1 Introduction
Zanthoxylum atchoum (Aké Assi), P.G. Waterman (Rutaceae) is an endemic straggling shrub distributed in humid forests of southern Ivory Coast. The plant is used to treat amenorrhea in traditional medicine. There has been no report of other phytochemical study and biological value of Zanthoxylum atchoum so far. In our continuous search for chemical bioactive constituents of Ivorian Zanthoxylum species [1], we investigated the methanol extract of Z. atchoum.
The present study deals with the isolation and structural elucidation of two new benzophenanthridine alkaloids, 6-nitronitidine (1) and 6-nitro-8-methoxy-7,8-dihydronitidine (2), and of two new indolopyridoquinazoline alkaloids, 3-hydroxydehydroevodiamine (3) and its zwitterionic form 3-hydroxylatedehydroevodiamine (4), along with 18 (5–22) known compounds.
This is the first report of a nitro group in the biosynthesis of new natural benzo[c]phenantridine alkaloids. We also report in the 1H NMR experiences an unusual hydrogen–deuterium exchange of the 8-methoxy hydrogens (CD3OD solvent) in compound 2. The antibacterial activities of compounds 1-4 were evaluated against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Enterococcus faecalis.
2 Experimental
2.1 Apparatus
NMR spectra were recorded in CD3OD using a Bruker Avance DRX-500 spectrometer (1H at 500 MHz and 13C at 125 MHz), and 2D-NMR experiments were performed using Bruker's standard microprograms (XWIN-NMR version 2.6 software). HR–ESI–MS and EI experiments were obtained using a micromass Q-TOF micro instrument (Manchester, UK) and water-micromass GCT (UK). Ultraviolet spectra were recorded in MeOH on a Philips PU 8720 spectrophotometer. Infrared spectra were measured using a Nicolet Avatar 320 FT-IR spectrometer. Chromatography was performed on silica gel 60 (63–200 μm, Merck). Preparative glass-backed TLC plates, coated with silica gel 60 F254 (Merck) were used. TLC spots were visualized under UV light (254 and 365 nm) followed by spraying with Dragendorff's reagent for alkaloids or with 50% H2SO4 for the detection of other compounds.
2.2 Plant material
The plant was identified and collected by Prof. Aké Assi in April 2003 in Yapo (Agboville), Ivory Coast. A voucher specimen (Aké Assi 14820) was deposited in the National Herbarium of Floristic Center of University HFB Cocody-Abidjan.
2.3 Extraction and isolation
A total of 920 g of air-dried powdered roots of Zanthoxylum atchoum were successively extracted with petroleum ether and methanol for 48 h. After removal of the solvent, the petroleum ether and methanol extracts were repeatedly chromatographed on silica gel to give 22 compounds (1–22). The petroleum ether extract (2 g) was chromatographed over a silica gel column using a gradient system of cyclohexane/chloroform (5:5 to 0:1) and then chloroform/methanol (1:0 to 9:1) to give: 19 (102 mg), 8 (4 mg) and 22 (406 mg) (in C6H12/CHCl3: 5:5), 21 (15 mg) (with 100% CHCl3) and 20 (60 mg) (eluted with CHCl3/CH3OH: 99:1). The methanol extract (5 g) was fractionated into seven fractions (F1–F7) by vacuum liquid chromatography on silica gel using a gradient of mixtures of C6H12/CHCl3 (1:0 to 0:1) and CHCl3/MeOH (9:1 to 5:5).
The 100% CHCl3 fraction F4 (1.3 g) was subjected to silica gel column chromatography (CC) eluting with an increasing gradient of C6H12/CHCl3 (1:0 to 0:1) and CHCl3/MeOH (1:0 to 99:1) to give nine compounds. The C6H12/CHCl3 fractions gave 11 (32 mg) (C6H12/CHCl3, 7:3), 10 (15 mg) (C6H12/CHCl3, 5:5), 6 (107 mg) and 17 (158 mg) (100% CHCl3). The CHCl3/MeOH fractions yielded compounds 9 (12 mg) (CHCl3/MeOH, 199:1), 7 (10 mg), 18 (23.4 mg) (CHCl3/MeOH, 99:1) and 14 (10 mg) obtained by preparative TLC protocol (CH3OH/NH4NO2, 9:1).
The fractions F5 + F6 (1.8 g) (CHCl3/MeOH, 8:2) were subjected to CC on silica gel, eluting with CHCl3/MeOH (1:0 to 5:5) to afford 60 subfractions (A1 to A60).
