1 Introduction
During the last decades, more and more attention has been paid to the problems concerning energy, such as depletion of fossil fuels, pollution of the environment, and energy security. The generally accepted conclusion is that new alternative energy sources are needed. One of the chemicals that could be considered as the fuel of 21st century is dimethyl ether (DME). DME has several advantages as a fuel or an energy carrier [1]. It can be used as a diesel substitute in engines currently produced [2,3]. DME combustion results in exhaust gas containing lower amounts of NOx, SOx and soot than conventional fuels. DME can be also used as a LPG-substitute fuel for heating and cooking. Thus, as an alternative fuel, the application of DME can address several problems connected with energy: security, conservation and accessibility, as well as environmental concerns [1].
DME can be produced by dehydration of methanol using solid acid catalysts following the exothermic reaction (Eq. 1) [4,5]:
| (1) |
This reaction is generally accepted as the initial stage of the methanol-to-olefins and methanol-to-gasoline processes. The acidity of the catalysts influences the distribution of the obtained products. Over the catalysts with strong acidic sites beside the formation of DME, the simultaneous formation of hydrocarbons as byproducts and subsequent coke formation was observed [5]. Accordingly, catalysts with moderate or weak acid sites are desirable for methanol dehydration at relatively low temperature [6]. Several materials have been tested as catalysts for this reaction [5]. Among the studied catalysts are:
- • zeolite-like materials (H-ZSM-5, H-[F]-ZSM-5, H-Y, AlPO4 [7], SAPO-5 [8]);
- • and oxides (mesoporous silica [9], TiO2–ZrO2 [10], γ-alumina, aluminosilicates, silica-titania and alumina-titania [6,11–13]).
The selected catalysts for methanol dehydration to DME and also their catalytic performance are presented in Table 1. The synthesis of DME from methanol over γ-alumina and silica-titania catalysts at 300 °C was studied by Yaripour et al. [11]. In general, silica-titania turned out to be not suitable for DME formation. On the other hand, both γ-alumina samples (commercial and prepared) showed similar catalytic performance and were selective and active for methanol dehydration. Yaripour et al. [6] also compared the catalytic performance of γ-Al2O3 to that of the aluminosilicates with different contents of silica at 300 °C. The catalytic performance of aluminosilicates depended on the silica content. All of the studied catalysts turned out to be active. Laugel et al. [8] studied synthesized zeolites (H-ZSM-5, H-[F]ZSM-5) with different textural properties and compared them to commercial catalysts (H-Y, SAPO-5 and γ-alumina). Zeolites having different structures exhibited different catalytic performances at 275 °C. The H-ZSM-5 catalyst showed high methanol conversion equal to 73%, while the conversion for samples H-Y and SAPO-5 was only 39 and 8%, respectively.
Selected catalysts used in methanol dehydration to DME and their catalytic performance.
| Catalyst | Reaction conditions | Methanol conversion (%) | Selectivity to DME (%) | SBET (m2·g−1) | Acidity (mmol·g−1) | Reference |
| γ-Al2O3 | Fixed-bed reactor; atmospheric pressure; T = 300 °C GHSV = 15,600 h−1 | 77.15 | - | 268.2 | 0.442 | [6] |
| Commercial γ-Al2O3 | 82.13 | - | 383.12 | 0.398 | ||
| Aluminosilicate 1% silica | 86.4 | - | 252.4 | 0.301 | ||
| Aluminosilicate 15% silica | 73.6 | - | 286.7 | 0.295 | ||
| γ-AlOOH | Fixed-bed reactor; atmospheric pressure; T = 300 °C GHVS = 5300 h−1 | 77.15 | 94.7 | 339 | 0.437 | [11] |
| Commercial γ-Al2O3 | 82.13 | 90.2 | 168 | 0.287 | ||
| Silica–titania (50% silica) | 16.4 | 2.2 | 46 | 0.109 | ||
| Silica–titania (75% silica) | 14.8 | 0.6 | 17 | 0.119 | ||
| H-ZSM-5 | Fixed-bed reactor; atmospheric pressure; T = 275 °C; WHSV = 237 h−1 | 73 | 99 | 335 | 1 | [8] |
| H-[F]-ZSM-5 | 57 | 99 | 370 | 0.34 | ||
| H-Y | 39 | 99 | 613 | 5.34 | ||
| SAPO-5 | 8 | 80 | 302 | 0.79 | ||
| γ-Al2O3 | 70 | 99 | 156 | 3.680 | ||
| AlPO4 | Fixed-bed reactor; atmospheric pressure; T = 300 °C GHSV = 5300 h−1 | 82 | 99 | 171 | 0.34 | [7] |
Lertjiamratn et al. [7] investigated AlPO4 as methanol dehydration catalyst at 300 °C. The main goal of their studies was to find the influence of a 10% water pretreatment on the catalytic performance. The investigations showed that the pretreatment with water at 200–300 °C improved the catalytic performance of AlPO4. This might be due to an increase in the acid strength and acidity of the catalyst.
