1 Introduction
Clidemia hirta (L.) D. Don (Melastomataceae; synonyms: Melastoma hirta L., M. elegans Aublet, C. crenata DC., C. elegans (Aublet) D. Don) is a shrub originating from South America and naturalised in Australia, Southern Asia, Sri Lanka, India, East Africa and several Pacific islands [1]. This plant is widely used in traditional medicine. For example, in Malaysia, crushed leaves of C. hirta L. mixed with saliva are applied as a poultice on wounds to stop bleeding. In Brazil, Clidemia species are used to treat Leishmania braziliensis skin infections [2].
C. hirta L. is used as a soap in French Guyana and its antimicrobial properties have been reported in ethnobotanical publications [3]. These properties were assayed by Meléndes and Capriles [4] and could be ascribed to the saponins found in its leaves and commonly used as antimicrobial compounds [5]. El Abdellaoui et al. [6] have recently identified arjunolic acid, a triterpenoid saponin, as one of the main antibacterial compounds present in this plant.
Moreover, an abundance of phenolics and flavonoids has been reported in its foliage [7] and its possible use as an antioxidant in cosmetics has been patented [8].
Plant cell cultures and calli cannot always produce the same secondary metabolites as those contained in whole plants [9–11] but shoot cultures can generally produce the set of molecules found in shoots grown in the wild [12]. One of the aims of the present work was to establish in vitro cultures of C. hirta in order to micropropagate them as clones to enable further cultures that can be used to discriminate which factors determine the contents and efficiency of this plant, between the genotype and the environmental culture conditions. Comparing the same clone under different conditions will reveal the influence of culture conditions while comparing different clones under the same conditions will indicate the natural variability of the content that can be attributed to the genotype. Another interest of in vitro culture is that it provides the possibility of harvesting the plant without impacting the wild population, thus avoiding any risk of overcollecting if the plant is used for industrial purposes. This study focuses on the establishment of C. hirta cultures in vitro and the impact of culture media on plant growth and production of phytochemicals in relation to their antibacterial and antioxidant properties.
2 Materials and methods
2.1 Plant material, surface sterilisation and culture conditions
C. hirta seeds were harvested in French Guyana (GPS coordinates: N04°33ʹ598ʺ; WO 52°12ʹ428ʺ). They were surface-sterilised for 20 min in a diluted commercial bleach solution (2% chlorine) with occasional shaking and finally washed three times (5 min) in sterile-distilled water. Seeds were then placed on solid culture media in square boxes (Duchefa). All the cultures were maintained at 25 °C under a 16-h photoperiod (30 μmol m−2 s−1 photosynthetically active radiation). Subculture was performed and/or data were collected after 40 days of culture. To obtain the dry weight, plantlets were lyophilised.
2.2 Culture media for plant micropropagation
Murashige and Skoog (MS) basal medium [13], modified Quoirin and Lepoivre (QL) medium [14] and Woody Plant Medium (WPM) [15] were used for in vitro micropropagation. Powdered basal media were purchased from Duchefa. To enable the comparison of mineral basal media, all the media contained Morel and Wetmore [16] vitamins, 30 g L−1 sucrose and were gelified with 2.8 g L−1 Phytagel (Sigma). The pH was adjusted to 5.7 before autoclaving.
The main features of the media are presented in Table 1.
Growth and shoot development of micropropagated C. hirta as a function of the composition of the culture medium.
| FW (mg) | DW (mg) | Roots/plant | Leaves/plant | [Chloro] (μg/g) | |
| MS | 101.37 ± 14.32c | 13.81 ± 1.56c | 1.75 ± 0.39b | 22.14 ± 2.36a | 32.51 ± 2.77c |
| WPM | 133.16 ± 10.58b | 18.24 ± 1.50b | 3.41 ± 0.28a | 12.91 ± 0.53c | 53.99 ± 1.92b |
| QL | 190.07 ± 18.24a | 24.52 ± 1.79a | 2.95 ± 0.30a | 16.58 ± 0.91b | 62.90 ± 4.45a |
2.3 Extraction
Leaf extracts of C. hirta were obtained by grinding 50 mg of aerial parts in 2 mL of 80% (v/v) ethanol (HPLC grade, Thermo) followed by 3 h of incubation at 45 °C under agitation (200 rpm) in a thermostated water bath and before filtration through a 0.45-μm filter prior to use.
