1 Introduction
Hemizigia bracteosa (Benth.) Brip. (ex Orthosiphon bracteatus (Benth.) Baker) (Lamiaceae) is an erected annual and sometimes perennial herb of about 90 cm tall, widespread in tropical and South Africa, in marshy grasslands in Senegal, Benin (ex Dahomey), North and South Nigeria and West Cameroon [1]. The flowers are white and fairly inconspicuous but the colorful purple of its bracts makes the plant a striking feature [2].
In Southern Africa the plants are burnt and the smoke or vapors inhaled to treat mental illnesses, for narcotic or divination purposes [3]. The leaves are smoked or chewed by the San in Botswana to give energy for dancing and as a stimulant [4]. The Shonas of Zimbabwe are reported to use powdered leaves orally to treat fits [5]. In Zimbabwe, the plant is used in association with other plants for treating or preventing HIV infection in general and maybe to reduce the viral load of patients infected with HIV and/or exhibiting symptoms of acquired human immunodeficiency syndrome (AIDS) [6]. The plant is very useful in West Africa [7]. It is used to treat malaria, to foment the body of patients suffering from fever and its leaves are used as a mosquito repellent [8]. The fresh aerial parts of the plant are also traditionally used by fumigation in Benin and the decoction of the leaves in addition with Dialium guineense, Pavetta corymbosa, Rytigynia canthiodes and Uvaria chamae is used orally to treat malaria [9,10]. Leaves are also used in drink preparation and were shown to possess some antimicrobial activities [11].
To the best of our knowledge there are no reports concerning the volatile components of the aerial parts of Hemizygia bracteosa (Benth.) Briq. The aim of this study is to describe the chemical composition of essential oils extracted from fresh aerial parts of this plant from Benin and the impact of the harvesting period on this chemical composition and on the essential oil extraction yield.
2 Experimental
2.1 Plant material
Aerial parts of Hemizygia bracteosa (Benth.) Briq. were collected in the morning, in the Botanical Garden of the Abomey-Calavi University (Republic of Benin). The fresh aerial parts were harvested in February 2009 (sample I), a period of very hot weather (35 °C), and in August 2009 (sample II) (21 °C), a colder period with occasional light rain. A voucher specimen (n°AA6391/HNB) of these aerial parts has been deposited at the University of Abomey-Calavi Herbarium.
2.2 Essential oil isolation
Five hundred grams (500 g) of fresh aerial parts were steam distilled for 3 h in an improved Clevenger-type apparatus [12]. The extraction of each aerial part (I and II) was carried out in triplicate. The essential oil yields were based on the fresh material.
2.3 Chemical analysis
Analysis of the oils was performed by GC/FID and GC/MS [13,14].
2.3.1 GC/FID analysis
The GC/FID analysis was carried out on a FOCUS GC (ThermoFinigan; Milan, Italy) using the following operating conditions: A DB5 column (25 m × 0.25 mm, df: 0.25 μm) (J&W Scientific Column of Agilent Technologies, N° US167072Ã, USA); injection mode: splitless; injection volume: 1 μL (TBME solution); flow of split: 10 ml/min; splitless time: 0.80 min; injector temperature: 260 °C; oven temperature was programmed as following: 50 °C – 250 °C at 6 °C/min and maintained at 250 °C for 5 min; carrier gas was helium with a constant flow of 1.2 mL/min; FID detector temperature was: 260 °C. The data were recorded and treated with the ChromCard software. The quantification was completed by the calculation of the areas under curve of the peaks (GC/FID, by the normalization process) and the identification of compounds by comparison of the retention indices with references.
2.3.2 GC/MS analysis
GC-MS analysis was carried out on a TRACE GC 2000 series (Thermo-Quest, Rodano, Italy), equipped with an autosampler AS2000 Thermo-Quest. The GC system was interfaced to a Trace MS mass spectrometer (ThermoQuest) operating in the electronic impact mode. A HP5 column (30 m × 0.25 mm, df: 0.25 μm) was used under the same operating conditions as above. The coupling temperature of the GC was 260 °C. The energy of the electrons was 70 eV and the source of the electrons at 260 °C. The data were recorded and analyzed using the Xcalibur 1.1 software (ThermoQuest). The mass spectra of the peaks were analyzed and compared with references and NIST/EPA/NIH database [15].
