1 Introduction
Helicenes are molecules with ortho-fused aromatic rings that adopt a helical shape as a consequence of the steric hindrance between the terminal rings [1]. The first hetero[5]helicenes were reported in 1903 by Meisenheimer and Witte, [2] and the first synthesis and resolution of carbo[6]helicene (hexahelicene) was performed by Newman in the 1950s [3a–c]. Since then, numerous methods have given access to a great variety of carbo-, hetero- and metallo-helicenes, [1] sometimes on a multi-gram scale [3d–f]. Using diverse stereoselective or resolution methods, a great panel of enantiopure helical π-conjugated scaffolds can be accessed [3,4]. Concomitant with the discovery of new efficient synthetic methodologies, experimental combined with theoretical studies of the steric, electronic, and optical properties of helicenes have shed new light on the potential applications of this class of inherently chiral molecules as chiral materials [5]. Indeed, the main property of helicenes is their helical structure which renders them chiral. Such inherently chiral topology combined with an extended π-conjugation provides helicenes with huge optical rotation (OR) values, intense electronic circular dichroism (ECD) spectra [6] and substantial circularly polarized luminescence (CPL) [7]. Strong chiroptical activity may find many applications in chiral materials [8]. The OR, ECD and CPL represent chiroptical properties which are characterized by differences between left- and right-handed circularly polarized light transmission, absorption and emission, respectively [9].
The development of functional devices at the molecular level has attracted considerable interest during the last few decades [10]. Molecular switches, in which there is a switching between two molecular states, are based on bistability, which means that the molecule has two stable states and can be resting in one of them. There are also other requirements for the construction of chiroptical switches such as chemical stability, non-destructive read-out, fast response times, reproducibility and fatigue resistance [11]. Interconversion of the two bistable chiral forms can result from responses of the chiral molecules to external stimuli such as heat, light, pressure, pH, chemicals, redox potential, solvent, etc. (Scheme 1).
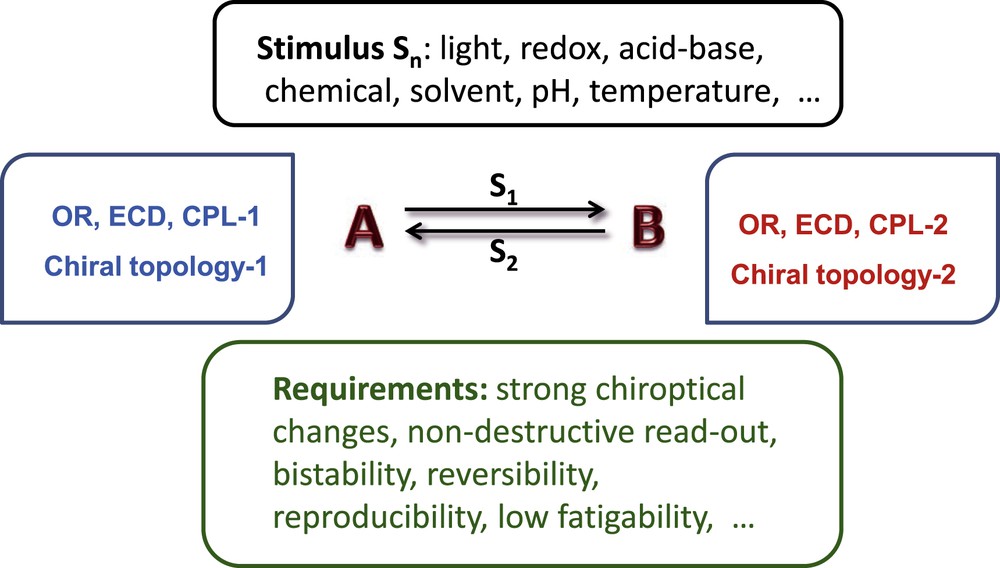
Principle of an efficient chiroptical switch.
The contributions to the field of chiroptical switches are stimulated by their potential applications as molecular memory elements, or logic operators, in which data storage and processing are the ultimate goal [11]. For example, systems that possess switchable functionality may lead to new prospects in molecular information processing and storage [12]. Chiroptical switches have also found applications in the detection of a multitude of different analytes with a high level of sensitivity, in asymmetric catalysis to offer switchable stereoselectivity [13] and in molecular motors since chirality can provide unidirectionality and original kinds of motions [11]. On the other hand, devices displaying circularly polarized luminescence (CPL) are of great interest because CPL activity may be a powerful method for addressing encoded information (cryptography) or for preparing 3D displays [7b].
Several recent examples of chiroptical switches have used helicenes in their designs. They are detailed in the next sections which are organized according to the input responsible for the switching process, namely helicene-based switches triggered by i) light, ii) redox and iii) acid/base stimuli. Helicene's unique attributes allow them to display several advantages such as higher solubility than more planar aromatic structures and large variations in electronic properties and chiroptical properties upon breakage or modification of the π-conjugation. Stability is an important requirement for switches, solubility allows handling in solution, and the possibility of large reversible changes in properties upon stimulation confers facile read-out.
2 Light-driven helicene-based chiroptical switches
Photoisomerization reactions of diverse chromophores (such as diarylethenes, spiropyrans, or azobenzenes) are good ways to photomodulate the chiroptical properties of a chiral molecule [11]. Diarylethenes and more thermally stable dithienylethenes (DTEs) are known to undergo reversible ring closure and opening upon irradiation with two different wavelengths, accompanied by an important variation of the optical properties [14]. With the use of appropriate chiral substituents, the cyclization can be diastereoselective and may be used in chiroptical switches [14,15]. A few examples of chiroptical switches bearing dithienylethenes and helicene moieties have been studied, taking advantage of these photochromic scaffolds to modulate the helical topology. In 1999, Dinescu et al. reported photochromic helical 1,2-dithienylethenes [16] that were built upon the rigid frame of dodecahydrophenanthrene 1, a thia[5]helicene analogue due to the ortho-fused multi-ring locked structure in the open form 1 (Scheme 2). The high molecular rigidity of the closed form 2 provided stability to the isomer formed after cyclization. The switching process consisted of photo-closing and photo-opening by irradiation at wavelengths corresponding to the absorption bands of the open (λ = 310 nm) and closed (λ > 450 nm) isomers. Although the authors did not study the process by chiroptical spectroscopy, they achieved a reversible thermally stable photochromism. Indeed, the colour of the benzene solutions changed from clear to yellow upon UV irradiation of the open form, and from yellow to colourless upon irradiation with visible light. The photochromic behaviour was also explored in an amorphous polymeric film. The authors attributed the efficiency of complete switching between these species to molecular rigidity and helical conformation and more than 10 cycles could be achieved in the absence of oxygen [16].
