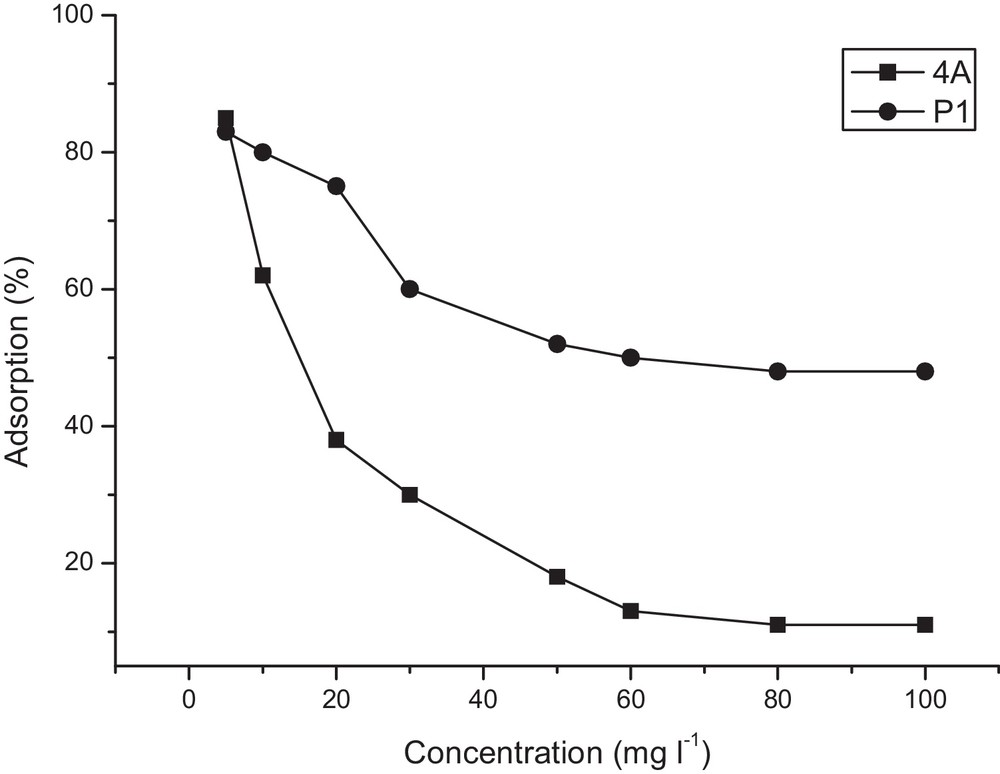
Influence of initial concentration on uranium (VI) adsorption onto 4A and P1 zeolites [T = 293 K, pH (4A) = 2, pH (P1) = 2.5, S/L = 1/100 and t = 2 h].
3.4 Effect of temperature
In order to study the influence of the temperature on the uranyl ion adsorption yield, a set of batch experiments were performed over the temperature range from 293 to 343 K. The results are shown in Fig. 6, where we notice that the temperature increase improved the adsorption process. In fact, the adsorption uranium (VI) percentage onto 4A and P1 zeolites reached 99% and 97%, respectively, at the temperature of 343 K. The process is therefore endothermic. This result is similar to that reported by Kütahyah et al. [21] in their study on uranium and thorium sorption on activated carbon.

Effect of temperature on uranium (VI) adsorption onto 4A and P1 zeolites. ([UO22+] = 10 mg·L−1, pH (4A) = 2, pH (P1) = 2.5, S/L = 1/100 and t = 2 h).
3.5 Adsorption dynamic of uranium (VI) onto 4A and P1 zeolites
The kinetic adsorption experimental study of uranium (VI) onto 4A and P1 zeolites has been carried out under the following conditions: ambient temperature, pH = 2.5, initial concentration 10 mg·L−1 and solid/liquid ratio R = 1/100.
The adsorption rate constants for all the models used, namely the pseudo first- and second-order, the intraparticle diffusion, were determined by linear regression of the data obtained experimentally. The results are shown in Fig. 7 and the figures representing the rate constants are compiled in Table 2. We notice that the adsorption is the fastest under the pseudo first-order model, which accurately fits well the experimental data. Wang et al. [22] suggested that adsorption of uranium (VI) on modified clay followed better the pseudo second-order model. In the case of clay, the interaction is mainly electrostatic.