2.4 Quantification of chlorophyll
The chlorophyll content was determined using a spectrophotometric assay [17]. Briefly, the sample was prepared by pipetting 1 mL of extract into a 15-mL centrifuge tube and adding 8 mL of acetone and 1 mL of water and centrifuging at 1000 g for 5 min. The absorbance was measured at 652 nm. The results were expressed as milligrams of chlorophyll per gram of fresh tissue.
2.5 Quantification of total phenolic compounds
The total phenolic content was analysed using the Folin–Ciocalteu (Sigma) reagent method as described by Dini et al. [18]. The C. hirta leaf extract was mixed with 0.5 mL of Folin–Ciocalteu reagent and 0.05 mL of 10% (v/v) Na2CO3, and then the absorbance was measured at 735 nm after 60 min of incubation at room temperature. Gallic acid (Sigma) was used as the standard for the calibration curve, and the total phenolic contents were expressed as milligrams gallic acid equivalents per gram of DW.
2.6 Quantification of total saponins
The total saponins were quantified by the method described by Dini et al. [18]: 0.5 mL of leaf extract of C. hirta was mixed with 0.5 mL of 8% (w/v) vanillin in ethanol and 5 mL of 72% (v/v) H2SO4 in water. The reagents were mixed at 60 °C for 20 min, cooled and then the absorbance at 544 nm was measured. A calibration curve was obtained using Saponin-1 (Sigma) as the standard saponin and the total saponin contents were expressed as milligrams saponin-1 equivalents per gram of DW.
2.7 Quantification of total flavonoids
The total flavonoid content was determined using the aluminium chloride colorimetric method as described by Saboo et al. [19]: 0.5 mL of leaf extract of C. hirta was mixed with 0.1 mL of 10% (w/v) AlCl3, 0.1 mL of 1 M potassium acetate and 2.8 mL of double distilled water. The mixture was incubated at room temperature for 30 min and the absorbance at 415 nm was read. Quercetin (Sigma) was used as the standard for the calibration curve, and the total flavonoid contents were expressed as milligrams quercetin equivalents per gram of DW.
2.8 HPLC analysis
Separation was performed on a Water liquid chromatography system (a Water 600 pump, a Water 600 controller with a degasser, a Water 2998 Photodiode Array (PDA) detector and Empower 3 Water software for processing) with the temperature set at 35 °C, using an ACE 5 AQ C-18 column (250 × 4.6 mm and 5 μm internal diameter) and detection set at 215 nm, 280 nm and 340 nm. Separation was achieved using a linear gradient composed of formic acid-acidified (0.02% v/v) HPLC-grade methanol (A) and water (B) in a mixture from 10:90 to 100:0 (A:B) for 70 min at a flow rate of 0.6 ml min−1.
2.9 Antibacterial activity
The antibacterial activity was tested as described in [6] on four bacterial strains: Escherichia coli ATCC 8739, Staphylococcus aureus ACTT 6538, Pseudomonas aeruginosa ATCC 16404 and Enterococcus hirae ATCC 8043. An overnight culture of each strain was diluted in Luria Bertani medium in order to reach an absorbance of 0.4 at 600 nm. Ethanolic extracts of C. hirta leaves diluted at 10, 5, 2.5, 1, 0.5 and 0.25 mg mL−1 in DMSO from 12 different plants were used for each micropropagation medium tested. They were added to bacteria for 1 h of culture at 37 °C and the same volume of DMSO was added as a control. Absorbance was read at 600 nm using a microplate spectrophotometer (Molecular Devices). The percentage of inhibition was calculated using the formula: [(A600 control − A600 extract)/A600 control] × 100. The experiment was carried out in triplicate. The MIC50 (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration) were determined using ED50plus v1.0 software.
2.10 Determination of free radical scavenging (DPPH method)
The ability of leaf extracts of C. hirta to scavenge the DPPH radical was measured as described by Brand-Williams et al. [20]. Aliquots (20 μL) of sample extract were mixed with 3 mL of 6.10−5 M DPPH solution (Sigma) and left in the dark at room temperature for 90 min. The absorbance of the resulting solution was measured at 515 nm. The ability to scavenge the DPPH radical was then calculated as the relative scavenging effect, expressed as the percentage inhibition of the scavenging DPPH radical by C. hirta leaf extract compared to water.