Khaleel [13] examined the performance of a titanium-doped alumina catalyst prepared by sol-gel method in the 180–300 °C temperature range. The study showed that the addition of 3–5 wt.% of titania to alumina had a positive effect on both the activity of the catalyst and its selectivity to DME. The author explained the enhanced catalytic performance by an increase in the acidity of the titania–alumina samples due to the introduction of titanium atoms into the alumina lattice. The positive effect of titania addition into heteropolyacids in DME production via methanol dehydration was also observed by Ladera et al. [14].
In this work, the catalytic performances of different modified vermiculites were studied. Vermiculite belongs to clay minerals. It is a hydrated phyllosilicate with a 2:1 layer type structure [15]. This material found various applications in a number of fields. The most interesting ones for the chemical industry concern the use of vermiculite as an adsorbent, a catalyst or a catalyst support. Especially modified vermiculite is a promising material when it comes to catalysis. Chemical and thermal modifications, such as acid treatment or clays pillaring, improve the catalytic properties of the final materials. Acid-treated vermiculite demonstrated to be an efficient acid catalyst in reactions, such as dehydration of 1-butanol and dealkylation of cumene [16,17]. On the other hand, Al-pillared vermiculite showed catalytic activity in reactions, such as hydroisomerisation of n-octane, selective reduction of NO by ammonia or hydroconversion of decane [16,18].
There are only a few reports that described mineral clays as catalysts for methanol dehydration. Sun Kou et al. [19] studied the catalytic activity of Zr-pillared betonites with or without the addition of an active component (Cu). The best methanol conversion obtained was ca. 80% with DME and hydrocarbons from C1 to C5 as the main products. Hoshimoto et al. [20] studied an Al-pillared montmorillonite that gave 20, 33 and 35% methanol conversion at 260, 300 and 340 °C, respectively, with 100% selectivity to DME. Montmorillonites were also investigated by Mishra and Parida [21]. An acid-activated vermiculite was studied by Ravichandran et al. [17]. They obtained olefins as the main product, but unfortunately the formation of DME was not discussed.
The main goal of this work was to study vermiculite-based catalysts in the reaction of methanol dehydration to DME. The application of such a material can be beneficial since vermiculite is a relatively cheap mineral. Additionally, its catalytic properties can be tailored by chemical and thermal modifications. Since there is no report on using vermiculite in methanol dehydration to DME, research in this area is promising.
2 Experimental
2.1 Catalysts preparation
The preparation of the catalyst was based on the procedure described by Caballero et al. [22] and Chmielarz et al. [18,23,24], and included five different modification steps. The vermiculite used in this study was supplied by Sigma Aldrich. The first step was the nitric acid leaching of starting vermiculite with 10 cm3 of 0.8 M HNO3 (per gram of vermiculite) for 4 h under stirring at 95 °C. Then vermiculite was washed and dried. Secondly, acid-treated vermiculite was calcined at 600 °C for 4 h under static air. Then, in a third step, the sample was treated with oxalic or citric acid (10 cm3 of 0.12 M acid solution per gram of vermiculite) at 80 °C for 3 h under continuous stirring. Afterwards, solids were washed and dried. Step 4 concerns Na+ exchange: in a typical procedure, vermiculites from step 3 (2 wt.% dispersion) were four times ion-exchanged with a 3 M solution of NaCl; each exchange was carried out for 2 h at 80 °C under continuous stirring and finally solids were washed and dried. In a final step (5), the solids were pillared with aluminium and titanium oligocations: a) a pillaring solution of aluminium oligocations was prepared by slow addition of an adequate volume of 0.4 M NaOH solution to 0.4 M AlCl3 until an OH/Al molar ratio of 2.4 was reached. Then the pillaring solution was aged at room temperature for 24 h. Subsequently, the pillaring solution was slowly added to the mineral suspension of Na-exchanged vermiculite (2–4% wt.) for 24 h at 80 °C; b) a pillaring solution of titanium oligocations was prepared by adding TiCl4 to 6 M HCl until reaching the final concentration of Ti4+ ions of 0.82 M. Then the solution was aged for 24 h. Vermiculites were treated with titanium by slowly addition of a pillaring solution to a mineral suspension of Na-exchanged vermiculites (2–4% wt.) at 30 °C for 6 h under stirring. Then the mixture was left to age for 18 h. In both cases, the amounts of oligocations were enough to supply 10 mmol Al/Ti per gram of vermiculite. The pillared vermiculites were finally calcined at 500 °C for 6 h under static air. The designation of the samples and preparation details are summarized in Table 2.
The designation of the samples and preparation details.
| Sample | Treatment with HNO3 | Calcination 600 °C | Treatment with complexing agent | Na-exchange × 4 | Pillaring agent |
| VER | - | - | - | - | - |
| V/S/Al | + | + | Oxalic acid | + | Ala |
| V/C/Al | + | + | Citric acid | + | Ala |
| V/S/Ti | + | + | Oxalic acid | + | Tib |
| V/C/Ti | + | + | Citric acid | + | Tib |
a Al: Al-hydroxycations.
b Ti: Ti-hydroxycations.