Fractions A25–A28 (68 mg, CHCl3/MeOH, 95:5) were separated by preparative TLC eluting with CH3OH/NH4NO2 9:1 to give 16 (16 mg) and 13 (9 mg).
Fractions A30–A33 (80 mg, CHCl3/MeOH, 9:1) gave 15 (10 mg).
Fractions A35–A40 (160 mg, CHCl3/MeOH, 85:15) gave 12 (102 mg) and 5 (14 mg) by preparative TCL (CH3OH/NH4NO2 9:1).
Fractions A42–A45 (110 mg, CHCl3/MeOH, 8:2) gave 1 (10 mg) and 2 (8 mg) by preparative TCL (CH3OH/NH4NO2 9:1).
Fractions A48–A58 (98 mg, CHCl3/MeOH, 7:3) afforded 3 (9 mg) and 4 (5 mg) by preparative TCL (CH3OH/NH4NO2 9:1).
2.3.1 2,3-(methylenedioxy)-6-nitro-10,11-(dimethoxy)-7-methylbenzo[c]phenanthridinium or (6-nitronitidine) (1)
Yellow powder; UV (MeOH) λmax: 231, 266, 312 nm; IR (KBr): 3408, 2924, 1613, 1521, 1496, 1351, 1277, 1036 cm−1; 1H (CD3OD + TFA, 500 MHz) and 13C NMR (CD3OD + TFA, 125 MHz) data see Table 1; EIMS m/z 393 [M]+; Tandem MS/MS 393: m/z 393[M]+, 346 [M–NO2–H]+, 332 [M–NO2–CH3]+, 318 [M–NO2–OCH3 + 2H]+, 301 [M–NO2–OCH3–CH3]+; HRESIMS m/z 394.1348 [M + H]+(calcd. for C21H18N2O6, 394.1347).
1H (500 MHz) and 13C NMR (125 MHz) spectroscopic data for compounds 1 and 2.
| 1 | 2 | |||
| Position | δC ppm | δH ppm [mult, J (Hz)] | δC ppm | δH ppm [mult, J (Hz)] |
| 1 | 106.0 | 8.17 (s) | 101.0 | 7.71 (s) |
| 2a | 153.5 | 152.0 | ||
| 3a | 154.0 | 152.5 | ||
| 4 | 108.0 | 7.68 (s) | 106.0 | 7.33 (s) |
| 4a | 132.0 | 130.0 | ||
| 5 | 128.0 | 8.63 (s) | 121.0 | 7.99 (s) |
| 6 | 144.0 | 144.0 | ||
| 8 | 155.0 | 9.82 (s) | 92.0 | 5.25 (s) |
| 8a | 122.0 | 121.0 | ||
| 9 | 110.0 | 7.93 (s) | 112.0 | 7.13 (s) |
| 10 | 155.0 | 150.0 | ||
| 11 | 160.0 | 150.0 | ||
| 12 | 105.0 | 7.41 (s) | 110.0 | 6.85 (s) |
| 13 | 119.0 | 119.0 | ||
| 12a | 133.0 | 127.0 | ||
| 14 | 138.0 | 141.0 | ||
| 14a | 123.0 | 130.5 | ||
| OCH2O | 103.0 | 6.33 (s) | 104.0 | 6.15 (s) |
| 10-OCH3 | 58.0 | 4.07 (s) | 57.0 | 3.93 (s) |
| 11-OCH3 | 58.0 | 4.12 (s) | 57.0 | 3.79 (s) |
| 8-OCH3 N-CH3 | 53.0 | 4.93 (s) | 55.0 40.0 | 3.50 (s) 2.68 (s) |
a Interchangeable, 1H and 13C recorded in CD3OD.
2.3.2 2,3-methylenedioxy-6-nitro-8,10,11-trimethoxy-7-methylbenzo[c]phenanthridine or 6-nitro-8-methoxy-7, 8-dihydronitidine (2)
Yellow powder; UV (MeOH) λmax: 271, 293, 307, 328; IR (KBr): 3408, 1615, 1502, 1470, 1384, 1284, 1209, 1036 cm−1; 1H (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz) data see Table 1; ESIMS: m/z 871 [M + Na + M]+, 447 [M + Na]+, 393[M- OCH3]+; HRESIMS m/z 425.1349 [M + H]+ (calcd. for C22H21N2O7, 425.1343).