2.11 Statistical analysis
All data presented in this study are the means and the standard deviations of at least three independent replicates. Comparative statistical analyses of groups were performed using one-way analysis of variance according to the data. All statistical tests were considered significant at p < 0.05. Pearson correlations, principal component analysis and agglomerative hierarchical clustering were performed with Excel-XLSTAT2014 using the means of the compound abundances or activities for each condition.
3 Results and discussion
3.1 Micropropagation of C. hirta
C. hirta seeds harvested in French Guyana were surface-sterilised and in vitro propagated. Two media designed for woody plants, QL medium [14] and WPM [15], and MS medium, being of general use, were used for the in vitro propagation of 40 individual seed-derived plantlets per medium. Initially, QL and WPM (Woody Plant Medium) were designed for trees (Prunus and Kalmia species, respectively) while the MS basal medium was first designed for tobacco plants [13]. These media differ mainly in their nitrogen contents, their NO3/NH4 ratio and their phosphate and sulphate ion contents (see Supplementary Table 1).
QL medium enabled the largest increase in both fresh and dry weights (Fig. 1A). Moreover, the plants looked healthier, especially regarding their foliage colour as confirmed by their chlorophyll contents (Table 1). WPM medium also enabled a satisfactory rooting rate, but the overall growth and the number of buds produced were slightly lower (about 25% less for bud production). The use of MS medium gave the poorest results: the plants were yellowish and although a similar number of leaves produced was noted, the plants looked stunted, their weight increase was very low, and some failed to produce roots. Moreover, some of the explants totally failed to produce new plants on MS medium.
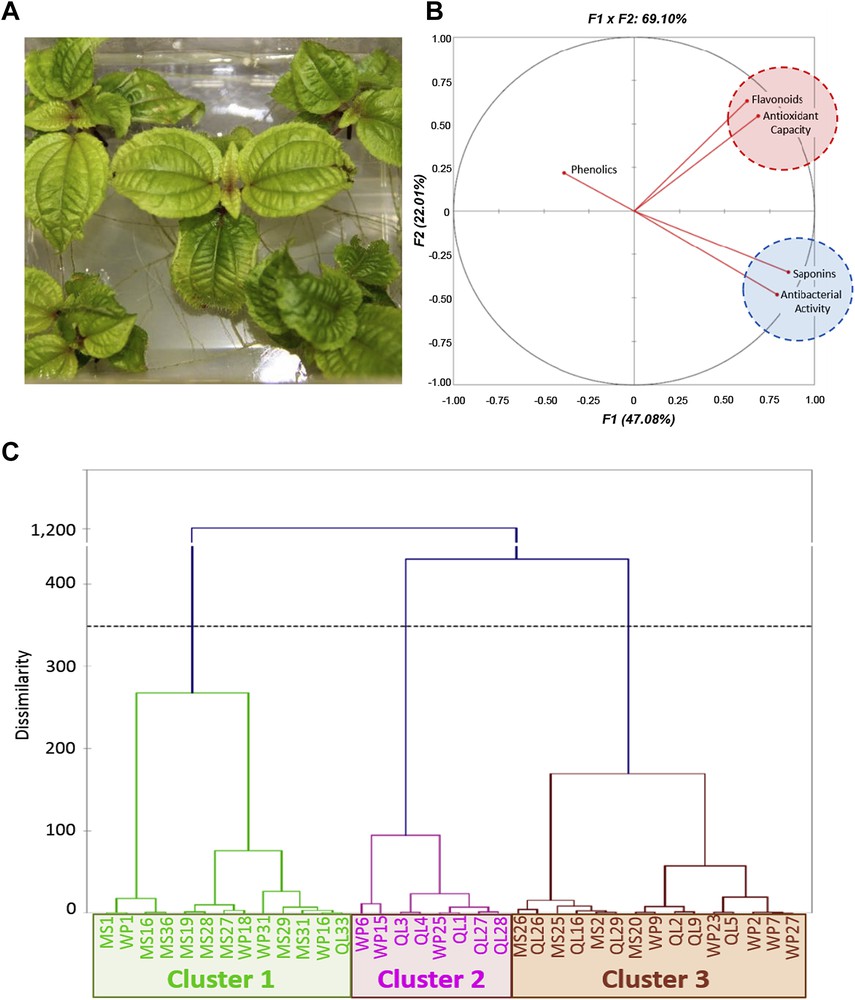
A. Clidemia hirta shoots subcultured for six weeks on WPM medium. B. Correlation circle for principal component analysis. The secondary metabolite contents, antioxidant capacities and antibacterial activities for 36 populations of C. hirta (12 populations per medium) were submitted for analysis by the PCA algorithm in Excel-XLSTAT, using the Pearson correlation matrix (significance level, 0.05). C. Agglomerative hierarchical clustering analysis. The secondary metabolite contents, antioxidant capacities and antibacterial activities for 36 populations of C. hirta (12 populations per medium) were analysed using the agglomerative hierarchical clustering algorithm in Excel-XLSTAT, and Pearson similarity coefficients. The chemical characteristics of the 3 clades are described in the text.