2.4 Identification of oil constituents
Individual components of the volatile oils were identified by comparison of their relative retention times with those of authentic standard references, computer matching against commercial library [15,16] and home-made library mass spectra made from pure substances and components of known oils [14]. Mass spectrometry literature data were also used for the identification, which was confirmed by comparison of the GC retention indices (RI) on a non-polar column (determined from the retention times of a series of n-alkanes “C8-C24” mixture). The Kovats indices (KI) calculated were in agreement with those reported by Adams [16]. A quantitative analysis of each oil component (expressed as percentages) was carried out by normalization measurements of peak area obtained by FID.
2.5 Chemicals
α-Pinene, β-pinene, camphene, p-cymene, myrcene, α-terpinene, γ-terpinene,1,8-cineol, terpinolene, borneol, citronellyl acetate, terpinene-4-ol, α-terpineol, geraniol, verbenone, carvacrol, thymol, bornyl acetate, α-copaene, β-caryophyllene, fenchone, thujone, trans-pinocarveol, trans-verbenol, lavandulol, myrtenal, trans-carveol, carvone, aromadendrene, allo-aromadendrene, γ-gurjunene, cis-ocimene, camphor, and n-alkanes “C8-C26” were obtained from Sigma-Aldrich Chemie (Germany), Acros Organics (New Jersey, USA), and FlukaChemie (Switzerland); α-thujene, sabinene, δ-3-carene, limonene, linalool, α-humulene, cis-pinane, α-phellandrene, p-cymenene, myrtenyl acetate, and valencene were purchased from Extrasynthese (Genay, France). All the chemicals were of analytical standard grade. tert-Butyl methyl ether and anhydrous Na2SO4 analytical grade were purchased from FlukaChemie and from UCB (Bruxelles, Belgium) respectively.
2.6 Statistical analysis
All data were expressed as mean ± standard deviation of triplicate measurements. The confidence limit was set at P < 0.05. Standard deviations did not exceed 5% for the majority of values obtained.
3 Results and discussion
3.1 Variation of essential oil yields
The oils extracted from samples I and II were obtained in small quantities with different yields (0.12 ± 0.01% and 0.25 ± 0.02%, respectively). The cold period would be favorable for quantity production of essential oil by Hemizygia bracteosa (Benth.) Briq. from Benin.
3.2 Variation of oil composition
A total of 65 compounds, representing 97% of hydrodistillate, were identified (Table 1).
Volatile compounds identified in the aerial part essential oils of Hemizygia bracteosa (Benth.) Briq. (Lamiaceae) from Benin.
| No | Compounds | aKI | KI | I | II |
| % ± bSD | % ± bSD | ||||
| 1 | 4-Hydroxy-4-methyl-pentan-2-one***o | 835 | 835 | – | 0.1 ± 0.04 |
| 2 | α-Thujene*h | 925 | 931 | – | 0.2 ± 0.05 |
| 3 | α-Pinene*h | 932 | 939 | – | 0.1 ± 0.08 |
| 4 | Sabinene*h | 972 | 975 | tr | 0.1 ± 0.01 |
| 5 | β-Pinene*h | 976 | 977 | 0.1 ± 0.02 | 0.1 ± 0.02 |
| 6 | Myrcene*h | 989 | 991 | 0.4 ± 0.13 | 0.3 ± 0.03 |
| 7 | α-Terpinene*h | 1017 | 1017 | tr | 0.1 ± 0.01 |
| 8 | p-Cymene*h | 1025 | 1026 | tr | 2.5 ± 0.04 |