2.3.3 3-hydroxy-8,13-dihydro-14-methyl-5-oxo-7H-indolo[2′,3′:3,4]pyrido[2,1-b]quinazolin-14-ium or 3-hydroxydehydroevodiamine (3)
Yellow powder; UV (MeOH) λmax: 214, 245, 330, 368, 381 nm; IR (KBr): 3063, 2920, 1692 cm−1; EI m/z: 318 [M]+.; 1H (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz); data see Table 2. Elementary analysis: C 62.36%, H 5.40%, N 9.01% for C19H16N3O2.
1H and 13C NMR data of compounds 3 and 4. δ in ppm, J in Hz.
| 3 | 4 | |||
| Position | δC | δH (mult, J) | δC | δH (mult, J) |
| 1 | 121.2 | 7.96 (d, J = 9.7) | 120.2 | 7.65 (d, J = 9.2) |
| 2 | 126.3 | 7.55 (dd, J = 9.7, 2.8) | 130.5 | 7.22 (dd, J = 9.2, 3) |
| 3 | 159.8 | 170.4 | ||
| 4 | 113.0 | 7.72 (d, J = 2.8) | 114.8 | 7.32 (d, J = 3) |
| 4a | 122.0 | 121.7 | ||
| 5 | 159.4 | 159.7 | ||
| 7 | 43.6 | 4.58 (t, J = 6.7) | 43.5 | 4.50 (t, J = 7.0) |
| 8 | 20.0 | 3.33 (t, J = 6.7) | 20.1 | 3.34 (t, J = 7.0) |
| 8a | 131.8 | 129.8 | ||
| 9 | 122.4 | 7.85 (d, J = 8.2) | 122.0 | 7.85 (d, J = 8.2) |
| 9a | 125.2 | 125.4 | ||
| 10 | 123.2 | 7.30 (t, J = 7.7) | 122.8 | 7.26 (t, J = 7.5) |
| 11 | 130.2 | 7.56 (t, J = 7.7) | 129.2 | 7.48 (t, J = 7.5) |
| 12 | 114.2 | 7.65 (d, J = 8.4) | 114.2 | 7.63 (d, J = 8.4) |
| 12a | 143.1 | 147.8 | ||
| 13a | 121.9 | 121.9 | ||
| 13b | 149.6 | 146.5 | ||
| 14a | 133.9 | 128.9 | ||
| NCH3 | 41.1 | 4.40 (s) | 40.8 | 4.35 (s) |
2.3.4 3-phenolate-8,13-dihydro-14-methyl-5-oxo-7H-indolo[2′,3′:3,4]pyrido[2,1-b]quinazolin-14-ium or 3-hydroxylatedehydroevodiamine (4)
Orange powder; EI m/z: 317 [M]+., 1H (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz); data see Table 2.
2.3.5 (–)-Evodiamine (6)
Yellow brilliant crystal; [α]D = –522°(C= 0.54, CH3OH),–642° (C = 0.67, CHCl3); UV (MeOH) λmax: 225, 272, 284, 293 nm; IR (KBr): 3582, 3269, 3063, 2920, 1637, 1605, 1480, 1424, 1306, 1031, 745 cm−1; ESI m/z: 304 [M + H]; 1H (CDCl3, 500 MHz): 2.50 (N-CH3, s), 2.98 (H-8, m), 3.30 (H-7b, ddd, 15.7, 10.7, 5), 4.89 (H-7a, ddd, 10.7, 5, 2.6), 5.92 (H-13b, s), 7.15 (H-1, d, 8), 7.21 (H-10, t, 7.5), 7.23 (H-3, t, 8), 7.27 (H-11, t,7.2), 7.43 (H-12, d, 8.0), 7.49 (H-2, t, 8), 7.60 (H-9, d, 8.2), 8.13 (H-4, d, 8), 8.31 (NH, s), 13C NMR (CDCl3, 125 MHz): 20.1 (C-8), 37.2 (CH3), 39.5 (C-7), 68.8 (C-13b), 111.3 (C-12), 113.6 (C-8a), 118.9 (C-9), 120.0 (C-10), 122.4 (C-1), 123.0 (C-11), 123.7 (C-4a), 124.1 (C-3), 126.2 (C-9a), 128.2 (C-13a), 128.9 (C-4), 133 (C-2), 136.7 (C-12a), 150.6 (C-14a), 164.7 (C-5).
2.4 Antibacterial assays
The assay for antibacterial activity against standard strains Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (29212), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 27853) was performed using the liquid microdilution growth inhibition method (Grare et al., 2006) [2]. Antibacterial agents used for positive controls were vancomycin for Gram+ and imipenem for Gram–strains. The MICs of test compounds were determined as described in the previous study by Yao-Kouassi et al. (2008) [3].
