1 Introduction
Cisplatin among platin salt based chemotherapies is of major clinical importance in the treatment of many solid cancers [1–6]. The main toxicity that hampers cisplatin utilisation and therefore patient survival is its nephrotoxicity. Like most tubulointerstitial acute nephritis, cisplatin induced tubulopathy lacks specific histological signs. This is critical because patients exposed to cisplatin are likely to accumulate several nephrotoxic drugs and to present multiple risk factors of tubular aggression.
In the first part of this investigation, we have demonstrated that μXRF using synchrotron radiation can be used to assess presence and spatial distribution of trace elements such as Pt and Zn in mice kidneys. Interestingly the Zn redistribution pattern as well as the Pt spatial distribution seems to be dependant on the injected Pt salt. As oxaliplatin and carboplatin are known to cause far less tubular damages than their cisNa conjugated counterparts we wondered whether the signal we observed could be specific to cisplatin induced nephropathy, and whether we could extrapolate the observations we made in the murine model to humans.
To further investigate this hypothesis we chose to explore two pathological kidney biopsies gathered in the Nephrology department of Tenon’s Hospital. Both patients presented acute renal failure attributed to acute tubular dysfunction after platin exposure.
2 Material and methods
All samples were investigated on the DiffAbs beamline at Synchrotron SOLEIL (France). Details regarding the experiments have been extensively given in different previous publications [7–12] and also in part I of this investigation.
Patient 1 was a 55 year old patient, his main comorbidities were: liver transplantation in 2005 for chronic alcoholic steatosis and sleep apnea syndrome. In July 2011 CT4aN2cM1 epidermoid carcinoma in the postcricoïd area was diagnosed. A treatment according to the EXTREME protocol (Erbitux, Cisplatin, 5Flurouracil) was initiated on 04/08/2011. Acute renal failure occurred few days after the first infusion, the serum creatinin increased from 96 to 607 μmol/l in 6 days. His usual medications by the time of his admission in the nephrology intensive care unit included: salicylate, valgancyclovir, gabapentine, tacrolimus, calciparin and pantoprazole. There was no clinical evidence of infection, hemodynamic constants were normal; the renal sonogram was normal, and prerenal etiology was ruled out. There was neither hematuria nor leucocyturia. There was non-selective proteinuria measured at 898 mg/mmol creatinine for only 388 mg/mmol creatinine of albuminuria. Immunological investigations were negative. A kidney biopsy was performed on 19/08/2014 (Supplementary data 1), 15 days after the last cisplatin infusion. Biopsy was cortical, 15 glomeruli were analyzable, 4 of them were sclerotic. Two glomeruli were ischemic. There was mild mesangial expansion in the others. Few tubular sections showed microvacuols as seen in anti-calcineurin toxicity. Less than 5% of the tubules were atrophic. The tubular epithelium was a bit flattened. There were almost no necrotic casts in tubules lumens. Interstitial fibrosis interested 10–15% of the parenchyma. There was no interstitial inflammation. Some arterioles showed severe chronic lesions. Immunofluorescence for IgG, IgA, IgM, C3, C1q, kappa, lambda was negative. This biopsy showed only mild tubular injuries regarding the severity of the renal failure. The retained diagnosis was acute renal failure secondary to cisplatin nephrotoxicity. Evolution was pejorative; hemodialysis was initiated on 30/08/2014 and was not disrupted until the patient’s death four months later.
Patient 2 was 57 at the time of the biopsy in 2011. He was diagnosed with T4N2M1 colon liberkhunian adenocarcinoma in January 2008. He was treated surgically and received multiple chemotherapies including: FOLFOX (folinic acid, oxaliplatin and 5Fluorouracil), FOLFIRI (Folinic Acid, 5Fluorouracile, Irinotecan), bevacizumab and capecitabin. In July 2011, Folfox (including 85 mg/m2 oxalipatin) and bevacizumab were reinitiated. On 16/9/11, during the 4th cure of FOLFOX, the patient presented shivering associated with hyperthermia and nausea. An empiric anti-biotherapy including tazocillin and vancomycin was initiated. Subsequently, he presented acute renal failure (serum creatinin raised from 90 μmol/l to 325 μmol/l and 804 μmol/l) with severe hyperkaliemia (6.6 mmol/l). The patient was transferred to the intensive care unit and extrarenal epuration had to be initiated. The renal sonogram was considered normal, and the blood and urinary ionogram couldn’t distinguish prerenal participation. Blood cell count in urine was negative, but there were hemoglobinuria and 3+ proteinuria on the urine dipstick. Clinically the patient presented rapidly progressing oligoanuria. The practician noticed acute hemolysis, thrombopenia and coagulation factor consumption. No schizocytes were noticed. Hyperthermia resolved within 24 h; microbiological investigations were negative and anti-biotherapy was disrupted. The Coombs test was 3+ positive for IgG and C3. A kidney biopsy was performed on September 22 (Supplementary data 2). Twenty-one glomeruli were analyzable on this cortico-medullar biopsy. Four glomeruli were sclerotic, two were ischemic, whereas the others didn’t show any sign of pathology. There was evidence of acute tubular injury with many necrotic debris in tubules lumen. Some of those debris showed a macrophagic inflammation response. There were mild non-specific interstitial inflammations. They were neither acute vascular injuries nor sign of acute microangiopathy. Immunofluorescence was negative for IgA, IgG, IgM, C3, C1q, fibrin, Kappa and Lambda. The final diagnosis was acute tubular necrosis secondary to oxaliplatin mediated hemolysis. Evolution was favorable and dialysis was disrupted on October 3rd.Renal function slowly normalized. Oxaliplatin was not readministrated, and the patient died from generalized cancer on October 1, 2012.