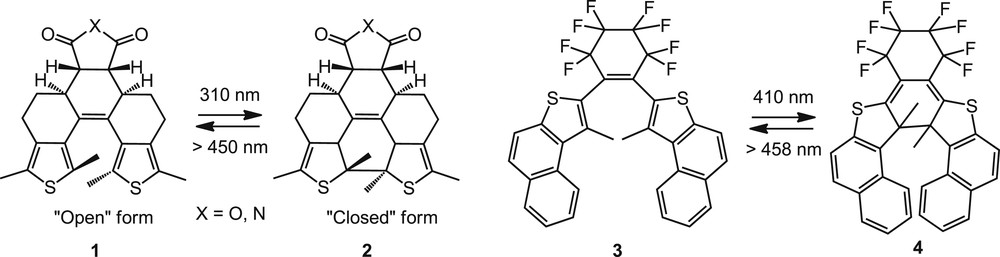
Racemic photochromic dithienylethene switches based on thia[5]- or thia[7]-helicenic derivatives either in the open (1,3) [16] or in the closed forms (2,4) [17a].
In 2001, Branda and co-workers reported the incorporation of the DTE photochrome within thia[7]helicenes [17]. When irradiated at 410 nm, the open-form DTE scaffold 3 [17a] (Scheme 2) underwent a conrotatory ring-closure, yielding the thia[7]helicene architecture 4 (74% in the photostationary state). The photochemical regeneration of the open form by irradiating at 458 nm destroyed the extended thia[7]helicene backbone. The photoswitching activity was studied through the changes in the UV–vis absorption spectra of the racemic system. Later on, an enantioenriched version was developed by the same authors, by attaching chirality at the end of both arms through pinene derivatives (5 in Scheme 3) [17b]. The photoswitchable behaviour of ring-opened and ring-closed forms was examined using their chiroptical properties (OR and ECD). The ring closure performed at 400 nm appeared 40% effective in the photostationary state and revealed a high level of stereoselectivity since only one single stereoisomer M-6 was obtained. The photoreaction appeared reversible with efficient ring opening at higher wavelengths without observable degradation. Because of a dominant chiral conformer, the ring-open system 5 displayed ECD and strong differences were observed in the ECD signal of the photostationary state 6 (Scheme 3b, inset), as well as in the optical rotary dispersion (ORD) spectra (Scheme 3b,c). Therefore, they satisfy the requirements of a successful chiroptical switch with large differences in the ECD and ORD spectral properties and stability of the ring-open and closed forms. This example nicely illustrates how the combination of an efficient photoresponsive molecular backbone and an inherently chiral architecture can greatly impact the environment and properties through efficient chiral induction processes.
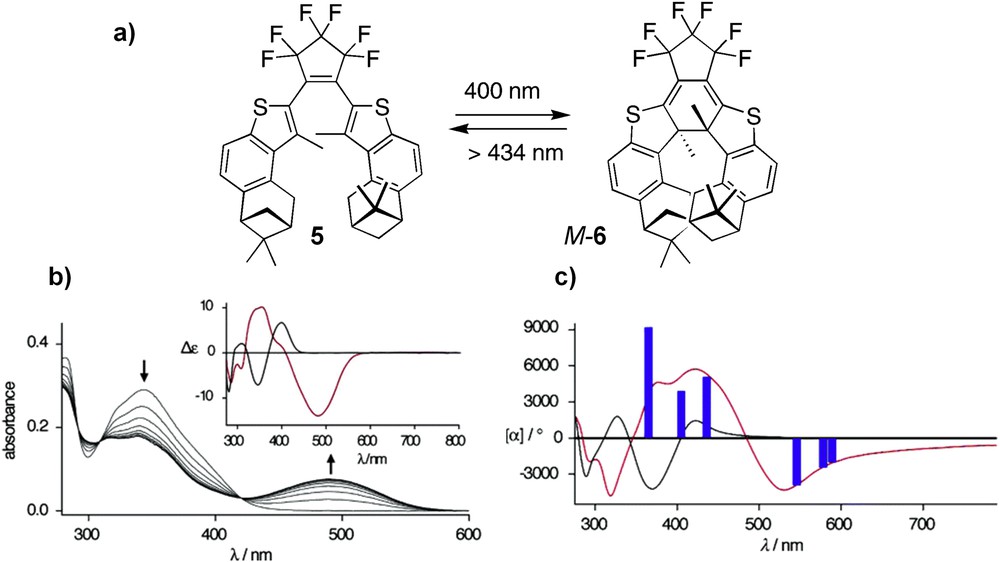
a) Stereoselective photocyclization of chiral dithienylethene 5 to thia[7]helicene M-6 [17b]. b) Changes in the UV–vis absorption spectra of a benzene solution of open form upon irradiation at 400 nm light. Inset: ECD spectra of open form in benzene (black trace) and of the photostationary state (red trace) generated at 400 nm light. c) Optical rotatory dispersion of open and closed forms (black and red traces respectively). Adapted with permission from Ref. 17b.
In 2007, Yokayama et al. developed similar dithienylethene photochromes to thiahelicene-like closed forms [18a]. In this case, the photoirradiation diastereoselectivity was achieved by introducing only one asymmetric carbon in the open form (7, Scheme 4). Very large differences in specific optical rotations between non-helicenic open form 7 and helicenic photostationary state 8 were obtained. The diastereoselectivity of the photochemical ring closure was analysed as a balance between stronger steric/electronic repulsions and 1,3-allylic strains. For example, upon photoirradiation at 366 nm, the enantiopure chiral open form 9 yielded M-10 with 57% conversion and 90% diastereomeric excess [18b].