2.2 Catalysts characterization
The structural properties of the catalysts were determined by XRD. The XRD patterns were recorded by D8 advanced diffractometer from Bruker, using a Cu Kα radiation source (λ = 1.5406 Å) in the 2θ range from 3 to 80°.
The textural parameters of the prepared samples were measured using low-temperature nitrogen sorption at −196 °C using an ASAP 2010 Micrometrics apparatus. The samples were vacuum-outgassed for 5 h at 300 °C. The specific surface area was determined using the BET method. The total pore volumes and micropore volumes were calculated using the t-plot method.
The thermogravimetric measurements for calcined materials and spent catalysts (after reaction) were carried out in the temperature range from 20 to 800 °C using a TG-DSC 92 Setaram apparatus under air and with a heating rate of 10 °C/min.
The SEM images of titania-modified samples were recorded on analytical FEG-SEM: JOEL 7001F apparatus equipped with Oxford light elements EDS detector. The EDS spectra were recorded for at least 3 different parts of each SEM image.
The acidity of the samples was determined by pyridine adsorption followed by IR spectroscopy on a Nicolet Nexus spectrometer. The samples were pressed into thin wafers (10–20 mg/cm2) and pretreated in an IR quartz cell at 450 °C for 3 h under vacuum (10−3 Torr). Afterwards, the samples were cooled down to 150 °C and contacted with pyridine (Peq = 1,5 Torr) for 10 min. Then, the pyridine excess was removed for 30 min under vacuum and the IR spectra were recorded; 64 scans with a resolution of 4 cm−1 were collected for each spectrum. The subsequent spectra were recorded at respectively 250, 350, and 450 °C. The concentrations of Brønsted and Lewis sites able to retain the pyridine at each temperature were determined using the integrated areas of the bands at 1541 and 1454 cm−1, respectively, the extinction coefficients determined by Emeis [25] and using the equation below:
2.3 Catalytic tests
Modified vermiculites were tested for the dehydration of methanol in a fixed-bed reactor. Prior to the experiment, each catalyst (0.2 g) was activated under air flow (5 L·h−1·g−1 of catalyst) for two hours at 450 °C and then cooled down to the desired temperature, if necessary.
The reaction was performed in the stream of N2 (100 cm3·min−1) as an inert gas. The flow of N2 was regulated by a mass-flow controller (MFC, Brooks 5850, E series). Methanol was supplied with a calibrated syringe pump from B|Braun at flow rate of 3 cm3·h−1 fed into a nitrogen stream and vaporized. The reaction products were analysed using a gas chromatographer (CP 9001 Chrompack) equipped with a flame ionization detector (FID). The product lines were electrically heated in order to avoid the condensation of the reaction products and of unreacted methanol. The reaction was carried out at 250, 350 and 450 °C. Additionally, the influence of methanol space velocity (WHSV of 11.9, 5.9, 3.9 and 1.9 h−1) and water on the catalytic performance was tested for the most active catalyst.
3 Results and discussion
3.1 Characterization of the samples
Fig. 1 presents the XRD patterns of the modified samples and raw vermiculite. The starting vermiculite exhibited several reflections at 6.2, 7.1, 7.4 and 8.8° 2θ, corresponding to distances of 14.24, 12.44, 11.94 and 10.04 Å, respectively. This indicates the presence of various cations in the interlayer space of vermiculite, such as Ca2+, Mg2+ or Na+. It is well-known that the position of the main reflection in the XRD pattern of vermiculite strongly depends on the type of cations placed in the interlayer space [24]. Alumina pillared samples exhibited the main reflection at lower values of 2θ than starting vermiculite (V/S/Al at 4.9° and V/C/Al at 5.2° 2θ). The position of these reflections indicates that samples were successfully pillared with alumina oligocations, which resulted in the increase of vermiculite basal distance from 14.24 Å for raw vermiculite to 18.00 and 16.98 Å, respectively, for samples V/S/Al and V/C/Al. Sample V/C/Al exhibited the reflection at around 5° 2θ with much lower intensity than V/S/Al. It also showed a reflection at 8.75° 2θ, indicating that this sample was only partially pillared. Similar results were obtained by Chmielarz et al. [23,24] and Caballero et al. [22]. Samples V/S/Ti and V/C/Ti had very similar XRD patterns. The absence of the main reflection at low values of 2θ and the presence of reflections at around 8° indicate that pillaring with Ti oligocations was unsuccessful. However, these two samples were treated as acid-activated vermiculites modified with titanium species and so tested in the reaction of methanol dehydration.

XRD patterns of modified vermiculites and raw material.