Although these two patients both required dialysis initially due to acute tubular injury few days after platin salt exposure, the mechanism underneath these acute kidney injuries is very different. As far as we can say, cisplatin exerted direct toxicity toward the tubular cells of patient 1, leading to their definitive destruction and to end stage renal failure. Patient 2 suffered acute intravascular hemolysis due to an immunoallergic mechanism. Renal failure is secondary to the haemoglobin precipitation in the tubules. Therefore we wondered whether we could identify the presence of Pt in these two kidney biopsies and whether it’s spatial distribution (among with Zn’s one) would be different.
3 Results and discussion
As described in Part 1 of this project concerning an animal model of platin induced nephritis, μXRF spectra were acquired in large areas of the two studied biopsy with micrometer scale resolution. They were digitally processed to isolate ZnKα and PtLα [13]. The intensity of the respective peaks was then used to generate maps representing spatial distribution of these elements in the two biopsies.
The striking result of this investigation comes from the fact that we were able to point out the presence of Pt in the human biopsy. To the best of our knowledge, this is the first time that such measurements were performed. Note that in the case of patient 1(Fig. 1a–b), Pt detection was performed 6 days after the last oxaliplatin infusion while for patient 2, the biopsy was performed more than 15 days after his first oxaliplatin infusion, and several dialysis. We were also able to detect Zn in the two patients’ biopsies (Fig. 1c–d).
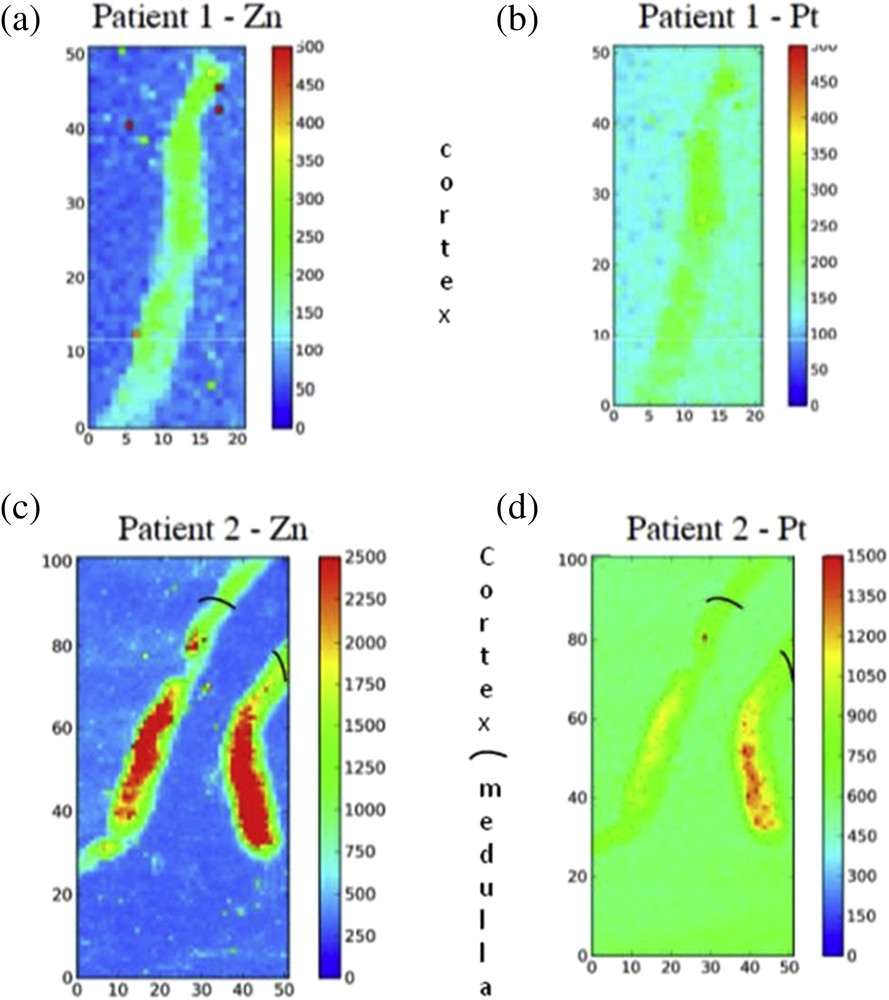
Zn and Pt XRF maps collected for patient 1 (a and b) and for patient 2 (c and d). Cortico medullar junction is shown in black.