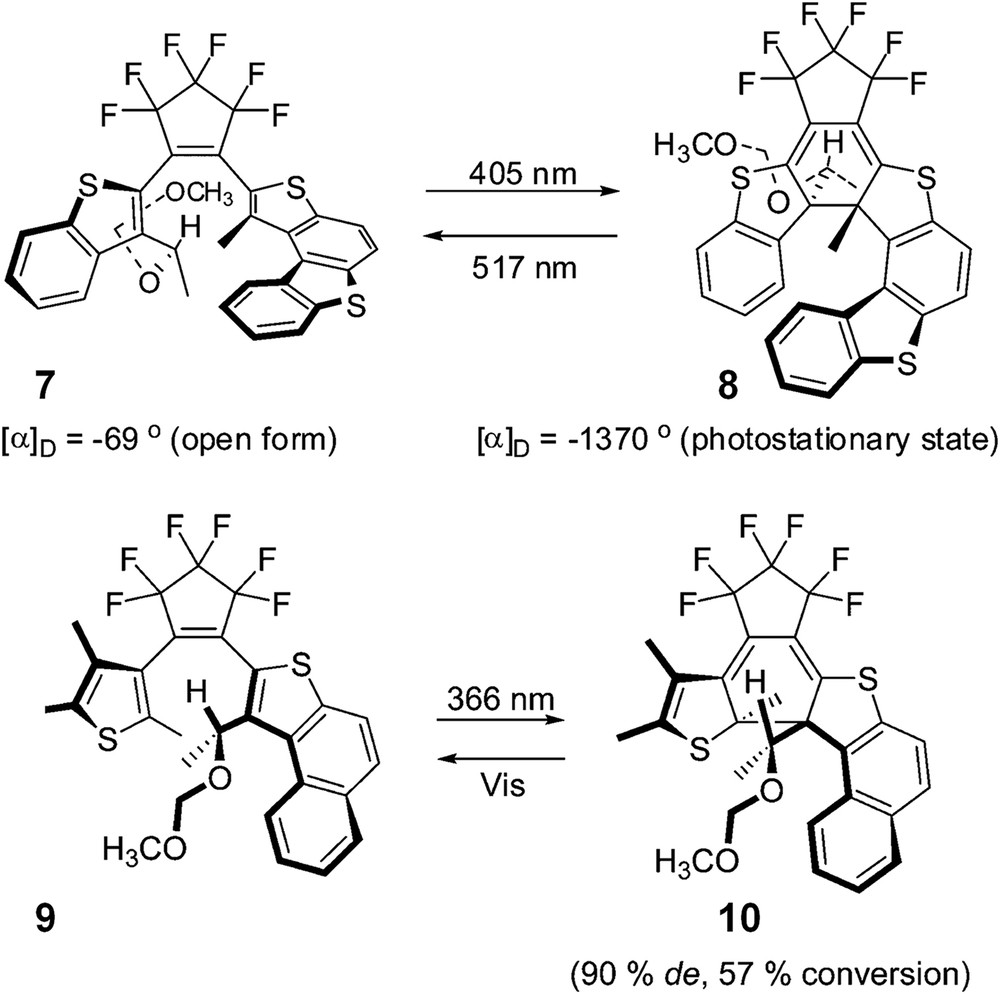
Helicenoid diarylethenes developed by Yokayama et al. Adapted with permission from Ref. 18a,b.
Other photochromic systems than DTE's have been used in helicene chemistry. For example, Moorthy et al. developed racemic helicenes such as 11 incorporating a chromene moiety which exhibited an original photochromism process [19]. Indeed, upon light irradiation of colourless 11, helical o-quinonoid intermediate 12 was formed and was responsible for the colour appearance and UV–vis absorption (Scheme 5) [19a]. Such systems were proposed as molecular logic gates with INHIBIT function for applications in optical data storage devices [19b].
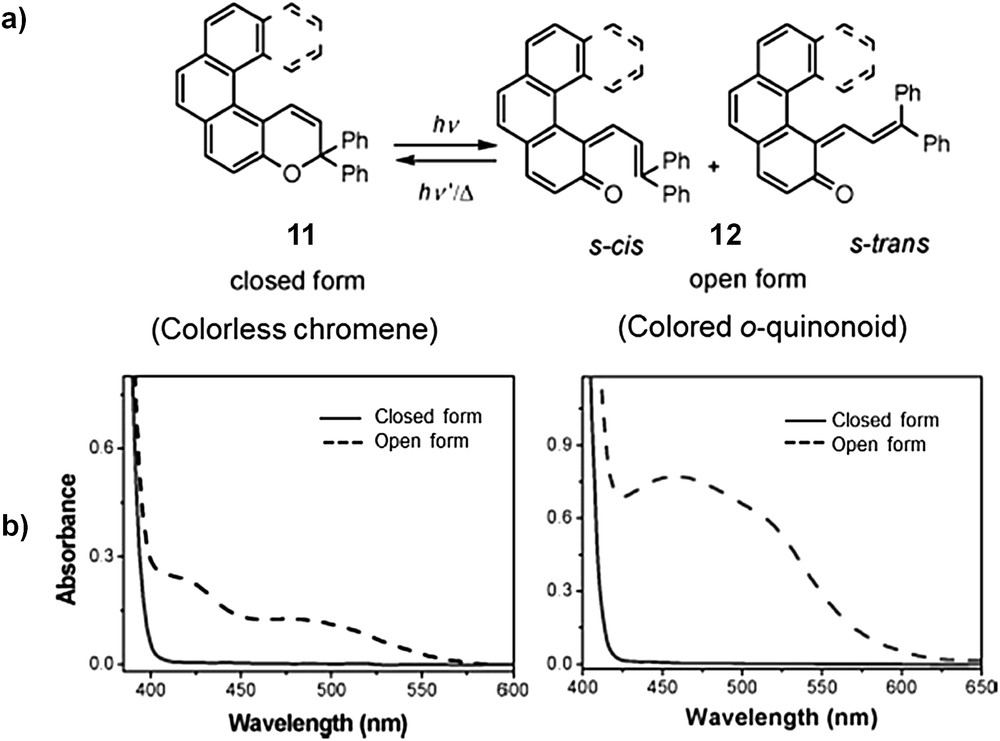
a) Helicene-chromene developed by Moorthy’s group [19]. b) UV–vis spectra of [4]helicene (left) and [5]helicene (right) chromene before (closed form) and after photoirradiation (open form). Adapted with permission from Ref. 19a.