Since pillaring with titania oligocations turned out to be unsuccessful, the titania-modified samples were examined by SEM-EDS microscopy in order to establish if titania was deposited on the catalyst. The SEM images with EDS spectra are presented in Fig. 2. The performed experiments confirmed that Ti was present and that the samples were homogeneous. Titanium species were well dispersed and no separated phase(s) was detected. The V/S/Ti sample exhibited a higher content of titania when compared with V/C/Ti sample. This effect was most probably connected with the treatment of samples with oxalic and citric acids. The oxalic treatment was much more effective and thus resulted in a better leaching of vermiculite lattice atoms. Due to that effect, oxalic acid-treated samples (V/S/Al and V/S/Ti) were successfully pillared with alumina and were doped with higher contents of titania, in contrast to citric acid-treated samples (V/C/Al and V/C/Ti).

(Colour online). SEM images and EDS spectra recorded for titania-modified samples–V/S/Ti and V/C/Ti.
Nitrogen sorption isotherms of starting vermiculite and modified samples are shown in Fig. 3. The isotherm of raw vermiculite corresponds to the type III of the IUPAC classification, and shows an N2 uptake increase at high relative pressure, revealing some interparticle mesoporosity. The isotherms of the modified vermiculites (2, 3, 4 and 5 in Fig. 3) correspond to the type IV of the IUPAC classification. The textural parameters of the studied samples obtained from BET and t-plot methods are summarized in Table 3. Raw vermiculite exhibited low specific surface area and total pore volume. Alumina pillaring resulted in the increase of the specific surface area by a factor of 10 to 20. Acid-treated titania-modified samples also exhibited improved textural parameters. Higher values of SBET and total pore volume were shown by the V/S/Ti sample, which may be connected with a higher Ti content in V/S/Ti sample, in comparison with the V/C/Ti sample. The acid treatment increased the specific surface area by one order of magnitude due to the partial leaching of atoms from the vermiculite structure. All modified samples additionally exhibited an increase in total pore volume (essentially mesoporous), although for Al-pillared samples, microporosity was also created.

(Colour online). Nitrogen adsorption–desorption isotherms of the parent vermiculite (1), and modified samples V/C/Ti (2), V/S/Ti (3), V/C/Al (4) and V/S/Al (5).
Textural parameters of vermiculite samples.
| Sample | SBET (m2/g) | Vtot (cm3/g) | Vmicro (cm3/g) |
| VER | 9 | 0.038 | 0 |
| V/S/Al | 209 | 0.149 | 0.058 |
| V/C/Al | 135 | 0.101 | 0.036 |
| V/S/Ti | 84 | 0.089 | 0.006 |
| V/C/Ti | 62 | 0.066 | 0.004 |
The thermogravimetric measurements of modified vermiculite samples were carried out in order to estimate the adequate calcination temperatures and to find out temperatures at which significant mass losses occur. All of the studied samples showed total weight loss between 6–9%. The differences between the samples can be associated with the different water contents. In each case, two regions of weight losses were present. The first, up to ca. 200 °C, can be attributed to water desorption. The second, from ca. 600 °C to ca. 800 °C, can be associated with high-temperature dehydroxylation of the octahedral layer of vermiculite and the formation of new silica phases [15]. The similar results were obtained by Perez-Maqueda et al. for Mg2+- and Na+-exchanged vermiculites [26], and by Sutcu for expanded vermiculite [27].
The IR spectra of pyridine adsorbed on Al-pillared and on titanium-modified vermiculites are presented in Fig. 4. After outgassing at 150 °C, seven bands were observed at 1448, 1490, 1546, 1575, 1610, 1621 and 1637 cm−1 for the samples V/S/Al, V/C/Al, V/S/Ti and V/C/Ti. The bands at 1546 and 1448 cm−1 are associated with the interaction of pyridine with Brønsted and Lewis acid sites, respectively [25]. Other bands observed in the IR spectra of the studied samples can be attributed to both types of acidity (1490 cm−1), or separately to pyridine adsorbed on Lewis sites (1580, 1610, and 1621 cm−1) and Brønsted sites (1635 cm−1) [22]. The calculated concentrations of Lewis and Brønsted acid sites are presented in Table 4. Lewis acidity was significantly higher for each sample than Brønsted acidity. The increase in the outgassing temperature resulted in the decrease of the number of acid sites. Brønsted sites disappeared completely at 250 °C. The number of Lewis acid sites decreased ca. 1.5 to 3 times with each increase in the outgassing temperature by 100 °C. According to Caballero et al. [22], Brønsted sites are connected with OH groups in the Si–O–Al bond in the tetrahedral layer, whereas the removal of H2O from the sample leads to the disappearance of such groups.

FTIR spectra of adsorbed pyridine of samples V/S/Al (A) and V/S/Ti (B).