3 Results and discussion
The root bark of Z. atchoum was extracted successively with petroleum ether and methanol. The extracts were chromatographed on silica gel to afford 22 compounds 1–22. Structural elucidation of these compounds was performed using spectroscopic methods, especially 1D and 2D-NMR, HR-EI MS, UV and IR.
Compound 1 was isolated as a yellow powder. The UV spectrum showed absorptions at 271, 293, 309, 328 and 390 nm, which are characteristic of the benzo[c]phenanthidine skeleton [4]. The HRESIMS spectrum displayed the molecular ion [M + H]+ at m/z 394,1348 (calcd for 394.1347), which, in combination with 1H and 13C NMR spectroscopy and IR data, indicated molecular formula C21H17N2O6.
The 1H-NMR spectrum (Table 1) of 1 revealed only ten singlet signals due to six aromatic proton resonances at δH 8.17 (1H, s, H-1), 7.68 (1H, s, H-4), 8.63 (1H, s, H-5), 9.82 (1H, s, H-8), 7.93(1H, s, H-9), and 7.41(1H, s, H-12), one methylenedioxy group at 6.33 (2H, s, O–CH2–O) and three methyl groups assignable to two methoxy groups at 4.07 (3H, s, 10-OCH3), 4.12 (3H, s, 11-OCH3) and one N-methyl group at 4.93. In agreement with the above, the 13C NMR of compound 1 showed resonances for 21 C-atoms, including 11 quaternary carbons, six methines, one methylene and three methyl groups (Table 1).
The 1H NMR and 13C NMR signals of compound 1 were closely related to those of nitidine (8), except for the absence of one aromatic proton and the presence of one more quaternary carbon, suggesting that 1 was certainly an ortho-pentasubstituted benzo[c]phenanthidine [4]. Total assignments of protons H-1, H-4, H-5, H-8, H-9, H-12 and of the methyl groups were clearly obtained from the NOESY and HMBC experiments (Fig. 1). The location of the N-methyl group and of proton H-1 were secured by the NOE interaction between H-1 at δH 8.17 and N-Me at δH 4.93; in addition, both protons and the deshielded proton at δH 9.82 (H-8) showed long-range connectivity with the signal at δC 138.0 (C-14). The HMBC interactions between the protons at δH 6.33, 8.17 and 7.68 (H-4) and carbons at δC 153.5 (C-2) and 152.9 (C-3) suggested that the methylenedioxy group was connected to C-2 and C-3 on the ring D. Furthermore, the NOESY spectrum displayed a significant cross-peak between H-4 and the proton at δH 8.63, which was unequivocally assigned to H-5, and the proton H-9 at δH 7.93 was assigned by its cross-peak between H-8. Thus H-12 was assigned to δH 7.41, confirming its long-range correlations with C-8a at δC 122.0 and C-13 at δC 119.0. The location of the two methoxy groups at C-10 and C-11 were established by HMBC correlations of protons at δH 4.07 to C-10 (δC 155.0) and 4.12 to C-11 (δC 160.0), and were confirmed by the NOESY correlations between H-9/Me-10 and H-12/Me-11. The HMBC correlations between H-5 and C-6 (δC 144.0) suggested that a heteroatom, presumably a nitro group, was certainly attached to C-6. This was in accordance with the upfield shift of C-6 and consequently inferred that 1 was a 2,3,6,10,11-ortho-pentasubstituted benzophenanthridine alkaloid. The IR spectrum of 1 showed two strong bands at 1521 and 1351 cm–1 which can be ascribed to N-O stretching of the nitro group. In addition, the presence of the nitro group was confirmed by the fragmentation MS/MS of the molecular ion [M]+ at m/z 393, which gave a series of ions at m/z 346, 332, 318 and 301, from which the loss of a nitro group was respectively deduced as follows: [M–NO2–H]+, [M–NO2–CH3]+, [M–NO2–OCH3 + 2H]+ and [M–NO2–OCH3–CH3]+. Thus, compound 1 was characterized as 2,3-(methylenedioxy)-6-nitro-10,11-(dimethoxy)-7-methylbenzo[c]phenanthridinium, to which we gave the trivial name 6-nitronitidine. This is the first occurrence of a natural benzylisoquinoline alkaloid with a nitro group on the C ring of the benzo[c]phenanthidine nucleus isolated from Zanthoxylum atchoum. The presence of the nitro group seems to be scarce in the plant kingdom, regardless of special phenanthrene derivatives mentioned in the Aristolochia genus [5]. Furthermore, several examples of C-6 substituted on the C ring of benzophenanthridines do exist, but it concerns 6-O-alkyl compounds in which the C ring is not completely aromatic [6,7].