Weaning in the greenhouse was satisfactory when using shoots collected 15–20 days after subculture. C. hirta is amenable to in vitro culture and, not surprisingly given its shrubby nature, media designed for ligneous species, such as WPM and QL, performed better than the MS medium widely used for non-ligneous plants. The best results were obtained using QL medium first designed for Prunus sp. [14]. The main differences between the mineral bases of these media are the amount and form of the nitrogen and the abundance in anions, MS being high in total nitrogen and QL high in phosphate and sulphate (Supplementary Table 1).
3.2 Phytochemical investigation and biological activities of C. hirta extracts
The phytochemical composition of leaf extracts of the micropropagated plants was then investigated. Plants cultivated on the three basal media exhibited different contents in saponins, phenolics and flavonoids (Table 2). The relative accumulation of the considered metabolites did not appear to be linked. Thus, it should be possible to find culture conditions that favour just one class of compounds.
Phytochemical accumulation and antioxidant capacities of the C. hirta extracts as a function of culture medium composition.
| Phenolics (μg/g GAEq) | Flavonoids (μg/g QEq) | Saponins (μg/g SEq) | DPPH inhibition (%) | |
| MS | 269.58 ± 36.22a | 180.79 ± 2.92b | 375.28 ± 30.22b | 37.74 ± 3.68b |
| WPM | 127.86 ± 17.49b | 204.04 ± 2.90a | 641.556 ± 35.17a | 56.26 ± 5.96a |
| QL | 273.92 ± 27.65a | 205.42 ± 4.96a | 644.32 ± 74.56a | 60.79 ± 3.91a |
The antioxidant capacity and antibacterial activity measured for each plant extract grown on different media are presented in Tables 2 and 3, respectively. C. hirta leaf extracts displayed an antibacterial effect against all the tested bacterial strains. The relatively low extract concentration (between 0.7 and 2.2 mg mL−1) required for the total inhibition of bacterial growth (MBC), whatever the strain, indicates the presence of highly active compound(s) in the extract. Given the low MBC/MIC ratio (between 1.5 and 2.3), this antibacterial activity could be ascribed to a bactericidal action of one compound, especially as these are extracts and not pure compounds (Table 3).
Antibacterial activity of the C. hirta extracts as a function of culture medium composition.