Acidity of studied samples.
| Sample | Temperature (°C) | Concentration | |
| Lewis sites (μmol·g−1) | Brønsted sites (μmol·g−1) | ||
| V/S/Al | 150 | 125 | 6 |
| 250 | 71 | 0 | |
| 350 | 26 | 0 | |
| 450 | 12 | 0 | |
| V/C/Al | 150 | 53 | 6 |
| 250 | 25 | 0 | |
| 350 | 10 | 0 | |
| 450 | 4 | 0 | |
| V/S/Ti | 150 | 34 | 3 |
| 250 | 19 | 0 | |
| 350 | 7 | 0 | |
| 450 | 4 | 0 | |
| V/C/Ti | 150 | 44 | 7 |
| 250 | 20 | 1 | |
| 350 | 7 | 0 | |
| 450 | 4 | 0 | |
| VER | 150 | 22 | 0 |
| 250 | 9 | 0 | |
| 350 | 3 | 0 | |
| 450 | 0 | 0 |
3.2 Catalytic tests
3.2.1 Al-pillared and titanium-modified vermiculite
Raw vermiculite and modified samples were tested in the reaction of methanol dehydration to DME. Fig. 5 presents the evolution of methanol conversion as a function of time on stream (TOS) observed for catalytic tests performed at 250, 350 and 450 °C with WHSV equal to 11.9 h−1. All catalysts show very low activity at 250 °C and medium-to-high activity at 350 and 450 °C. At the latter temperatures, methanol conversion has the following sequence: V/S/Al > V/C/Al > V/S/Ti ≈ V/C/Ti. The best catalytic performance was found for the sample pillared with aluminium (V/S/Al) at 450 °C with initial conversion above 55%. Samples V/S/Ti and V/C/Ti showed a very similar performance, but were much less active than pillared vermiculites, the highest methanol conversion obtained being ca. 18%. Low activity may arise from several reasons. Firstly, specific surface area as well as the total pore volume of samples modified with titanium was much lower than for the pillared ones. Secondly, there are no pillars in their structure. It is believed [15,22] that for interlayered clays, pillars are the source of Lewis acidic sites. Lower Lewis acidity for Ti-samples in comparison with Al-pillared ones (per gram of catalyst) is confirmed by acidity measurements (Table 4). The V/S/Ti sample loaded with higher amount of titanium species exhibited higher values of textural parameters. However, its catalystic performance was very similar to that of the V/C/Ti sample, indicating that there is no direct correlation between textural parameters and methanol conversion for titania-modified samples.
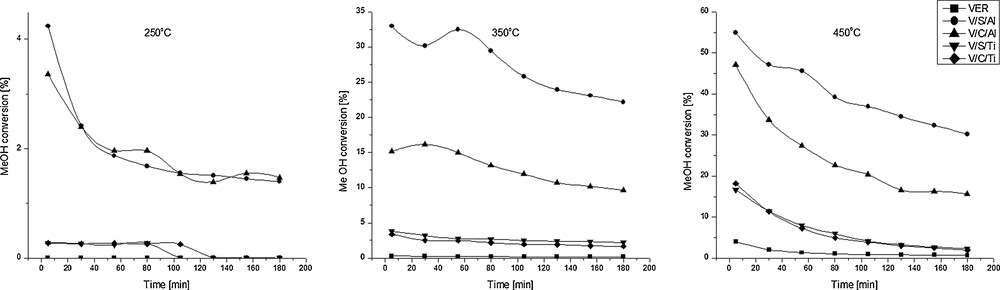
Conversion of methanol as a function of time at 250, 350 and 450 °C for samples V/S/Al, V/C/Al, V/S/Ti, V/C/Ti and raw vermiculite; WHSV = 11.9 h−1.
There is a correlation between the Lewis acidity of the studied catalysts and the rate of methanol conversion presented in Fig. 6. Indeed, it seems that for reactions carried out at 350 °C (and at 450 °C to a lesser extent), methanol conversion strongly depended on the acidity of the catalyst. Since the relation between methanol conversion and the number of Lewis acid sites at both 350 and 450 °C is not linear, it might be concluded that other factors such as texture were also influential besides catalyst acidity. Nevertheless, the concentration of Lewis acid sites followed the sequence: V/S/Al > V/C/Al > V/S/Ti, proving that the higher the sample's acidity, the higher the rate of methanol conversion.

Average methanol conversion vs. no of Lewis acid sites; WHSV = 11.9 h−1; temperatures: 250, 350 and 450 °C.
All reaction products were analysed as a function of time on stream and it was observed that at lower temperatures (250 and 350 °C), only DME was formed, which is in agreement with literature data suggesting that DME formation is favoured at lower temperatures [28]. The product distribution obtained at 450 °C for samples V/S/Al and V/S/Ti is shown in Fig. 7. Al-Pillared and Ti-modified samples present quite different distributions. The former led to the formation of a variety of products at the beginning of the reaction, while only DME and small amounts of methane were registered after 30 mins. On the other hand, titanium-modified samples showed high selectivity to DME, decreasing with time on stream. The yield of methane increases as the reaction proceeds. These differences can be tentatively ascribed to the presence of additional Lewis sites on alumina pillared samples. Moreover, the lack of pillars in V/S/Ti results in textural differences, such as lower specific surface area, as well as lower total and microporous volumes.