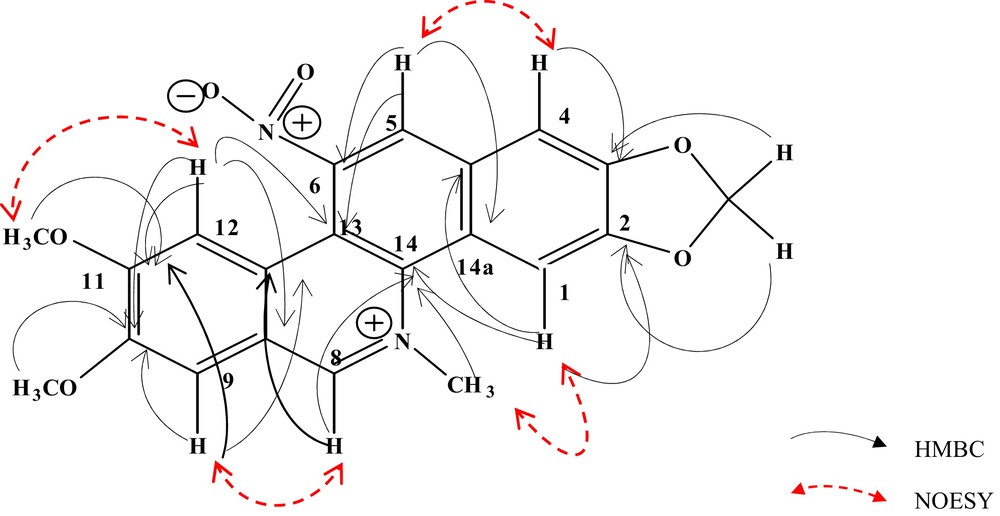
(Color online.) Selected HMBC and NOESY correlations for compound 1.
Compound 2 was obtained as a yellow amorphous solid. Its molecular formula was deduced as C22H20N2O7 by positive-ion mode HRESIMS [M + H]+ at m/z 425.1349 (calcd for 425.1349). Comparison of the NMR data (Table 1) of 2 to those of 1 indicated a slight modification of the iminium bond CN at C-8. Instead of the aromatic methine proton at C-8, the signals detected were those of the methine proton at δH 5.25 (1H, s, H-8) and of a methoxy group at δH 3.50 (3H, s, 8-OCH3) in the 1H-NMR spectrum. The location of this methoxy group was confirmed by the NOE correlation between the –OMe and H-8. Compound 2 seems to be the pseudo base of 1 and was characterized as 6-nitro-8-methoxy-7,8-dihydronitidine (Fig. 2).
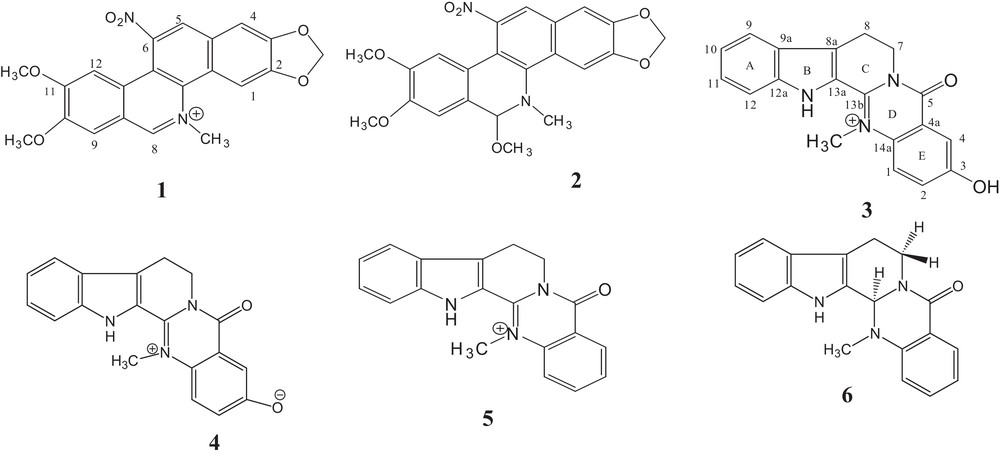
Structures of compounds 1–6.
