1 Introduction
Hydroxy-l-proline (HP) represents an excellent molecular scaffold for the synthesis of diverse multi-functionalized unnatural amino acids. Such pyrrolidines are challenging targets from a synthetic point of view and have a high potential as biologically active molecules [1]. The introduction of a hydroxyl group at the 3- or 4-position of l-proline could lead to helical oligopeptides displaying on their outer surface the hydroxyl functionality available for further potential conjugation [2]. The present study reports on the stereoselective synthesis of 3-substituted 4-hydroxyproline derivatives via 1,3-dipolar cycloadditions. The obtained scaffolds, therefore, present the hydroxyl group at the 4-position, and a 1,2,3-triazole connected to the pyrrolidine scaffold through a carbon–carbon bond, at the 3-position.
Menthone-derived nitrones incorporating a glycine equivalent [3] have now demonstrated the potential of 1,3-dipolar cycloadditions with alkenes to create in a single step three contiguous stereogenic centers with strict stereocontrol [4–9]. Various alkenes have been used for the synthesis of diastereoisomers of 4-hydroxyisoleucine [5]. This is a molecule found in fenugreek (Trigonellafoenum-graecum), a plant traditionally used as an antidiabetic remedy thanks to its insulinotropic properties. An efficient synthesis of (αS,3R,4S)-3-glycinyl-4-hydroxypyrrolidine and 3-substituted 4-hydroxyproline derivatives was also reported with a total control of the stereochemistry (Fig. 1) [6,7]. This strategy is based on a cascade of cycloaddition–cyclization using (E)- or (Z)-1,4-dichlorobut-2-ene and chiral nitrones as glycine equivalents [6,7]. Another approach in which the nitrone cycloaddition is followed by a Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) provides rapid access to enantiopure 1,2,3-triazolylisoxazolidine derivatives [8]. A recent strategy towards cycloalkylglycines has been demonstrated [9].
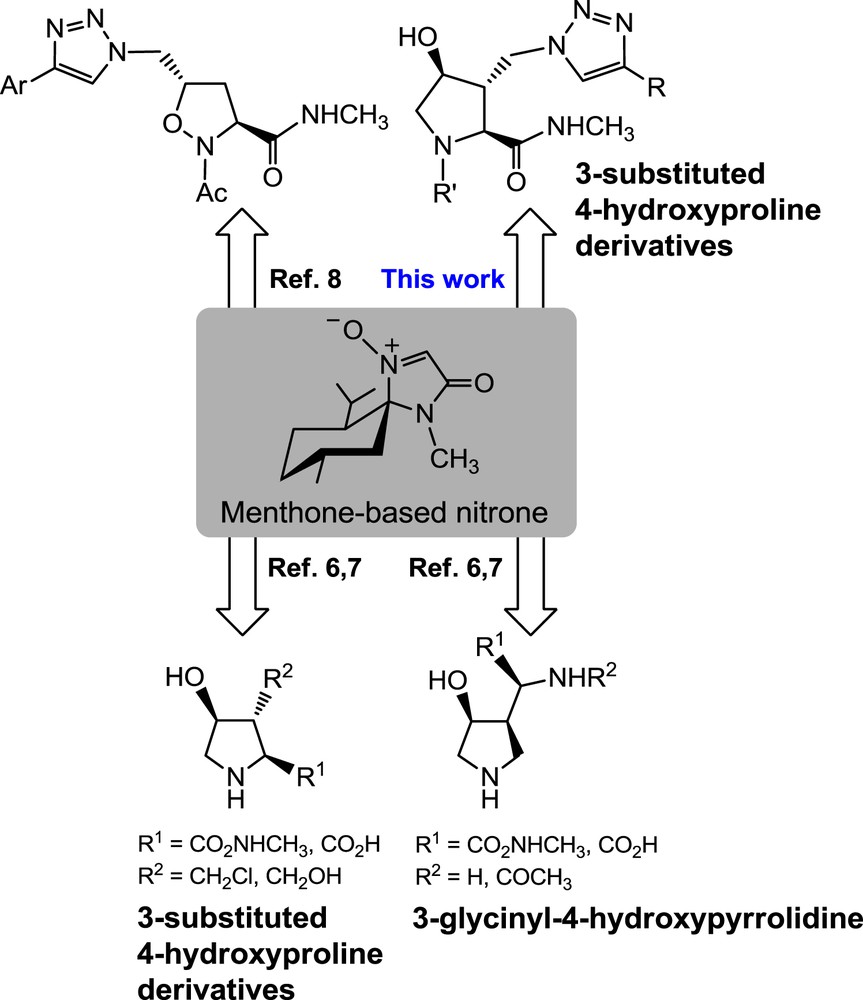
Selected scaffolds obtained from a common menthone-based nitrone precursor.
The general strategy towards unnatural amino acids originating from menthone-based nitrones as chiral auxiliaries for a glycine derivative can now be applied to the stereoselective synthesis of unprecedented 4-hydroxy-3-substituted proline derivatives in only 6 steps at a high overall yield (Fig. 1). The synthesis proceeds through 1,3-dipolar cycloaddition of the menthone-based nitrone and (Z)-1,4-dichloro-2-butene, followed by a regioselective azidation of a chlorine atom and the subsequent installation of a 1,2,3-triazole moiety by microwave-assisted CuAAC. Hydrolytic cleavage of the menthone chiral auxiliary followed by catalytic reduction of the N–O bond led to an amine intermediate that readily cyclized intramolecularly through nucleophilic displacement of the remaining chlorine atom [6,7].