| MIC50 | MBC | MBC / MIC ratio | ||||||||||
| Sa | Eh | Ec | Pa | Sa | Eh | Ec | Pa | Sa | Eh | Ec | Pa | |
| MS | 0.8 ± 0.1b | 1.3 ± 0.2c | 2.2 ± 0.2b | 1.2 ± 0.1b | 1.7 ± 0.2b | 2.3 ± 0.3b | 5.8 ± 0.7b | 2.8 ± 0.3b | 2.2 ± 0.2b | 1.8 ± 0.1a | 2.7 ± 0.3a | 2.5 ± 0.3a |
| WPM | 0.5 ± 0.1a | 0.5 ± 0.1a | 1.2 ± 0.1a | 1.2 ± 0.2b | 0.8 ± 0.13a | 0.8 ± 0.1a | 2.6 ± 0.3a | 2.3 ± 0.3b | 1.7 ± 0.1a | 1.7 ± 0.1a | 2.3 ± 0.3a | 2.6 ± 0.6a |
| QL | 0.6 ± 0.2ab | 0.8 ± 0.1b | 1.0 ± 0.2a | 0.7 ± 0.1a | 0.7 ± 0.1a | 1.25 ± 0.2a | 2.2 ± 0.3a | 1.3 ± 0.1a | 1.5 ± 0.2a | 1.6 ± 0.1a | 2.5 ± 0.3a | 2.1 ± 0.2a |
A principal component analysis was conducted and the obtained correlation circle projection accounted for 69.1% (F1 + F2) of the initial variability of the data (Fig. 1B). The projection of the flavonoid content and the antioxidant capacity appeared to be strongly positively correlated. According to the Pearson correlation matrix, the antioxidant capacity was also positively correlated with the flavonoid content and, although less strongly, with the saponin content (Table 4). Moreover, the projection of the saponin content and the antibacterial capacity appeared to be strongly positively correlated (Fig. 1B). According to the Pearson correlation matrix, this bactericidal and/or bacteriostatic activity was linked only to the presence of saponins (Table 4) and not to the antioxidant capacity (Table 4). The phenolic content, however, did not appear to be linked to any of the studied biological activities (Table 4).
Pearson correlation matrix.
| Variables | Phenolics | Saponins | Flavonoids | Antiox | MIC | MBC |
| Phenolics | −0.172 | −0.021 | −0.076 | 0.181 | 0.158 | |
| Saponins | 0.227 | 0.380 (0.022) | −0.748 (<0.0001) | −0.520 (0.001) | ||
| Flavonoids | 0.545 (0.001) | −0.186 | −0.138 | |||
| Antiox | −0.277 | −0.064 | ||||
| MIC | 0.612 (<0.0001) | |||||
| MBC |
The best results in terms of secondary metabolite accumulation were obtained with WPM and QL media (Table 1). Despite their similar growth in vitro on WPM and QL media, plants cultivated on QL medium were richer in phenolics than those cultivated on WPM, while the contents in saponins and flavonoids were similar.
It is not surprising to observe that the plants cultivated on WPM and QL outperformed those cultivated on MS basal medium for the free radical scavenging capacity (Table 4) given the correlation of this antioxidant ability with flavonoids and saponins. WPM and QL also provided the samples with the best antibacterial activity (Table 3), which again seems logical given its positive correlation with the saponin content.
The agglomerative clustering analysis (Fig. 1C) shows three clusters: cluster 1, with weak antibacterial and antioxidant activities, is mainly constituted of plants grown on MS medium (62%); cluster 2, with intermediate biological activities, groups plants growing on QL medium (62.5%) and WPM (37.5%); and cluster 3, exhibiting high antioxidant and antibacterial activities, consists of plants grown on WPM and QL medium (73.3% of cases, Fig. 1C) with plants grown on QL predominating in this cluster (40% of cases, Fig. 1C). This analysis also indicates the natural variability of the content that can be attributed to the genotype, as evidenced by the presence of the QL33 clone in cluster 1 and some MS clones in cluster 3 (Fig. 1C).
4 Conclusion
It is now accepted that growing plants in vitro can produce secondary metabolites with an efficiency that can exceed that of greenhouse-grown plants [21] or even wild plants [22]. In our hands, the quantity of secondary metabolites was strongly affected by the medium used for micropropagation. Low nitrogen and high anion contents were found to be more efficient both for growth and development and for secondary metabolite production, thus affecting the biological activities due to the positive correlation between antioxidant capacity and flavonoid content and between antibacterial activity and saponin content. The antibacterial activity of the extract is mainly bactericidal possibly due to the presence of arjunolic acid, an antibacterial saponin recently reported [6]. In conclusion, although adjusting media for in vitro plant culture generally focuses on growth regulators, if micropropagated plants are required for the production of secondary metabolites, particular attention should be paid to the basal culture medium. The present study represents a first step in understanding the conditions for secondary metabolite production in C. hirta and how these influence the biological activities of the corresponding extracts with a view to their future use in cosmetic or pharmaceutical applications.
Acknowledgements
This work was funded by the Region Centre Val de Loire, the Ville d'Orléans and the Conseil General du Loiret. The authors also thank the Cosmetic Valley for its support. Émilie A. Leclerc is kindly acknowledged for her critical reading of this manuscript.