| 9 | Limonene*h | 1029 | 1031 | tr | 0.3 ± 0.04 |
| 10 | 1,8-Cineole*o | 1033 | 1033 | 0.1 ± 0.01 | 0.2 ± 0.01 |
| 11 | (Z)-β-Ocimene*h | 1038 | 1036 | – | 0.2 ± 0.05 |
| 12 | (E)-β-Ocimène*h | 1047 | 1050 | tr | 0.1 ± 0.03 |
| 13 | γ-Terpinène*h | 1059 | 1062 | 0.4 ± 0.01 | 1.6 ± 0.04 |
| 14 | cis Sabinene hydrate*o | 1067 | 1067 | tr | – |
| 15 | p-Cymenene*h | 1091 | 1091 | 0.1 ± 0.03 | 0.1 ± 0.03 |
| 16 | Linalool*o | 1097 | 1096 | tr | – |
| 17 | Lavandulol*o | 1165 | 1165 | 1.1 ± 0.01 | 0.1 ± 0.01 |
| 18 | Terpinene-4-ol*o | 1182 | 1178 | 0.6 ± 0.05 | tr |
| 19 | Terpinen-4-ol acetate*o | 1300 | 1300 | 0.1 ± 0.02 | 0.1 ± 0.02 |
| 20 | α-Cubebene**h | 1348 | 1347 | 0.3 ± 0.04 | 0.2 ± 0.04 |
| 21 | α-Copaene**h | 1378 | 1379 | 2.3 ± 0.02 | 1.5 ± 0.05 |
| 22 | β-Panasinsene**h | 1383 | 1383 | 0.1 ± 0.05 | 0.2 ± 0.05 |
| 23 | β-Bourbonene**h | 1386 | 1388 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 24 | β-Elemene**h | 1391 | 1391 | 7.4 ± 0.05 | 3.3 ± 0.05 |
| 25 | β-Caryophyllene**h | 1422 | 1418 | tr | 3.6 ± 0.06 |
| 26 | (Z)-β-Farnesene**h | 1426 | 1426 | 0.1 ± 0.03 | 0.1 ± 0.01 |
| 27 | β-Copaene**h | 1430 | 1430 | 0.1 ± 0.01 | tr |
| 28 | Guaia-6,9-diene**h | 1443 | 1443 | 0.4 ± 0.02 | tr |
| 29 | (E)-β-Farnesene**h | 1456 | 1454 | 64 ± 0.04 | 67 ± 0.04 |
| 30 | α-Humulene**h | 1458 | 1457 | 1.7 ± 0.03 | 0.6 ± 0.03 |
| 31 | (2E) Dodecenal***o | 1466 | 1466 | – | 0.1 ± 0.03 |
| 32 | Massoïlactone***o | 1474 | 1474 | 0.2 ± 0.04 | 0.4 ± 0.04 |
| 33 | γ-Himachalene**h | 1477 | 1477 | 1.8 ± 0.03 | tr |
| 34 | Germacrene-D**h | 1484 | 1480 | 0.3 ± 0.04 | 1.5 ± 0.04 |
| 35 | β-Selinene**h | 1486 | 1485 | 0.3 ± 0.06 | 0.8 ± 0.02 |
| 36 | α-Selinene**h | 1492 | 1491 | 0.2 ± 0.01 | 0.5 ± 0.01 |
| 37 | Valencene**h | 1495 | 1494 | 0.2 ± 0.01 | 0.8 ± 0.01 |
| 38 | α-Muurolene**h | 1498 | 1496 | 2.7 ± 0.03 | 0.3 ± 0.03 |
| 39 | β-Himachalene**h | 1503 | 1501 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 40 | Germacrene-A**h | 1510 | 1508 | 0.1 ± 0.01 | 0.3 ± 0.01 |
| 41 | γ-Cadinene**h | 1514 | 1513 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 42 | δ-Cadinene**h | 1519 | 1519 | tr | 1.9 ± 0.03 |
| 43 | 7-epi-α-Selinene**h | 1522 | 1522 | 0.1 ± 0.01 | 3.1 ± 0.01 |
| 44 | Cadina-1,4-diene**h | 1534 | 1533 | tr | 0.1 ± 0.03 |
| 45 | cis-Cadineneether**h | 1554 | 1554 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 46 | Elemicin***o | 1557 | 1557 | 0.2 ± 0.02 | 0.2 ± 0.02 |
| 47 | Germacrene-B**h | 1560 | 1559 | 0.2 ± 0.01 | 0.5 ± 0.01 |
| 48 | cis-Caryophyllene oxyde**o | 1566 | 1564 | 0.3 ± 0.01 | 0.1 ± 0.01 |
| 49 | Dendrolasin**h | 1568 | 1569 | 0.1 ± 0.02 | 0.2 ± 0.04 |
| 50 | Spathulenol**o | 1580 | 1581 | 0.3 ± 0.01 | 0.3 ± 0.03 |
| 51 | trans-Caryophyllene oxyde**o | 1584 | 1584 | 0.1 ± 0.05 | 0.9 ± 0.05 |
| 52 | Humulene-1,2-epoxyde**o | 1612 | 1608 | 0.7 ± 0.05 | 0.2 ± 0.05 |
| 53 | 1,10-diepi-Cubenol**o | 1616 | 1616 | 0.1 ± 0.03 | 0.1 ± 0.08 |
| 54 | trans-Nerolidol**o | 1625 | 1625 | 6.2 ± 0.04 | 0.1 ± 0.01 |