Azobenzenes have also been widely used as photoresponsive cis-trans compounds in the design of chiroptical switches [20]. Wang et al. [21] designed a [5]helicene analogue 13, consisting of a helical o-terphenyl molecule grafted with a photoresponsive azobenzene moiety and an electroactive imide group, therefore acting as a dual-mode chiroptical molecular switch (Scheme 6). The strong specific rotation measured at 436 nm could be tuned through trans-cis photoisomerization of the azobenzene moiety using UV and visible light. Reversible electrochemical modulation (vide infra) between M-trans-13 and [M-trans-13] •- was also achieved through the reversible redox reaction occurring at the imide group. Large chiroptical read-out signals were observed during the redox cycles as indicated by the strong molar ellipticity changes at 454 nm (Scheme 6) [21]. Photomodulation studies were also conducted in the solid state, using a thin film of polycarbonate containing 2 wt % of P-13, where a switching behaviour similar to that in solution was observed.
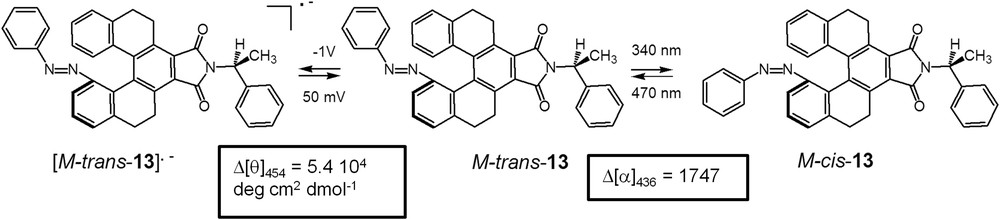
Dual photochromic and redox chiroptical switch developed by Wang et al. in its cis and trans azobenzene forms [21].
3 Redox-driven helicene-based chiroptical switches
In redox-triggered chiroptical switches, the chiroptical properties can be modulated by oxidation or reduction [13, 22], induced either by an electrochemical potential or by chemical oxidizing/reducing agents. It is important that sub-products from chemical reagents and additives such as electrolytes do not interfere with the detection method. Polymers, coordination complexes and organic molecules have been used to display redox chiroptical switch behaviour [22]. It is indeed possible to obtain a strong chiroptical signal and to achieve good switching activity by using helical polymers with chiral substituents or dopants. In coordination complexes, redox processes cause electronic changes at the metal and conformational changes in the ligands may occur which can be used in chiroptical switches. Finally, organic molecules can show important changes in their chiroptical properties upon oxidation/reduction in one or two-electron processes. The same principles are valid for the design of helicene-based redox-triggered chiroptical switches. Pioneering work in the electrochemical modification of helicene structures was achieved in 1993 by T. J. Katz et al. who prepared helicene oligomers M-(−)-14 using cobaltocene's chemistry (Fig. 1). These organometallic oligomers revealed efficient tuning of their ECD spectrum upon reduction of CoIII to CoII [23].
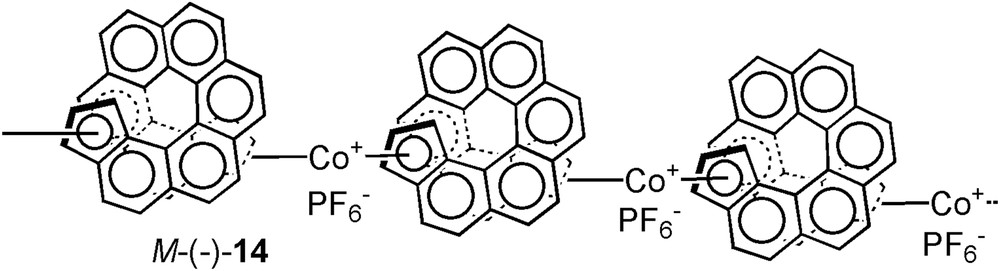
First example of oligomeric organometallic CoIII complexes M-(−)-14 bearing bis-η5-helicenic ligands and displaying redox-tuning of the ECD [23].
Our group became involved in the field of chiroptical switches in 2010, when we started the investigation of platinahelicenes, which incorporate one or several platinum centres in their helical backbone [24]. These platinahelicenes, such as 15 in Scheme 7, display strong chiroptical properties and efficient phosphorescence at room temperature (rt.), and the presence of the platinum ion allows for the tuning of their properties. Indeed, the oxidation of PtII ion into PtIV with iodine yielded enantiopure air-stable PtIV-[6]helicenes P-16. The phosphorescence was quenched upon oxidation and the chiroptical properties (OR and ECD) were significantly modified (Scheme 7). The PtIV species P-16 could be reduced back to the PtII complex P-15 by using Zn powder, thus recovering the previous chiroptical properties. However, this PtII/PtIV-[6]helicene couple cannot be considered as a redox chiroptical switch since it does not display the reversibility and reproducibility requirements (purification steps needed).
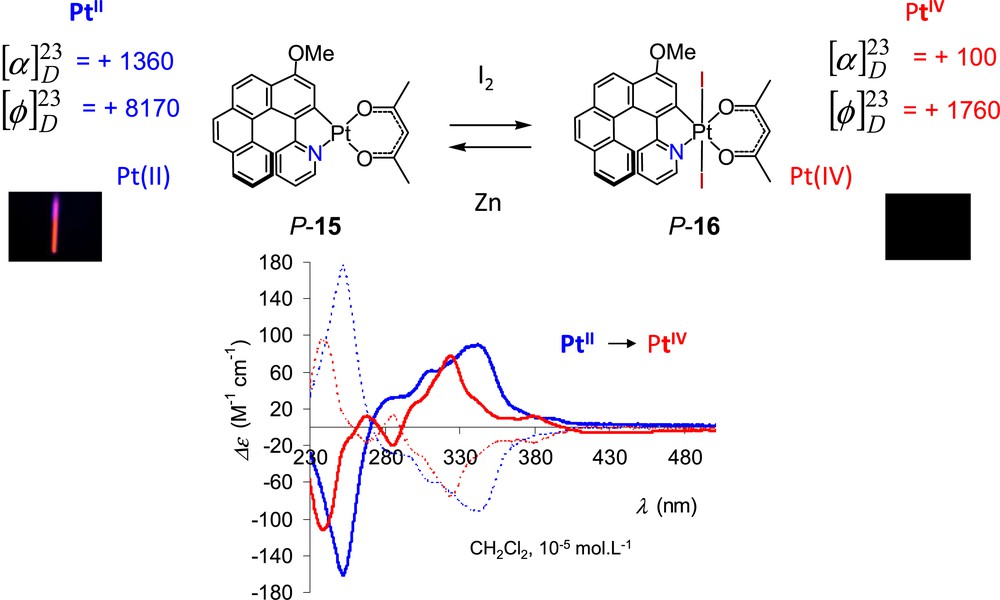
Redox tuning of the chiroptical properties (specific and molar rotations and ECD) and luminescence of platina[6]helicene 15 [24].