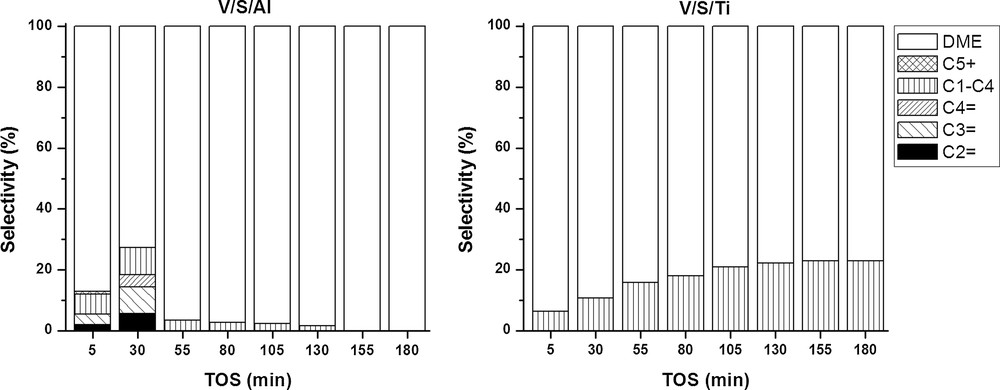
Product distribution obtained for samples V/S/Al and V/S/Ti at 450 °C; WHSV= 11.9 h−1.
All of the samples exhibited mainly acid sites of Lewis type. Though the basicity of the samples was not examined, aluminosilicates exhibit acid–base pairs on the surface. The acid sites are associated with the presence of Al3+, Fe3+ cations, while the basic sites are attributed to O2−anions of aluminosilicates [15,29]. The mechanism of the methanol dehydration reaction is still not yet well known. However, from many reaction paths described in the literature [13,28,30–32], all proposed mechanisms require adjacent acid–base pairs of Lewis or Brønsted type. As water is produced during the reaction, the Lewis acid sites can be transformed in situ into Brønsted acid sites [28]. Moreover, since the methanol dehydration to DME is a structure-sensitive reaction, it can be used for the characterization of the acid properties of the catalyst [13,31,32]. Since with all samples, DME was obtained as the main product (Fig. 7), which confirms the presence of acid–base pairs and is consistent with acidity measurements, an additional relation between methanol conversion and textural parameters can be established. Methanol conversion decreased for samples that present lower values of textural parameters (Table 3). The sample with the highest values of specific surface area and porous volumes showed the best catalytic performance. Moreover, there was a strong influence of microporosity on the reaction. For catalyst samples with poor microporosity (V/S/Ti and V/C/Ti), methanol conversion was much more inferior than for Al-pillared ones. Raw vermiculite, which does not exhibit any microporosity, did not convert methanol at all. Fig. 5 also shows that all samples showed a noticeable decrease in activity with time on stream, due to coke formation.
The results of thermogravimetric experiments carried out for the spent catalysts tested at 250, 350 and 450 °C are presented in Fig. 8. All studied samples exhibited weight losses between 7 and 12 wt.%. Two regions of weight loss can be distinguished. The first one, up to ca. 200 °C, can be associated with the desorption of adsorbed water. The second one, from ca. 250 °C, is attributed to the oxidation of coke deposits, since it is accompanied by an exothermic peak. Thus, the loss of catalitic activity is most probably caused by the deposition of carbon on the surface of the catalyst. Based on the results of the catalytic tests, the deactivation rate at 450 °C was calculated as the ratio of the difference between methanol initial and final conversion to its initial conversion. The comparison of the deactivation rates is given in Table 5. Titania-modified samples were deactivated much faster than the Al-pillared ones, pointing one more time that the textural parameters of samples had a significant influence on the catalysts’ performance. The lower porosity of titania-modified samples could result in faster blocking of the space available for the reaction and thus cause faster deactivation.

(Colour online). TG profiles of samples V/S/Al (A), V/C/Al (B), V/S/Ti (C), V/C/Ti (D) measured for the spent catalysts after reaction at 250, 350 and 450 °C; WHSV = 11.9 h−1.
Comparison of the deactivation rate of tested catalyst samples at 450 °C. WHSV = 11.9 h−1.
| Sample | Initial MeOH conversion (%) t1 = 5 min | Final MeOH conversion (%) t2 = 180 min | Deactivation rate (%) |
| V/S/Al | 55.0 | 30.2 | 45 |
| V/C/Al | 47.0 | 15.6 | 66 |
| V/S/Ti | 16.6 | 2.3 | 86 |
| V/C/Ti | 18.2 | 1.9 | 89 |
The comparison of the catalytic performances of modified vermiculites with other clay minerals [19–21] and of catalysts typically used in the methanol dehydration to DME (Table 1) is not easy, since different reaction conditions have been used. Modified vermiculites studied in this work were active at higher temperatures than the catalysts described in the literature. However, they showed a selectivity to DME of around 100%, which is beneficial, because it eliminates the problem of product separation in case of DME production on an industrial scale. When compared with other layered clays Al-pillared, vermiculite-based pillared catalysts seem to be more promising.