| 55 | epi-Cubenol**o | 1629 | 1629 | 0.2 ± 0.01 | 0.1 ± 0.02 |
| 56 | Ledol**o | 1632 | 1632 | tr | 0.1 ± 0.01 |
| 57 | epi-α-Cadinol**o | 1643 | 1643 | 0.5 ± 0.04 | 0.4 ± 0.01 |
| 58 | Himachalol**o | 1654 | 1653 | 0.1 ± 0.01 | tr |
| 59 | α-Cadinol**o | 1656 | 1655 | 1 ± 0.04 | 0.2 ± 0.04 |
| 60 | neo-Intermedeol**o | 1660 | 1660 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 61 | 14-Hydroxy-9-epi-(E)-caryophyllene**o | 1670 | 1670 | 1.6 ± 0.05 | 0.1 ± 0.05 |
| 62 | (Z)-α-trans-Bergamotol**o | 1708 | 1705 | 0.2 ± 0.03 | 0.1 ± 0.03 |
| 63 | Khusimol**o | 1713 | 1713 | 0.1 ± 0.03 | tr |
| 64 | Crysolide***o | 1723 | 1720 | 0.1 ± 0.01 | 0.1 ± 0.01 |
| 65 | p-Cresoloctanoate***o | 1777 | 1775 | tr | 0.2 ± 0.03 |
| Total | 97.7 ± 1.12 | 96.9 ± 1.59 |
The oils were characterized by four major chemical groups: hydrocarbon and oxygenated monoterpenes; hydrocarbon and oxygenated sesquiterpenes with high amounts of hydrocarbon sesquiterpenes in all studied seasons (86.9 ± 0.61% in the cold season and 82.8 ± 0.56% in the hot season).
We observed the presence of a higher percentage of monoterpenes (and particularly hydrocarbons) in the sample collected in August (5.7 ± 0.37%) as compared to the sample collected during the hot season (1 ± 0.09%). The opposite is observed concerning oxygenated sesquiterpenes (2.8 ± 0.4% and 11.5 ± 0.41%, respectively) (Table 2). Non terpenic compounds represented 0.5 ± 0.07% of the essential oil collected during the hot season and comprised massoïlactone (0.2 ± 0.04%) and crysolide (0.1 ± 0.01%), while we found massoïlactone (0.4 ± 0.04%), p-cresol octanoate (0.2 ± 0.03%), 4-hydroxy-4-methyl-pentan-2-one (0.1 ± 0.04), (2E) dodecenal (0.1 ± 0.03%) and crysolide (0.1 ± 0.01%) representing 1.1 ± 0.17% of the extract of the cold season sample (Table 2).
Seasonal variation of the composition of the essential oils of Hemizygia bracteosa (Benth.) Briq.
| Chemical groups | I | II |
| % ± aSD | % ± aSD | |
| Hydrocarbon monoterpenes | 1 ± 0.09 | 5.7 ± 0.37 |
| Oxygenated monoterpenes | 1.9 ± 0.09 | 0.4 ± 0.04 |
| Monoterpenes | 2.9 ± 0.18 | 6.1 ± 0.41 |
| Hydrocarbon sesquiterpenes | 82.8 ± 0.56 | 86.9 ± 0.61 |
| Oxygenated sesquiterpenes | 11.5 ± 0.41 | 2.8 ± 0.4 |
| Sesquiterpenes | 94.3 ± 0.97 | 89.7 ± 1.01 |
| Others | 0.5 ± 0.07 | 1.1 ± 0.17 |
Extract I (59 compounds) obtained from the aerial parts harvested during the hot season was characterized by the presence as main constituents of (E)-β-farnesene (64 ± 0.04%), β-elemene (7.4 ± 0.05%), trans-nerolidol (6.2 ± 0.04%), α-muurolene (2.7 ± 0.03%), α-copaene (2.3 ± 0.02%) together with γ-himachalene (1.8 ± 0.03%), α-humulene (1.7 ± 0.03%), and 14-hydroxy-9-epi-(E)-caryophyllene (1.6 ± 0.05%).
Extract II obtained during the cold season (63 constituents) was characterized by a high concentration of (E)-β-farnesene (67 ± 0.04%) along with β-caryophyllene (3.6 ± 0.06%), β-elemene (3.3 ± 0.05%), 7-epi-α-selinene (3.1 ± 0.01%), p-cymene (2.5 ± 0.04%), δ-cadinene, (1.9 ± 0.03%), γ-terpinene (1.6 ± 0.04%), α-copaene (1.5 ± 0.05%) and germacrene-D (1.5 ± 0.04%).