Pseudo second-order kinetic plots for uranium (VI) adsorption onto 4A and P1 zeolites (T = 293 K, [UO22+] = 10 mg·L−1, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100).
Kinetic parameters for the adsorption of uranium (VI) onto 4A and P1 zeolites (T = 293 K, [UO22+] = 10 mg·L−1, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100).
| Zeolites | Pseudo first-order | Pseudo second-order | Intra particule diffusion | |||||||
| qe (cal) (mg g−1) | k1ads (min−1) | R2 | qe (cal) (mg g−1) | k2ads (g mg−1·mn−1) | h (mg g−1·min−1) | R2 | qe (exp) (mg g−1) | Kp (mg g−1 mn−0.5) | R2 | |
| 4A | 1.078 | 4.37 × 10−2 | 0.995 | 10.03 | 5.85 × 10−2 | 5.865 | 0.993 | 0.912 | 8.79 × 10−2 | 0.558 |
| P1 | 2.146 | 3.45 × 10−3 | 0.976 | 9.33 | 1.12 × 10−1 | 9.750 | 0.961 | 2.373 | 10.41 × 10−2 | 0.422 |
3.6 Study of the uranium adsorption equilibrium
The equilibrium adsorption isotherms are important for investigating the adsorption capacity of UO22+ onto 4A and P1 zeolites, and the type of adsorption. The different characteristic coefficients data obtained, concerning the models used: Langmuir, Freundlich and Dubinin–Raduchkevitch constants as well as the corresponding correlation coefficients are given in Table 3. According to these data, it emerges that the Langmuir model fits best the results of our adsorption experiments; the isotherms for 4A and P1, respectively, are displayed in Fig. 8. The adsorption energy (Ea) calculated from the constant of adsorption energy (Kads) is useful for estimating the type of adsorption reaction. If the value of Ea is less than 8 kJ·mol−1, the nature of adsorption is physical. But if it ranges between 8 and 25 kJ·mol−1, the adsorption is an ion exchange [23–25]. In the present case and from the data in Table 3, the Ea values for 4A and P1 zeolites indicate clearly an ion exchange mechanism.
Values of the Langmuir, Freundlich and Dubinin–Radushkevich (D–R) constants for the adsorption of uranium (VI) species onto 4A and P1 zeolites (T = 293 K, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100).
| Zeolites | Langmuir | Freundlich | Dubinin–Radshkevich (D–R) | ||||||||
| Q0 (mg·g−1) | b (L·g−1) | RL | R2 | n | kf (L·g−1) | R2 | Qmax (mg·g−1) | kads | R2 (J2·mole−2) | Ea (mg·g−1) | |
| 4A | 1 | 2.4 × 10−4 | 0.112 | 0.997 | 6.54 | 0.5 | 0.943 | 1.60 | 15.6 × 10−9 | 0.876 | 22.36 |
| P1 | 3.72 | 1.6 × 10−4 | 0.134 | 0.984 | 2.10 | 0.63 | 0.953 | 21.63 | 8.9 × 10−9 | 0.890 | 11.18 |

Langmuir isotherm for uranium (VI) adsorption onto 4A and P1 zeolites [T = 293 K, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100].
The Langmuir parameters were used to calculate the RL factor; this coefficient represents the affinity between the material and the adsorbate. The effect of the initial uranyl concentration on the RL factor was studied and the data are shown in Fig. 9. It is clear from these results that the affinity that is referred to is more important for high initial uranium (VI) concentrations. Moreover, RL data between 0 and 1 suggest that the adsorption is favoured [19,26].

Separation factor RL for uranium (VI) adsorption onto 4A and P1 zeolites [T = 293 K, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100].
3.7 Uranyl ion UO22+ diffusion study
In order to determine the uranyl ion diffusion coefficients during the adsorption process onto 4A and P1 zeolites, the Fick's laws of diffusion were used [1,18].
The plot of the quantity (qt/qe) against t1/2 (Fig. 10) and after linear regression were drawn, the diffusion coefficients Di were determined; they have been found to be 1.36 × 10−12 and 0.075 × 10−12 m2/s for zeolites 4A and P1, respectively. These results indicate that uranyl ions diffuse better in 4A zeolite, which can be explained by the difference in the chemical composition, the cations’ distribution, the Si/Al atoms ratio in the zeolite tetrahedron and the pores aperture (4A: 4 × 4 Ǻ and P1: 4.5 × 3.1 Ǻ). The diffusion activation energies were calculated from the plot of ln D versus 1/T; the values are compiled in Table 4. We notice that the activation energy of diffusion (Edif) onto P1 is smaller than that onto 4A, indicating that uranyl ion UO22+ diffuses more easily through the pores of zeolite 4A, whose porous volume is larger than that of P1 (0.16 mL·g−1 and 0.17 mL·g−1). In order to explain and to understand the experimental results, Kristou et al. [7] reported that the ability of zeolite to exchange cations with a solution is strongly related to the dimensions of the channels and the size that the UO22+ cations in solution have. The ionic radius of uranyl ion is equal to 1.8 Ǻ. This size is much less than the dimension of 4A and P1 channels, indicating that uranium (VI) ions in the solution have an access to their exchangeable sites.