In 2012, we reported the first strictly speaking helicene-based redox-triggered chiroptical switch [25], by designing a carbo[6]helicene grafted with a vinyl-ruthenium moiety 17 (Scheme 8). Interestingly, the electroactive metal centre which is π-conjugated with the helicene core through a vinyl moiety enhances the chiroptical properties and allows for their redox-tuning in a reversible way without modifying the ortho-fused π-system. By using a spectro-electrochemical cell (Optically Transparent Thin Layer Electrochemical - OTTLE cell), the switch showed multistep reversible oxidation/reduction steps at low potentials (0.2/-0.2 V vs. Fc/Fc+) and significant changes in the ECD at 340, 500 nm and in the near-infrared region (around 1000 nm), as a result of the electrochemical input (Scheme 8). Theoretical calculations indicate that the vinylhelicene ligand has an important role in these processes by supporting part of the charge density which is not totally localized on the metal atom, thus behaving as a non-innocent ligand. An enantiopure di(ruthenium-vinyl)helicene system was also prepared by us and its redox-switching activity was also examined [25].
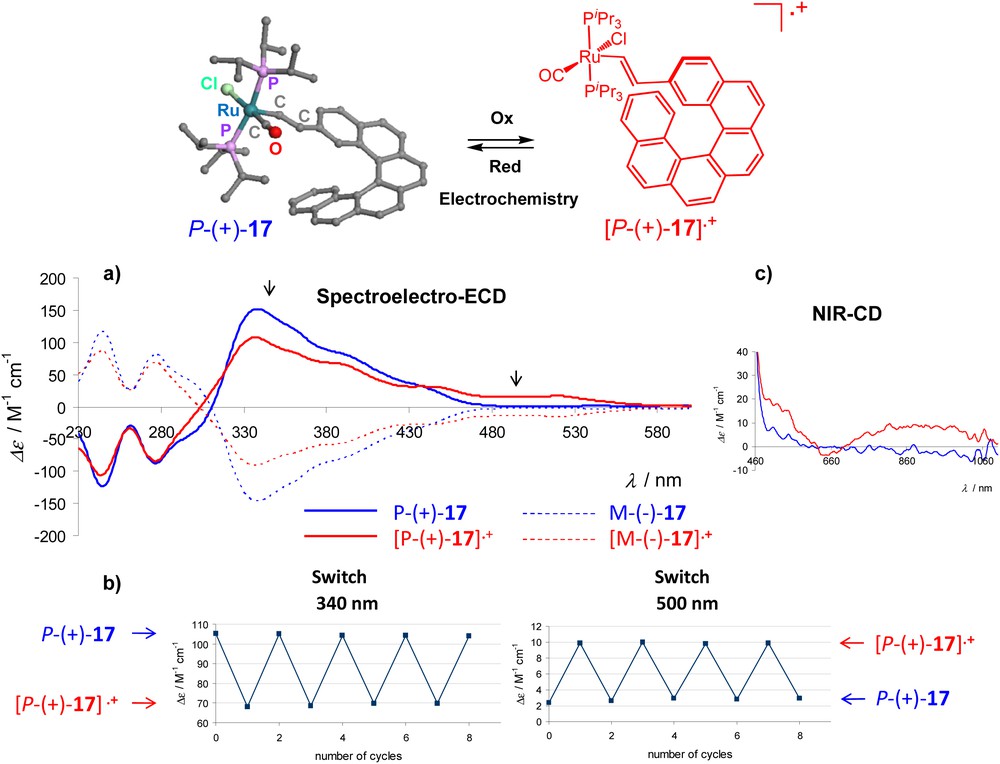
ECD spectrum of the Ru-vinylhelicene P-17 (blue) and of its oxidized species [P-17]•+ (red) in dichloromethane, with an electrolyte, at rt. in an OTTLE. b) Redox chiroptical switching observed by ECD spectroscopy at 340 and 500 nm c) NIR-CD of neutral and oxidized species [25].
In 2010, Rajca et al. reported the oxidation of a [7]thiahelicene P-18 (a helical β-oligothiophene, Fig. 2) giving rise to a radical cation which was configurationally stable at rt. [26]. The electrochemical oxidation was carried out in an OTTLE cell observing in real time the changes in the absorption spectra, ECD, and EPR (they observed the appearance of an unpaired electron). Although the bistability and reversibility were not examined in detail, these results paved the way to new organic redox-triggered chiroptical switches. Indeed, later on, several enantiopure organic redox-triggered chiroptical switches based on organic helicene derivatives were prepared and displayed good reversibility and reproducibility as observed by ECD spectroscopy in the UV–vis and NIR region. For example, in collaboration with Avarvari’s group, we reported an organic redox chiroptical switch based on a helicenic skeleton fused with an electroactive tetrathiafulvalene (TTF) moiety (M-19, Fig. 2) [27]. Stable radical-cation species [M-19]•+ were formed upon oxidation and the system displayed redox-tuneable chiroptical properties. The shifts and changes in ECD (in UV–vis and NIR region) were totally recovered over several cycles of electrochemical oxidation/reduction in an OTTLE cell, demonstrating the efficiency of non-metallic electroactive helicenes as chiroptical switches. A new kind of metal-free redox-triggered helicene-based chiroptical switch was recently reported by our group and Diederich et al. [28]. It consisted of a 1,2-ortho-quinone [6]helicene P-20 (Fig. 2) that could be reduced to the stable semiquinone radical anion [P-20]•- and oxidized back reversibly. The changes in ECD and UV–vis absorption were followed by spectro-electrochemistry, and the switching was reversible over several cycles. Interestingly, the reduced semiquinone species was able to recognize the two enantiomers of chiral binaphthyl phosphoric acid. This diastereoselective process was evidenced by ENDOR experiments [28]. Note that in both examples 19 and 20, significant intramolecular charge transfers have been found to be responsible for the strong optical modifications.