3.2.2 The influence of methanol hourly space velocity on activity and product distribution
The effect of WHSV on the reaction was studied for the two most active catalysts of each series: V/S/Al and V/S/Ti. The reaction was carried out at 450 °C with WHSVs of 11.9, 3.9 and 1.9 h−1 for sample V/S/Al. Sample V/S/Ti was tested with space velocities of 11.9 and 3.9 h−1.
Fig. 9 presents the evolution of methanol conversion as a function of time on stream for a V/S/Al sample tested with different space velocities. The deactivation is very fast for all the used conditions and, as expected, the test carried out with a WHSV of 1.9 h−1 gave the highest conversion rates. Due the high deactivation occurring at the initial time, the conversions observed with a TOS of 5 min are very similar.
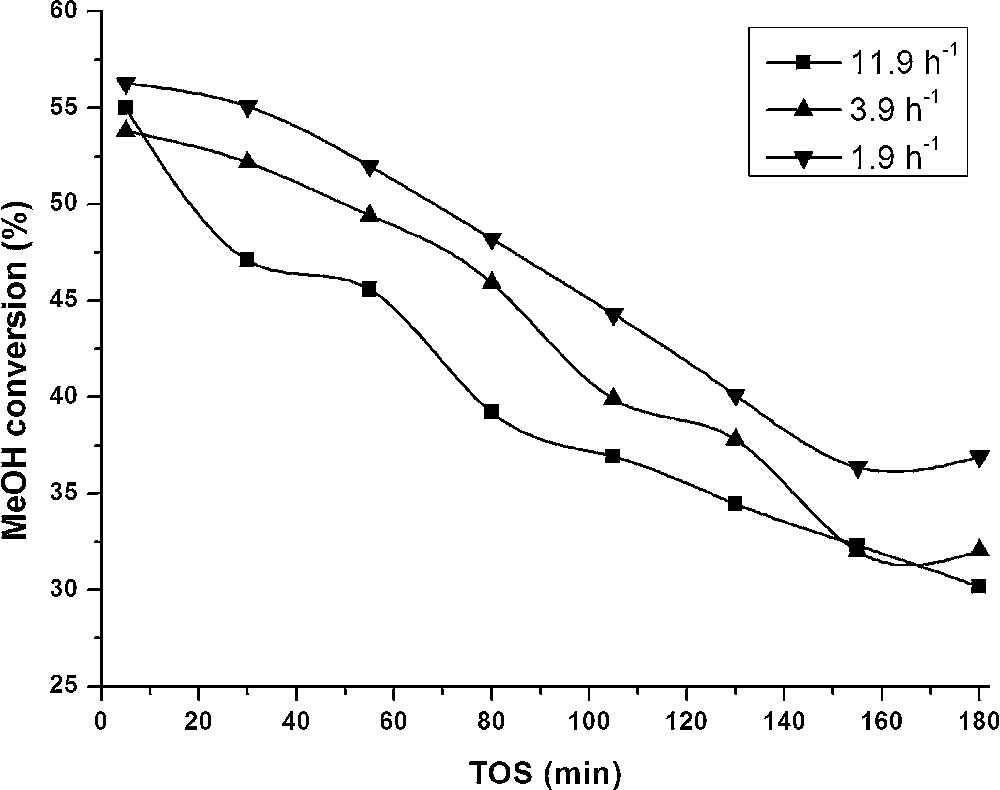
Methanol conversion as a function of time on stream for different values of WHSV (11.9, 3.9, 1.9 h−1) for sample V/S/Al; temperature: 450 °C.
Selectivity and product distribution evaluated for reactions carried out with different methanol space velocities are shown in Fig. 10 and Table 6. It should be noticed that although methanol conversion was not strongly influenced, the selectivity to DME changed drastically. The initial selectivity (at t = 5 min) dropped from ca. 90% for a WHSV of 11.9 h−1 to ca. 40% for a WHSV of 1.9 h−1. Instead of DME, high amounts of hydrocarbons were produced, such as methane, ethylene, propene, and different C4 products. This result suggests that DME is an intermediate in the formation of hydrocarbons. Similar observations were made for MTG reaction, applied in the industry to produce gasoline [33]. The formation of this type of hydrocarbons, namely olefins, led to higher coke formation and thus faster deactivation of the catalyst.

Product distribution for different values of space velocity: 11.9 and 1.9 h−1; sample V/S/Al; temperature: 450 °C.