This kind of product is well known among the quaternary benzo[c]phenanthridine alkaloid-free bases and detailed structural and NMR studies have already been reported by Dostal et al. [8,9]. Surprisingly enough, the signal of this methoxy group at δH 3.5 was gradually vanished within 24 h, whereas the signal of the residual solvent (MeOH) at δH 3.38 was increased (Fig. 3). Fig. 3 shows the 1H-NMR spectrum of 2, in which t1 and t3 represent respectively the beginning and the end of the experiments. It is remarkable that the protons of the 8-OCH3 group were H–D exchanged during the 1H-NMR experiments by the deuterated solvent (CD3OD) (Fig. 3). This situation made very difficult the NMR interpretation because of the bewildering assignment of the 8-OCH3 or OH group in benzophenanthridine pseudo-base compounds. In our study, deuterium retention of the 8-OCH3 to 8-OCD3 was confirmed by mass spectra with 3 amu more than in 1.
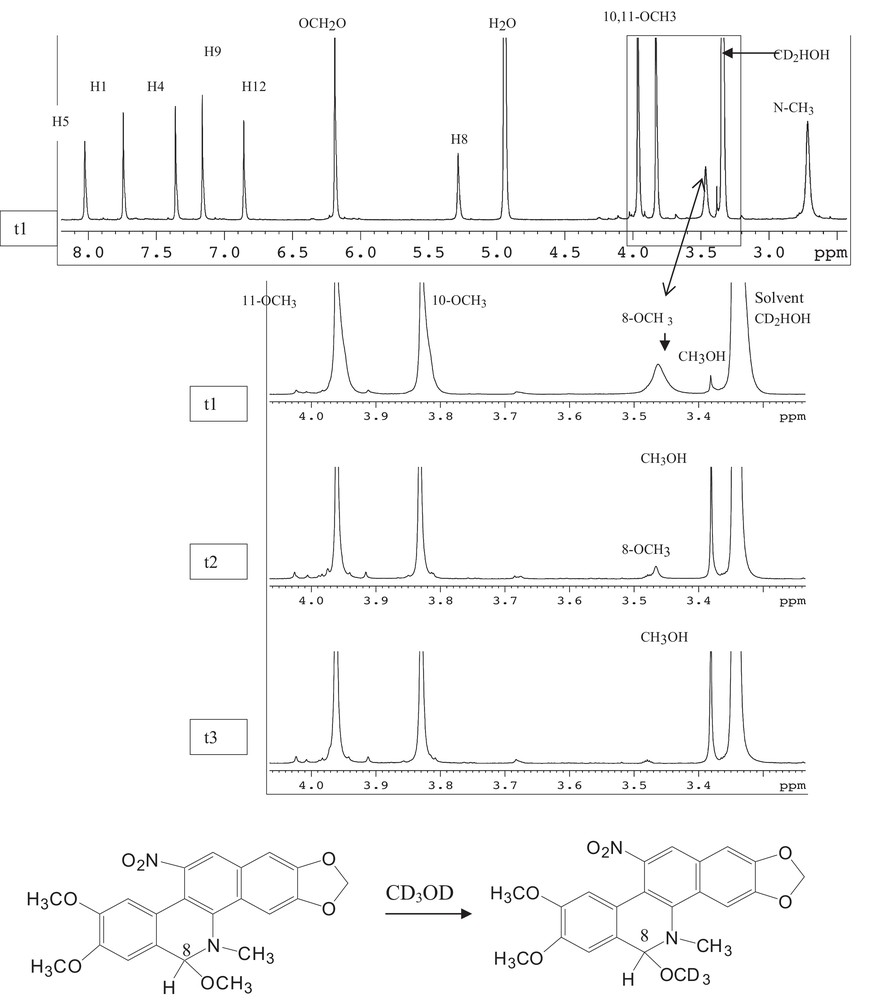
H-D exchanged in the 1H-NMR experiments by CD3OD solvent.