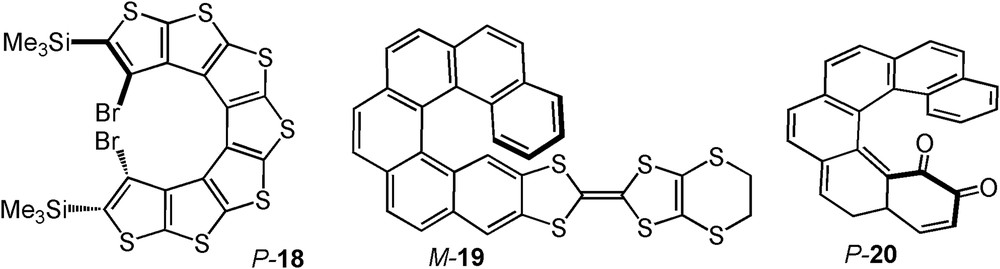
Examples of organic helicenes bearing electroactive moieties for the development of redox-triggered chiroptical switches [26–28].
Teply and co-workers have studied the electrochemical behaviour of configurationally stable helical-shaped condensed N-heteropolyaromatics named helquats, such as 21 in Scheme 9, which consist of a structural combination of helicenes and viologens [29]. With these structures, they reported the most intense chiroptical switching response in the field of helicenoids to date [29b,c]. The system works at three stages, with two consecutive reductions from di-cation, mono-cation to the fully reduced neutral form. The radical cation formation upon first reduction or oxidation was monitored by EPR spectroscopy. The electrochemical transformation caused very large changes in the ECD spectra. The cycling between reduction and oxidation potentials even caused changes in the signs of ECD bands or an ON/OFF switching of the ECD signal depending on the read-out channel selected (wavelength) [29b].
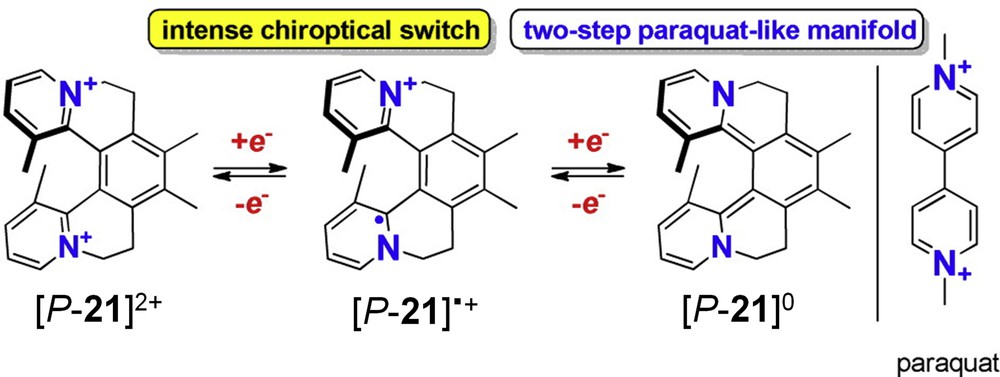
Helicene-paraquat derivative 21 developed by Teply et al. that display strong chiroptical redox-switching activity. Adapted with permission from Ref. 29b.
While the former examples did not display any changes in their molecular geometry, a series of redox chiroptical switches based on the electrochemical C-C bond formation/breaking has been developed by Suzuki and collaborators since 2001 [30]. Such systems consist of a closed form, a dihydro[5]helicene derivative with intrinsic helical chirality, and an open one corresponding to a biaryl compound with axial chirality. For instance, the dihydro[5]helicene P-22 was opened by two-electron oxidation to a stable axially chiral and strongly coloured dicationic species P-23 (Scheme 10a). Upon two-electron reduction, a good reversibility and bistability were achieved, taking into account the importance of the configurational stability and low racemization in the process. Between the two states, large differences in the UV–vis and ECD spectra were observed [30a,b]. The same authors investigated these systems as multimodal switches. For example, in a multi-input/multi-output system, they investigated fluorescent dihydro[5]helicene derivative P-24 with appropriate aromatic substituents, which upon oxidation is stereospecifically converted into the binaphthyl dication derivative P-25 (Scheme 10b) that is no longer fluorescent and which displays different UV–vis and ECD spectra [30e]. This open oxidized species also showed solvatochromism, with different colours and ECD spectra depending on the polarity of the solvent. Therefore, they have a three-way-output response system upon electrochemical input (UV–vis, ECD, fluorescence) and the system is also considered as a multi-input one because the solvent could tune the properties in UV–vis and ECD spectra. Finally, Suzuki et al. reported similar bistable chiroptical switches based on a neutral binaphthylic diolefine as the open form that closed upon oxidation to a dicationic dihydro[5]helicene [30c,d,f]. For example, they described in 2008 a molecular system with three states [30f]. Binaphthylic diolefine M-26 was oxidized to stable dicationic helicene-type (M,R,R)-27, and this second state could be converted into fluorescent species (M,R,R)-28 by treatment with NaHCO3 (Scheme 10c). The fluorescent helicene-type state could be converted back to the dication by the addition of acid. This is a case of a four-ways output response system (UV–vis, ECD, fluorescence and fluorescence detected ECD - FDCD) with electrochemical and pH inputs (vide infra).