The effect of space velocity on methanol conversion and selectivity for V/S/Ti sample. Temperature 450 °C. Pure methanol feed.
| WHSV (h−1) | Conversion of MeOH (%) t1 = 5 min, t2 = 180 min | Selectivity to DME (%) t1 = 5 min, t2 = 180 min |
| 11.9 | 16.61 | 93.66 |
| 2.34 | 77.07 | |
| 3.9 | 28.13 | 94.07 |
| 5.09 | 77.51 |
Concerning the tests performed with the V/S/Ti catalyst, it was observed that, as opposed to Al-pillared vermiculite, the change in methanol space velocity did not affect selectivity to DME–the values obtained for WHSV 11.9 and 3.9 h−1 were similar (results not shown). This result may be associated with the difference in porosity, because titania-modified samples exhibited much lower microporosity than Al-pillared vermiculites. Additionally, no effect on product distribution was observed, the only by-product obtained in both cases being methane.
3.2.3 The influence of water on activity and product distribution
The effect of water on the reaction of methanol dehydration to DME was tested for the catalyst with the best catalytic performance. The reaction was carried out at 450 °C using a mixture of 80 wt.% methanol in water (3 cm3 h−1) and the results were compared with those obtained with pure methanol. The experiments were carried out in order to establish the influence of water on the catalyst's activity, as it has been proved that water could have either negative or positive effect on methanol conversion depending on the catalyst's properties [7].
From Fig. 11, it is possible to observe that, at the beginning of the reaction, methanol conversion was similar in both cases and was equal to ca. 55%. With the increase in TOS, MeOH conversion decreased drastically in the presence of water, as compared to pure methanol feed. Deactivation caused by water was also observed by Xu et al. [12], who concluded that water competed with methanol over the active sites and permanently blocked them. The tested catalyst was additionally deactivated by coke formation.

Effect of water addition into the feed (80 wt.% methanol in water; 3 cm3/h) on methanol conversion. Temperature: 450 °C; sample V/S/Al.
Selectivity to DME at the beginning and at the end of the reaction (TOS = 5 or 180 min., respectively) is compared for the feed with and without the addition of H2O in Table 7. It may be concluded that the addition of water into the feed affected not only the activity of the catalyst, but also its selectivity to DME. For pure methanol feed, the selectivity to DME was equal to 87% at TOS = 5 min, while for the mixture of 80% of methanol in water, the selectivity decreased to ca. 60%. Product distribution was also affected: more hydrocarbons, such as methane, ethylene, propene and different C4 products were obtained when the feed contained water. This may be the reason for faster catalyst deactivation by coke formation from hydrocarbon products. At TOS = 180 min selectivity to DME in both cases was close to 100%.
The effect of water addition into the feed on the selectivity to DME for V/S/Al sample.
| Feed | TOS (min)) | Selectivity to DME (%) |
| Pure MeOH | t1 = 5 | 87.03 |
| t2 = 180 | 99.33 | |
| 80 wt.% MeOH/ 20 wt.% water | t1 = 5 | 59.62 |
| t2 = 180 | 100 |
Conclusions
Intercalation and acid activation of vermiculite resulted in the formation of porous materials characterized by increased specific surface areas and pores volumes. All the materials obtained were tested as catalysts for methanol dehydration to DME.
The following conclusions may be drawn from the present study.
- a) IR measurements showed that the samples did not possess any strong Brønsted acidity. Based on that fact, it can be concluded that in the case of the prepared samples, the reaction of methanol dehydration to dimethyl ether essentially proceeds via Lewis acid sites.
- b) All samples were active in the methanol dehydration and selective to DME. Their catalytic performance depended on the modification procedure. Al pillaring was found to result in more active catalysts than the modification with TiO2. Methanol conversion at 250, 350 and 450 °C formed the following sequence: V/S/Al > V/C/Al > V/S/Ti > V/C/Ti, following the same trend as that observed in both surface area and number of Lewis acid sites.
- c) At lower reaction temperatures (250, 350 °C), the selectivity to DME was equal 100%, while at 450 °C, the selectivity to DME varied from 70 to 100% depending on the catalyst used.
- d) The best catalytic performances were observed for the V/S/Al sample, with methanol conversion rates higher than 30% for high TOS (at the beginning of the reaction 55%) and selectivity to dimethyl ether > 99%.
- e) Raw vermiculite showed very low conversion of methanol at 450 °C and was not active at 250 °C.
- f) The change in hourly space velocity did not have any significant influence on the catalytic conversion of Al-pillared vermiculite, but instead changed product distribution and selectivity at the beginning of the reaction. Lower space velocities increase the conversion of methanol by ca. 10% and drastically decreased the initial selectivity to DME.
- g) The addition of water into the feed had a negative effect on both methanol conversion and selectivity to DME and also resulted in faster catalyst deactivation.
The tested modified samples were active at higher temperatures with respect to the materials described in the literature. However, the performed research allowed us to establish that, in order to enhance the catalytic performance, vermiculite-based catalysts should be modified by the addition of appropriate promoters to tailor the acid-base properties. Additionally, the contact time should be shortened (higher values of WHSV should be used) in order to avoid the consecutive products (hydrocarbons) and thus establish higher selectivity to dimethyl ether.
Acknowledgements
The authors thank Portuguese FCT for financial support (SFRH/BPD/91397/2012) and AGH grant 11.11.210.213.