2 Results and discussion
The synthesis of the mono-azido-mono-chlorinated scaffold 1 [6] has been reported. Discussion on the regioselectivity of mono-azidation is provided [6,7]. Copper-catalyzed 1,3-dipolar cycloaddition under microwave irradiation of azide 1 with various functionalized alkynes gave the corresponding 1,2,3-triazolyl-functionalized isoxazolidines 2a–f with high yields (Scheme 1). Acidic cleavage of the chiral auxiliary in 2a–f under the action of a mixture of acetic acid, acetic anhydride and a catalytic amount of sulfuric acid brought about methylamides 3a–d,f with good yields. In the case of isoxazolidine 2e, the same reaction was attempted but without success. The structure of isoxazolidine 3a was unambiguously determined by a single crystal X-ray diffraction analysis [10] (Fig. 2). It provided reliable data for the structural characterization of this series of intermediates.
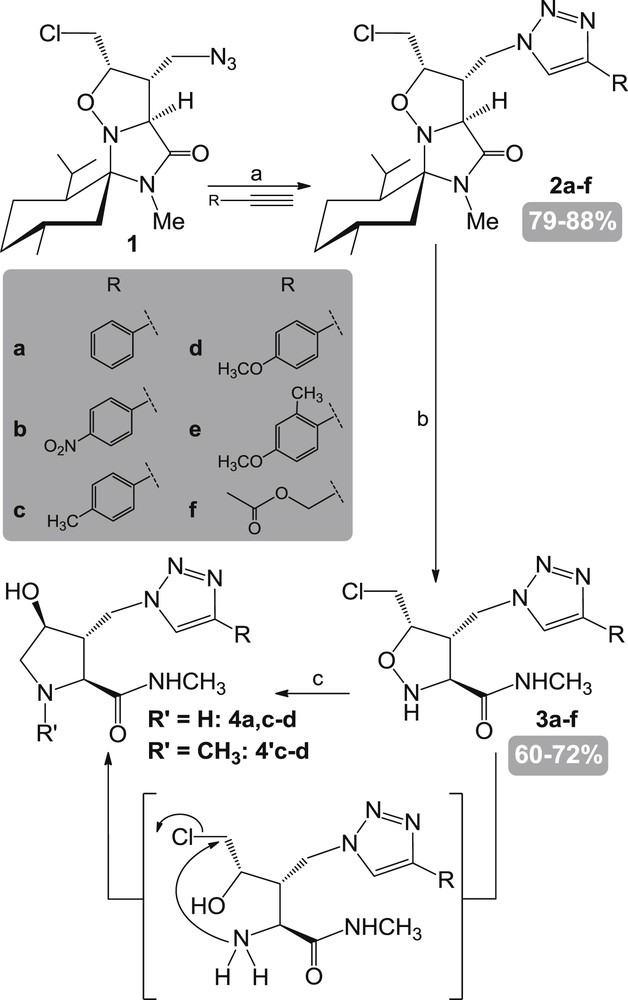
Synthesis of enantiopure 3-substituted 4-hydroxyproline derivatives. Reagents and conditions: (a) iPr2NEt, CuI, μW, DMF, 110 °C, 15 min; (b) AcOH, Ac2O, cat. H2SO4, 50 °C, 6.5 h; (c) H2 (1 atm), Pd(OH)2, MeOH, rt, 4 days.

ORTEP representation of the crystal structure of 3a.
4-Chloromethyl isoxazolidines 3a–f can be readily converted to the corresponding pyrrolidines 4 upon reduction of the N–O bond and subsequent nucleophilic displacement of the chlorine atom by the generated amine functionality [6]. Indeed, reductive cleavage under hydrogenolysis conditions of the N–O bond of the 4-chloromethyl isoxazolidine 3a led to the enantiopure methylamide 4a in an almost quantitative yield. Meanwhile, catalytic hydrogenolysis applied to compounds 3c–d led to a separable mixture of 4c–d (27 and 15%) and 4′c–d (70 and 84%). In the case of compounds 3b,f, the reaction led to a complex mixture of inseparable products. For compound 3b, the opening of the N–O bond by catalytic hydrogenation (H2, Pd/C) induced an inseparable mixture of products. The NO2 group was most probably reduced to NH2 and further unexpected reactivities may be observed. In the case of compound 3f, the acetate might be cleaved by intra- or intermolecular reaction with the generated amine leading to an inseparable mixture of products. Two other conditions for the cleavage of the N–O bond were then applied [Mo(CO)6, CH3CN–H2O [11]; Zn, AcOH [3b]] for these compounds but without success.
The obtained N-methylated products 4′c–d were reported in the N-alkylations of amines carried out using different alcohols as the solvent and with 10% Pd/C or 20% Pd(OH)2/C as the catalyst at room temperature [12].
3 Conclusion
In conclusion, 1,3-dipolar cycloaddition of a menthone-based nitrone to (Z)-1,4-dichloro-2-butene occurred with the simultaneous creation of three contiguous stereogenic centers to afford a dichlorinated isoxazolidine-based cyclo-adduct. Regioselective substitution of the chlorine atom by an azide group, followed by another azide–alkyne 1,3-dipolar cycloaddition (CuAAC) induced enantiopure 1,2,3-triazolyl-functionalized isoxazolidines. Acidic cleavage of the chiral auxiliary led directly to the 3-methylamide isoxazolidines. An amino group was then generated by reductive cleavage of the isoxazolidine N–O bond which triggered an intramolecular cyclization. This rapid and stereoselective synthetic strategy provides reliable access to a series of enantiopure 3-substituted 4-hydroxyproline derivatives.
Acknowledgements
Financial support from the University of Monastir, University of Lyon and Centre national de la recherche scientifique (CNRS) are gratefully acknowledged. The authors thank Dr. F. Albrieux, C. Duchamp and N. Henriques from the Centre Commun de Spectrométrie de Masse (ICBMS UMR-5246) for their assistance and access to mass spectrometry facilities.