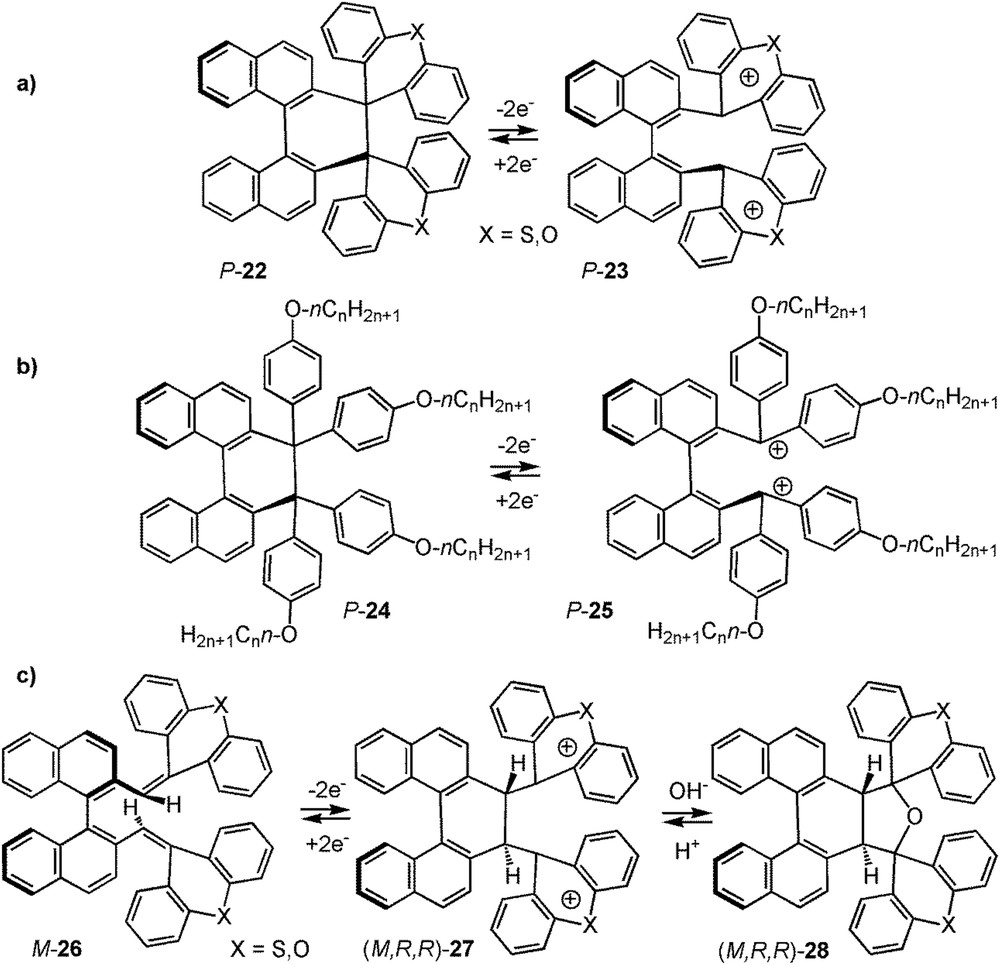
Selected binaphthylic/[5]helicenic molecular systems developed by Suzuki et al. which act as multimodal chiral switches [30].
4 Acid-base triggered helicene-based chiroptical switches
To our knowledge, very few examples of acid-base triggered helicene-based chiroptical switches have been reported to date [29c,30f,31,32]. Our group reported enantiopure osmium–vinylhelicene complex P-29 and an osmium-carbene helicene P-30 that are reversibly inter-convertible by using an acid or a base and therefore act as an acid-base chiroptical switch [31]. We could indeed achieve the formation of the osmium-carbene helicene derivative P-30 by means of HCl addition, and the recovery of the osmium–vinylhelicene complex P-29 by adding Et3N (Scheme 11). Upon such transformations, the OR values and ECD bands significantly change due to the modification of the electronic interaction between the osmium centre and π-helicenic platform. Although the low stability of these organometallic osmium–helicene complexes did not allow very good reversibility and reproducibility, this work was a good proof of concept for the use of carbenic complexes as acid-base chiroptical switches.
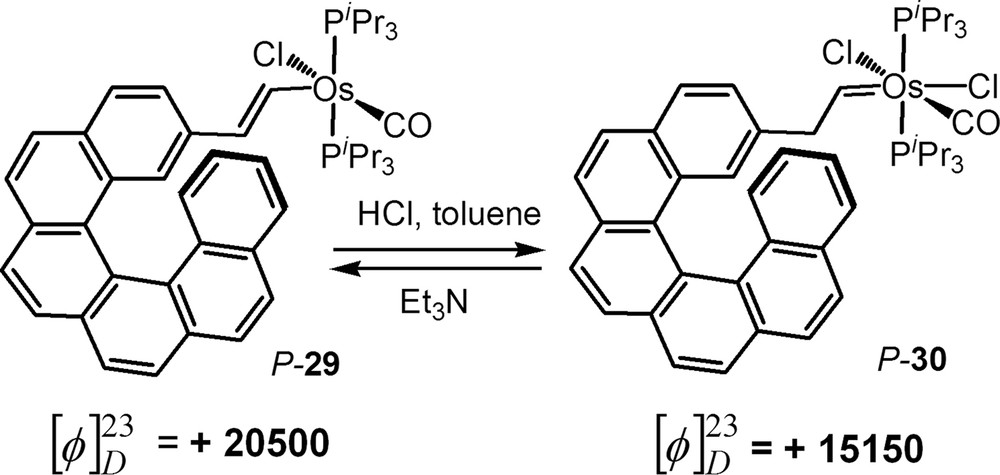
Acid/base-triggered switch based on a helicene-vinyl-osmium/helicene-vinyl-osmium binary system [31].
Recently, we also reported a multiresponsive acid/base chiroptical switch [32] based on 2-pyridyl-aza[6]helicene scaffold 31 (Scheme 12) acting as a helicenic 2,2′-bipyridine system which can be protonated through its bipyridyl core. Upon progressive addition of acid aliquots, isosbestic points were found in the UV–vis and ECD spectra, and double protonation occurred. Strong changes in emission wavelengths were observed, with a 160 nm red-shift of the fluorescence, while CPL activity remained similar (gCPL ∼ 2.5 × 10−3 [9] for both neutral and protonated P-31). The reverse process could be achieved by using a base, thus yielding an efficient switch with multimodal read-out (UV–vis, ECD, fluorescence, CPL). The corresponding cycloplatinated complexes P- and M-32 (see Scheme 12) displayed UV–vis, ECD, rt. phosphorescence and CPL activity and enabled the comparison of their switching abilities with organic ligands P- and M-31. Upon gradual monoprotonation of the free pyridyl group in 32, clear isosbestic points were also found in UV–vis absorption and ECD spectra. However, emission remained at a similar wavelength with a slight increase in the dissymmetry factor (gCPL ∼ 2 × 10−3, for protonated P-32, twice as high as the neutral form P-32, Scheme 12). Thus, this example enabled us to compare the CPL switching behaviour of a fluorescent organic species and a phosphorescent organometallic one [32]. Note that switchable CPL functionality may have important applications in the field of molecular information processing and storage [7b].