Pt and Zn seemed to be equally distributed in the biopsy of patient 1. Pt and Zn seemed to be concentrated in the medulla of patient 2. In our experience in mice, Pt and Zn are found in the cortex after cisplatin infusion only, and we believe that this is somehow related to cisplatin induced tubulopathy. This observation likely explains the asymmetry observed in the patient 2 sample. Unfortunately in absence of analyzable medulla in patient 1 tissue, we cannot definitely confirm our hypothesis.
Following the approach defined for mice in part I of this study, the fluorescence maps for the different samples were analyzed using Intensity Correlation Analysis (ICA) methods [14,15] in order to highlight possible spatial correlations between Zn and Pt (Fig. 2). Although this proves the feasibility of this measurement, the absence of medulla in the patient 1 biopsy is clearly a limitation of such an approach.
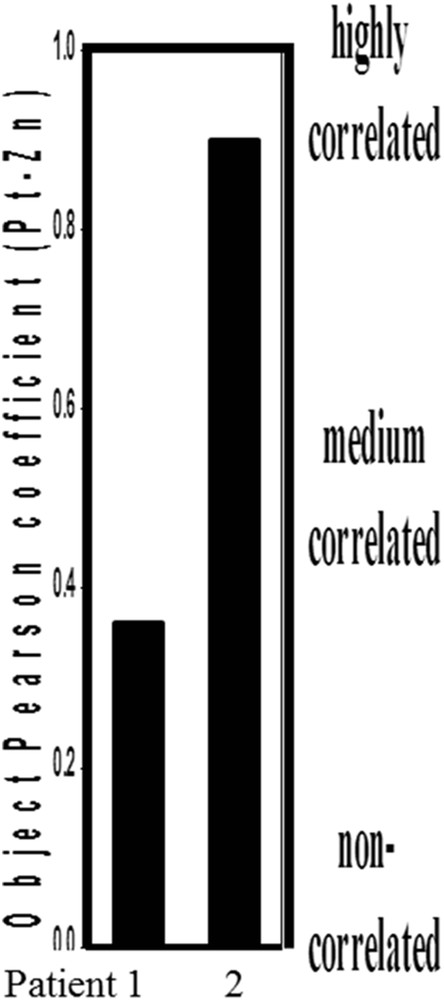
Pearson correlation coefficients between Zn and Pt obtained through statistical analysis for the patients 1 and 2.
4 Discussion
For the first time to the best of our knowledge, we have shown that μXRF can detect Pt and Zn at a micrometer scale nondestructively in human kidney biopsy. From a clinical point of view, routine renal anatomopathology is quite unequipped to assess the participation of a specific drug in acute tubular necrosis. μXRF allowed us to physically detect the potential nephrotoxic agent in the tissue of interest. Although the tubular lesions of patient 1 were very discrete after standard staining, which contrasted with the severity of the renal failure, μXRF could identify traces of remanent Pt in the cortex. Such measurements may thus be of primary importance for the clinician.
Moreover, the spatial correlation between Pt and Zn may also be discussed using such an approach. Whether the zinc we measured is linked to zinc renal metabolism modification, protective mechanisms such as heavy metal detoxification metallothionein induction, a witness of platin induced damaged DNA linked protein recruitment such as ZNF143 or to deleterious mechanisms such as activation and accumulation of profibrotic metalloproteases such as MeprinA is unclear. However, cisplatin seems to be the only Pt salt able to induce a redistribution of renal Zn to the cortex, and this is probably an interesting new pathway to explore.
We are aware that this study is preliminary and contains several limitations. Renal biopsies grant access to a very small sample of kidney tissue and the representativity of the sample is a constant concern. Nevertheless, an advantage of such an approach is that we are able to investigate the paraffin bloc through a non-destructive technique. Therefore it does not prohibit further exploitation of the sample by the anatomopathologist. In this experiment the lack of medullar tissue in the patient 1 biopsy hampers greatly our ability to reproduce the cisplatin specific Pt and Zn spatial distribution pattern we identified in mice. Nevertheless, we believe that the unique ability of μXRF to dissect matter could be of great utility in the understanding and diagnosis, and therefore the prevention of Pt related nephrotoxicity.
5 Conclusion
This set of experiments shows that synchrotron mediated μXRF is a suitable tool to detect Pt in kidney biopsy and consequently probably so on any organ exposed to Pt. Therefore, μXRF could also be of major interest to decipher the mechanism beyond Pt induced neurotoxicity, ototoxicity. Pharmacoavailability of chemotherapies is a major concern because some treatment failures are explained by poor tumor penetration of the active molecule. Thus μXRF could be an elegant way to map the distribution of Pt inside cancerous cells at the micrometer scale. Pt and Zn are only two of the numerous trace elements μXRF can detect. Heavy metal intoxication, diagnosis and toxicity mechanisms could probably also benefit from this innovative technique.