Compound 3 was isolated as a yellow amorphous solid. Our EI mass spectra evidenced the positive ion [M]+ at m/z 318, which is consistent with the molecular formula C19H16N3O2. The IR spectrum of 3 implied the presence of hydroxyl and NH groups (3000–3063 cm−1) and of the amide carbonyl group (1692 cm−1). The UV spectrum showed absorptions bands at 250, 330 and 380 nm, with a bathochromic shift observed in alkaline medium at 417 nm, suggesting the presence of a phenol group. The 1H-NMR spectrum of 3 showed four aromatic protons of an 1,2-ortho-disubstituted phenyl ring at δH 7.85 (1H, d, J = 8.2 Hz, H-9), 7.30 (1H, t, J = 7.7, H-10), 7.56 (1H, t, J = 7.7 Hz, H-11), 7.65 (1H, d, J = 8.4 Hz, H-12), three aromatic proton signals at δH 7.96 (1H, d, J = 9.7 Hz, H-1), 7.55 (1H, dd, J = 9.7, 2.8 Hz, H-2) and 7.72 (1H, d, J = 2.8 Hz, H-4), revealing the presence of an ABX system in a 1,3,4-trisubstituted phenyl ring, one methyl singlet group at δH 4.40 (3H, s, N-CH3), and two methylene groups at δH 4.58 (2H, t, J = 6.7 Hz, H-7) and 3.33 (2H, t, J = 6.7 Hz, H-8). All these data readily help us to identify a typical 7, 8-dihydropyridoquinazoline nucleus [10,11]. The 13C-NMR spectrum of 3 showed 19 carbon signals due to eight quaternary carbons, seven methines, two methylenes, N-methyl and carbonyl groups. The 1H and 13C NMR spectra (Table 2) of 3 were similar to those of dehydroevodiamine (5) [12], except for the increase of chemical shift of C-3. According to the preceding data, 3 was suggested to be a 3-hydroxy analog of 5 and similar to those described by Li et al., 2001 [13]. The protons H-9 and H-1 were assigned by the NOESY spectrum, which showed respectively cross-peaks between H-9/H-8 and H-1/N–CH3 (Fig. 4). The location of the hydroxy at C-3 was confirmed by the HMBC experiment, which showed correlations between the protons H-1, H-2 and H-4 and C-3 (δC 159.8) (Fig. 4). Thus, on the basis of spectral evidence, the structure of compound 3 was elucidated as a salt of 3-hydroxy-8,13-dihydro-14-methyl-5-oxo-7H-indolo[2’,3’:3,4]pyrido[2,1-b]quinazolin-14-ium, to which we gave the trivial name of 3-hydroxydehydroevodiamine.
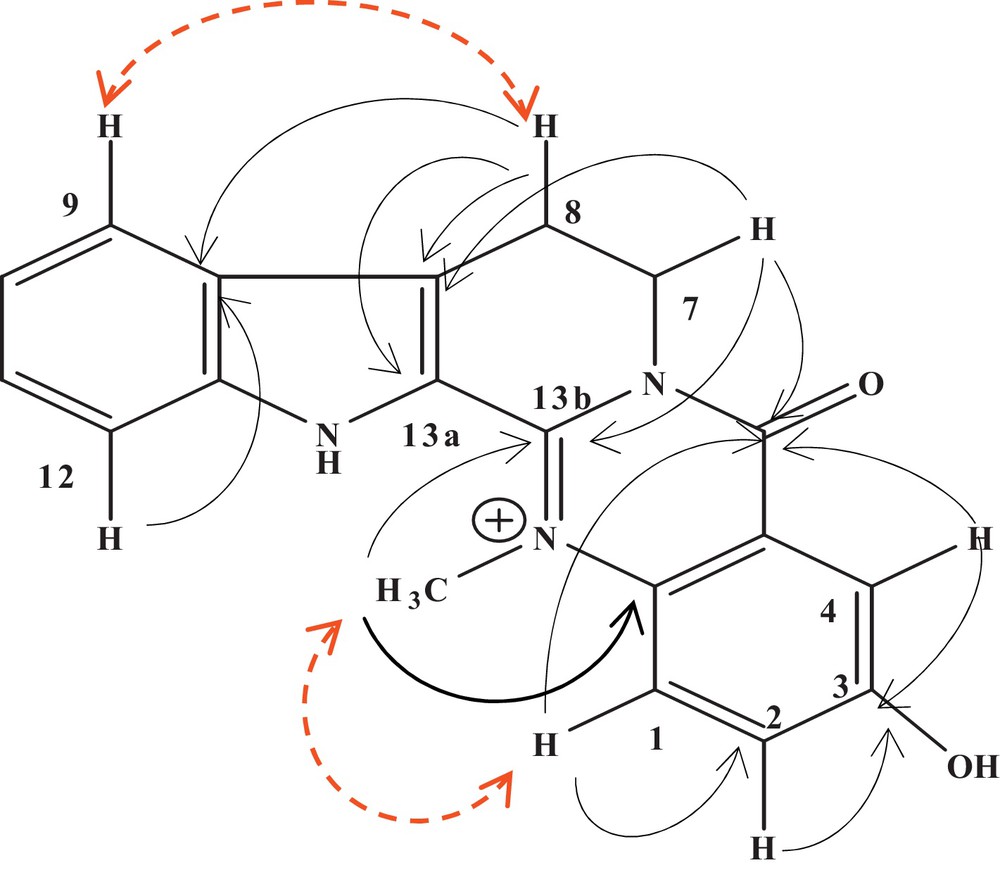
(Color online.) Selected HMBC and NOESY correlations for compounds 3.