The concentration of all other constituents was less than 1.2%. Each extract was thus characterized by known compounds but the main components may differ quantitatively; for I, (E)-β-farnesene, β-elemene, and trans-nerolidol, and for II (with different levels), (E)-β-farnesene and β-caryophyllene were the major constituents. This is the first report of these components in the essential oil of Hemizygia bracteosa (Benth.) Briq.
If we compare the essential oils of the two samples, we can see that the differences between samples were noticed especially on the level of three sesquiterpenes: β-elemene, β-caryophyllene and trans-nerolidol. (E)-β-farnesene (Fig. 1) was the predominant compound in both samples with a level higher than 60% (67% in sample II and 64% in sample I). This acyclic sesquiterpene olefin, was previously identified in high levels (>70%) in the essential oil of Hemizygia petiolata Ashby (Lamiaceae) from South Africa [17] and was found in small amounts in essential oils of hundreds of species of both gymnosperms [18] and angiosperms [19–21].
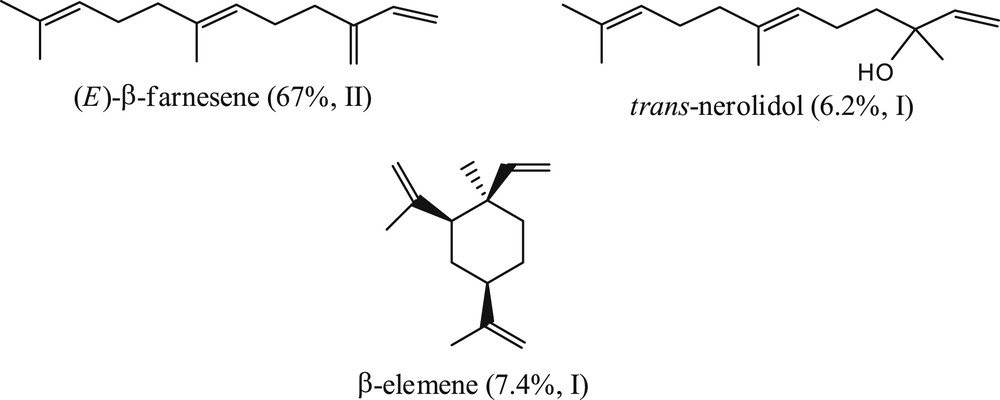
Major essential oil constituents of Hemizygia bracteosa (Benth.) Briq. samples harvested in February 2009 (I) and in August 2009 (II).
(E)-β-Farnesene is also released by aphids as an alarm pheromone upon death to warn away other aphids. Several plants, including potato species, have been shown to synthesize this pheromone as a natural insect repellent [22]. Recently, this compound has been reported to be involved in pea aphid (Acyrthosiphon pisum) wing induction [23] while its insecticidal activity at high doses has also been demonstrated [24].
β-Elemene (7.4%, Fig. 1) is an anticancer drug extracted from the traditional Chinese medicinal herb Rhizoma zedoariae. It has been used efficiently in China to treat certain types of tumors [25] and was evaluated in clinical trials in the United States. Studies indicated that β-elemene had a broad-spectrum anticancer activity, with effectiveness in treating leukemia, brain tumor, breast cancer and liver cancer [25]. Furthermore, it had low toxicity, was easy to administer, and was well tolerated and accepted by cancerous patients [25]. It also showed therapeutic potential for rheumatoid arthritis [26].
The third major constituent of our oils, nerolidol (6.1%, Fig. 1), was approved by the U.S. Food and Drug Administration as a food flavoring agent. It exhibited antineoplastic, antinociceptive and anti-inflammatory activities [27,28], and it was tested as a skin penetration enhancer for the transdermal delivery of therapeutic drugs [29]. Rodrigues-Goulart et al. [30] reported its activity against the malaria parasite. Arrudan et al. [31] described the leishmanicidal activity of nerolidol and its inhibitory effect on the biosynthesis of isoprenoids.
4 Conclusion
GC/FID and GC/MS analyses allowed us to identify in the essential oils of Hemizygia bracteosa (Benth.) Briq., 65 compounds. The main constituents were (E)-β-farnesene (67 ± 0.04%), β-elemene (7.4 ± 0.05%) and trans-nerolidol (6.2 ± 0.04%). Biological studies of these essential oils could help to clarify their biological properties.
Acknowledgments
This work was supported by the CUD (Commission Universitaire pour le Développement) and the CIUF (Coopération Institutionnelle Universitaire Francophone) (now ARES-CCD) through a grant given to S. Kpoviessi.