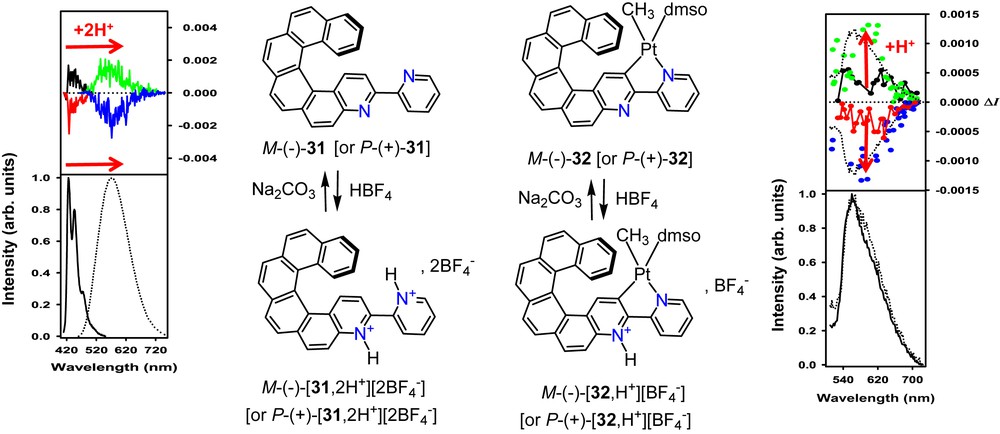
Acid-base triggered switching of CPL of enantiopure P and M organic (31) and organometallic (32) helicene-bipyridine derivatives. Left: CPL (top) and total emission (bottom) of fluorescent 3-(2-pyridyl)-4-aza[6]helicene P-31 (black), and M-31 (red) enantiomers to respectively P-[31,2H+][2BF4−] (green) and M-[31,2H+][2BF4−] (blue). Left: CPL (top) and total emission (bottom) of phosphorescent complex P-32 (black), and M-32 (red) enantiomers to respectively P-[32,H+][BF4−] (green) and M-[32,H+][BF4−] (blue) [32].
Finally, very recently, Teply et al. developed a helquat system displaying pH-switchability. Indeed, by using phenol substituents in the helquat structure, the changes in pH-triggered very important and reversible changes in the ECD response [29c].
5 Conclusions and perspectives
In this review, we have presented the few examples of helicene-based chiroptical switches that have been reported to date in the literature. The reviewed examples are mainly triggered by light, redox or acid/base inputs, but there are alternative stimuli such as the solvent. In most of the cases, the read-out signal used to compare the chiroptical properties of the two binary states is the ECD response but other chiroptical methods can be used such as FDCD, ORD or CPL activity. Different wavelengths can be used, from the UV to the near-IR regions and large differences in chiroptical responses can be accomplished. Another important feature is the possibility to have multimodal input accompanied with multiple read-out systems.
Further developments of helicene-based switches may allow these chiral systems to be used in devices. Such an example has already been demonstrated by Katz et al. who studied electro-optic switching of helicene derivative forming a nematic liquid crystalline phase [33]. Future advances are also predictable in the area of luminescent materials and the use of CPL as a read-out signal combined with the ECD and OR activity, which could be a powerful method for addressing encoded information (cryptography) or for 3D displays. Multi-output systems are very appealing as prototypes of molecular logic operators since they may act as parallel operating logic elements. Further enhanced chiroptical switches are desired that feature higher differences in chiroptical properties between the different states. Only a limited number of examples have been reported to date but the doors are already open in electroactive, purely organic and metallahelicenes, and the field will undoubtedly develop to take advantage of their potential.
More important goals of this research would be to conceive chiral materials displaying some functions that can be tuned upon an external stimulus, e.g. systems mimicking fundamental biological processes. In addition, being able to modulate a catalytic activity or a chiral recognition process by an external stimulus is a fascinating area of research. Finally, production of motion at a molecular level may pave the way to new models of molecular machines [11,12], as has been nicely illustrated in Feringa's helical unidirectional molecular motors (Fig. 3a) [34] and in Kelly's triptycene-[4]helicene systems which act as molecular “ratchets” (Fig. 3b,c) [35].
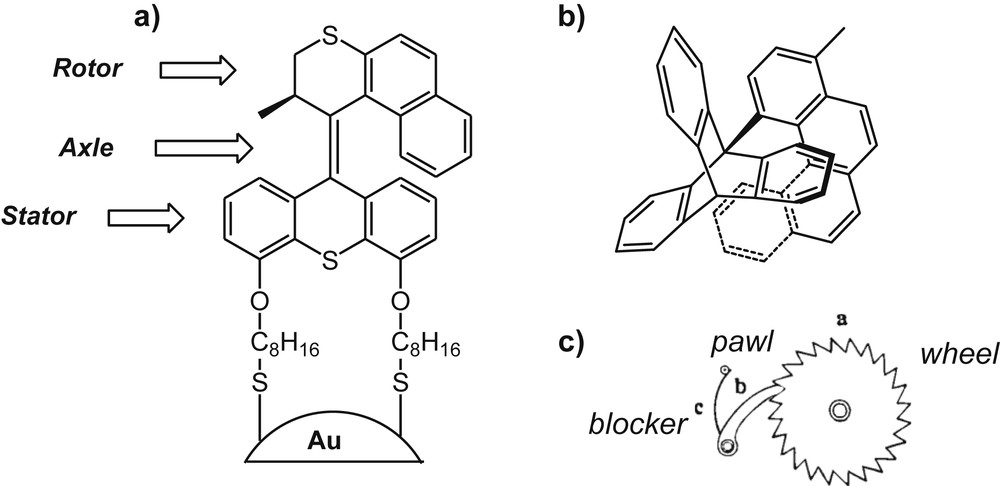
Two examples of molecular devices prepared from helical molecular systems [34,35].
Acknowledgements
We thank the Ministère de l’Éducation Nationale, de la Recherche et de la Technologie, the Centre National de la Recherche Scientifique (CNRS), the ANR (ANR-12-BS07-0004-METALHEL-01) and The Région Bretagne (SAD 2014–CHIROPEM) for H.I.'s grant. J.C. warmly thanks Prof. Régis Réau for his scientific contribution and for longstanding fruitful discussions and all the researchers and students who have been involved in this research.