Compound 4 was obtained as an orange amorphous solid. A molecular ion peak at m/z [M]+ 317 suggested its molecular formula C19H15N3O2, which was one proton less than in that of 3.
Furthermore, the NMR data of 4 were similar to those of 3. However, the 13C NMR spectrum of 4 showed the presence of a deshielding C-3 at δC 170.4 instead of 159.8; this means that 4 is the zwitterionic 3-phenolate form of 3, named 3-phenolatedehydroevodiamine (Fig. 2).
The known compounds (5–23) were readily identified, from their spectral data and by comparison with reported corresponding compounds in the literature, as dehydroevodiamine (5), evodiamine (6) [14], decursinol (7) [15], asarinin (8) [16], oxynitidine (9), norchelerythrine (10) [17], tridecanonchelerythrine (11) [18], nitidine (12), chelerythrine (13), methoxychelerythrine (14), fagaridine (15), methoxyfagaridine (16), arnottianamide (17), pellitorine (18), sesamin (19), skimmianine (20), stigmasterol (21), and lupeol (22). Compounds 12–23 were previously found in other Ivorian Zanthoxylum species [1]. We describe the structural elucidation and configuration of 13b αH of compound 6 which displayed negative values: [α]D = –522° (c 0.54 in CH3OH) and–642° (c 0.67 in CHCl3) for optical rotation, like that of the synthesized one [19]. Nevertheless, the isolated evodiamine described in literature was optically inactive or displayed positive optical rotation values [20–22].
The isolated new compounds (1–4) were evaluated for their antibacterial activities against four pathogenic microorganisms, E. coli, P. aeruginosa, S. aureus, and E. faecalis; the results are summarized in Table 3. Among them, compound 2 was the most active one, with MICs of 4, 8, 16, and 32 μg·mL−1 against S. aureus, E. faecalis, P. aeruginosa, and E. coli, respectively (Table 3). Compound 1 exhibited mild activity against these four bacteria with MICs of 32 μg·mL−1. This result suggested that the pseudo base of these isolated nitro benzophenanthridine is more active than the ammonium quaternary 1. A similar antimicrobial effect was observed with sanguinarine and chelerythrine and their pseudo bases [23].
Antibacterial activity of isolated compounds 1–4.
| Test materials | MIC μg·mL−1 | |||
| S. aureus | E. coli | E. faecalis | P. aeruginosa | |
| 1 | 32 | 32 | 32 | 32 |
| 2 | 4 | 32 | 8 | 16 |
| 3 | 64 | 64 | 64 | 64 |
| 4 | 64 | 128 | 64 | 128 |
4 Conclusions
This work constituted the first phytochemical study of Z. acthoum. The methanolic extract of the roots led to the isolation of 11 benzophenanthridines, four indolopyridoquinazoline, one furanoquinoline alkaloids, two lignans, one coumarin, one amid, one triterpene, and one phytosterol. Among them, 6-nitronitidine (1), 6-nitro-8-methoxy-7,8-dihydronitidine (2), 3-hydroxydehydroevodiamine (3), and 3-phenolatedehydroevodiamine (4) were characterized as new compounds. This paper reports the first natural nitro group on the benzophenanthridine nucleus. This work allowed us to understand the fickle behavior of benzophenanthridine bases towards solvents, in particular deuterium retention of the OCH3 to OCD3, which made difficult the interpretation of the spectra. The antibacterial activities against two gram-positive (S. aureus and E. faecalis) and two gram-negative (E. coli and P. aeruginosa) strains for compounds 1–4 were tested. Compound 2 showed strong antibacterial activity against S. aureus at the concentration of MIC50 4 μg·mL−1.
Acknowledgements
The authors thank Prof. C. Lavaud and Dr. J.-M. Nuzillard for profitable scientific exchanges, and are grateful to CNRS UMR 7312 France and Government of Ivory Coast for financial support. The authors would like to pay a tribute to the Prof. Aké Assi L. (University FHB Cocody-Abidjan) for the identification of the plant.