qt/qe vs. t1/2 plots of the early stage of the adsorption of uranium onto 4A and P1 zeolites (T = 293 K, [UO22+] = 10 mg·L−1, pH (4A) = 2, pH (P1) = 2.5 and S/L = 1/100).
Diffusion activation energy Edif, diffusion ccoefficient D0 and activation entropy ΔS* onto 4A and P1 zeolites.
| Zeolites | Edif (kJ·mol−1) | D0 (m2·s−1) | ΔS* (J·mol−1·K−1) |
| 4A | 40.376 | 4.58 × 10−9 | –49.684 |
| P1 | 25.289 | 1.37 × 10−14 | –89.123 |
As for the entropy of activation (ΔS*), the values obtained and reported in Table 4 are negative; therefore, the mechanism under which the diffusion took place is an association mechanism. The basic internal structure of the zeolites did not undergo any change. This result reflects that a good agreement was seen on 4A and P1 zeolites X-ray patterns before and after UO22+ adsorption (Fig. 1b and Fig. 2b). Also, it is similar to that reported by Akyl et al. [3] in their study on the distribution of uranium on zeolite X and investigation of the thermodynamic parameters for this system.
3.8 Thermodynamic study
The resolution of the Van’t Hoff equation by plotting ln Kd against 1/T [1,24] led to the determination of the adsorption enthalpy and entropy ΔH°ads and ΔS°ads through linear regression, the results are shown in Table 5. The positive values of the adsorption enthalpy indicate that the uranium (VI) adsorptions onto 4A and P1 zeolites are endothermic processes, which is also indicating by increasing adsorption with increasing temperature. The negative Gibbs free energy values prove the feasibility as well as the spontaneity of the adsorption.
Thermodynamic parameters for uranium (VI) adsorption onto 4A and P1 zeolites.
| Zeolites | ΔH°ads (kJ·mol−1) | ΔS°ads (J·mol−1·K−1) | ΔG°ads (kJ·mol−1) | |||
| 293 K | 303 K | 313 K | 323 K | |||
| 4A | 39.14 | 112.95 | –17.42 | –23.18 | –25.11 | –27.04 |
| P1 | 24.12 | 136.06 | –15.75 | –19,82 | –21.18 | –22.16 |
3.9 Experimentation on real effluents
The following step of our work consisted in testing 4A and P1 zeolites in genuine solutions. A set of experiments was carried out at the adsorption optimum conditions previously obtained in the study of the synthetic solutions.
The effluents were taken at different points along the process of uranium purification and concentration in the uranium ore treatment. The yellow cake, which is a mixture of oxides whose composition is expressed in U3O8, is the first uranium compound produced at this stage of uranium ore refining; it is then sent and used as a raw material in the purification process, where it is converted into a more concentrated compound called ammonium di-uranate (ADU) or ammonium uranyl carbonate (AUC). The processes that is referred to concern different unit operations such as extraction, re-extraction, washing, precipitation; generating several effluents that need to be treated. In this respect, three solutions with initial concentrations of 100, 85 and 80 mg·L−1 were used. The results are shown in Table 6; it emerges that our zeolites 4A and P1 effectively removed uranyl ion UO22+ with a yield higher than 96%.
Experimental data on radioactive waste treatment by adsorption onto 4A and P1 zeolites.
| Zeolite | Solution 1 (100 mg·L−1) | Solution 2 (85 mg·L−1) | Solution 3 (80 mg·L−1) |
| Uptake uranium adsorption | |||
| 4A Condition pH: 2 T: 343 K | 96.66 | 96.31 | 98.12 |
| P1 Condition pH: 2.5 T: 343 K | 90.82 | 90.60 | 91.61 |
4 Conclusion
In the present comparative study, the ability of two locally produced zeolites, namely 4A and P1, were tested for the adsorption of uranyl ion UO22+ in aqueous solutions.
Both zeolites (4A and P1) were synthesized and characterized. The equilibrium time was determined and found to be 60 min under the following condition: T = 293 K, pH = 2.0 and 2.5 for 4A and P1, respectively, initial uranium (VI) concentration C0 = 100 mg·L−1, and a solid–liquid ratio = 1/100. However, the optimal operating time taken by the subsequent experiments was 120 min. The effect of the parameters that are referred to on the adsorption yield was studied. The two zeolites adsorb effectively uranium (VI) and pH is the most important parameter. The maximal adsorption yield was obtained at pH = 2.0 for zeolite 4A and 2.5 for the zeolite P1.
Our experimental data fit best the pseudo first-order, with a correlation coefficient of nearly one, and our equilibrium adsorption capacities qe (cal) are close to the experimental ones qe (exp) for both zeolites produced. As for equilibrium, the Langmuir model was found to describe best our experimental results for both zeolites. It is also shown that the adsorption capacity onto P1 is higher than onto 4A. Besides, the figures obtained for the dimensional factor 0 < RL < 1 indicate that the adsorption is favourable.
The low values of the diffusion activation energy Edif showed that the uranyl ion diffuse easily through the zeolites 4A and P1 pores. The negative values of the Gibbs free energy showed clearly the feasibility of the adsorption phenomenon; however, adsorption is more possible onto zeolite 4A (ΔG°ads = –27.04 kJ·mol−1) than onto P1 (ΔG°ads = –22.16 kJ·mol−1). The standard adsorption entropies and enthalpies were found to be positive, indicating that the adsorption onto our two zeolites is spontaneous and endothermic.
Eventually, a set of adsorption experiments were conducted onto our locally made zeolites 4A and P1 with a genuine low-level radioactive effluent. The results are encouraging, as the adsorption yield reached exceeds 96%. In conclusion, the different results obtained suggest a chemical nature of the adsorption (chemisorptions), which makes its recycling hard.


