1 Introduction
Formation of crystals in renal tubules is the obligatory initial step in stone formation. However, crystalluria, that is, the presence of crystals in urine, is a natural phenomenon resulting from urine supersaturation and may only reflect transiently low urine volume and/or high solute output. Indeed, the presence of crystals made of common constituents such as calcium oxalate (CaOx), calcium phosphate (CaP), or uric acid (UA) may occur in nonlithiasic subjects and is not a pathological finding per se, although the occurrence of crystalluria was found to be significantly more frequent in stone formers than in healthy subjects [1–3].
The search for crystalluria and analysis of the characteristics of urinary crystals is of interest in the laboratory evaluation of a stone former in several ways: (i) CaOx, CaP, and UA present as different crystalline phases which reflect different lithogenic risk factors; (ii) unusual morphology or abundance of crystals made of common constituents may orient toward peculiar etiologies such as conditions associated with massive hyperoxaluria; (iii) certain crystal species such as struvite, cystine, dihydroxyadénine (DHAd), xanthine, or drugs are not found in normal urine and are indicative of specific pathological conditions; and (iv) serial determination of crystalluria is of help in the follow-up of patients with severe forms of nephrolithiasis, as it reflects the activity of stone disease and response to therapeutic measures.
In patients who suffer from stone disease, crystalluria studies may specifically contribute to the diagnosis by comparison to extensive metabolic evaluation. For example, DHAd is not commonly measured in urine of affected patients, whereas crystalluria is found in almost all patients and establish unambiguously the diagnosis. In cystinuric patients, the diagnosis may be established either by the observation of cystine crystals or by measuring cystine excretion in urine. However, for the therapeutic management of the patients, cystine concentration or excretion is not sufficient to predict stone recurrence, whereas the presence of cystine crystals in urine and the global crystal volume (GCV) of cystine may be a good marker for predicting the risk of stone recurrence [4–6]. In calcium stone formers, neither the 24 h excretion of calcium, oxalate, phosphate, and citrate nor their respective concentrations are able to predict the presence of crystals and then the risk of stone recurrence, which may be deduced from crystalluria studies [7]. Thus, crystalluria examination is clinically relevant for either the diagnosis of crystalline pathologies or the management of patients.
2 Protocol for crystalluria evaluation
To be informative, crystalluria analysis should follow an adequate methodology and be performed in patient's usual conditions of life and nutrition.
Urine sample should be taken in the fasting state, on first-voided morning urine, or on the second micturition, because urine produced during the night is the most concentrated and therefore carries the highest probability of crystal formation.
The recommended protocol is described elsewhere [8–10]. In short, urine sample should be brought to the laboratory within 2 h of voiding, kept at room temperature, and processed without delay [11].
First, urine pH is measured with a pH meter (preferably) or with double-color scale strips with a precision of 0.1 unit, and specific gravity is measured using a densitometer (preferably) or a multichannel strip with a correction for urine pH [12]. Then, a sample of urine is homogenized by gentle shaking (not centrifuged), homogenized mixture is put in a Malassez cell, and is examined by polarized light microscopy. Microscopic examination includes urine cytology, and comprehensive crystalluria evaluation is based on the identification of all crystal species, numeration and measure of the size of crystals and aggregates and, if relevant, the assessment of crystal volume. Fourier transform infrared spectroscopy is used whenever needed to confirm the nature of unusual crystals.
Statistical comparisons regarding biochemistry of urine with or without crystals (ANOVA) and the distribution of crystals as a function of urine pH classes (chi-square test) were performed with the NCSS statistical package (J. Hintz, Gainesville, FL). A P value less than 0.05 was considered as statistically significant.
3 Nature and morphology of urinary crystals and correlations with etiology
Urinary crystals have a great variety of morphologies. Several textbooks dealing with the urinary sediment or crystal analysis may help to identify metabolic or drug crystals [13–15]. From a clinical point of view, crystals are reflecting supersaturation of urine regarding one or several chemical species. In our experience, correlations between crystalluria and etiology of nephrolithiasis have been established from microscopic examination of approximately 25,000 first morning urine samples from 3000 patients for whom urine biochemistry parameters were determined in the same samples and full etiological evaluation was available. In addition to the chemical nature of common forms of nephrolithiasis, identification of the crystalline forms of CaOx, CaP, or UA crystals provides important information that may orient toward specific pathophysiological conditions, some corresponding to severe forms of nephrolithiasis.
3.1 Common types of urinary crystals
3.1.1 Calcium oxalates
Crystalluria made of CaOx crystals is the commonest form of crystalluria found in stone formers. The main determinant of CaOx crystal formation is urine supersaturation owing to excessive concentration of calcium and oxalate ions, reflected by their molar product (pCaOx). A very low concentration of low molecular weight inhibitors such as citrate and magnesium ions may also induce crystalluria [16,17]. However, CaOx crystals do not come under a homogeneous category, as they present commonly in two different forms, CaOx monohydrate (COM, or whewellite) and CaOx dihydrate (COD, or weddellite) according to the respective concentrations of Ca and Ox ions (Fig. 1). A third form, namely CaOx trihydrate or caoxite may be scarcely found in urine (Fig. 2).
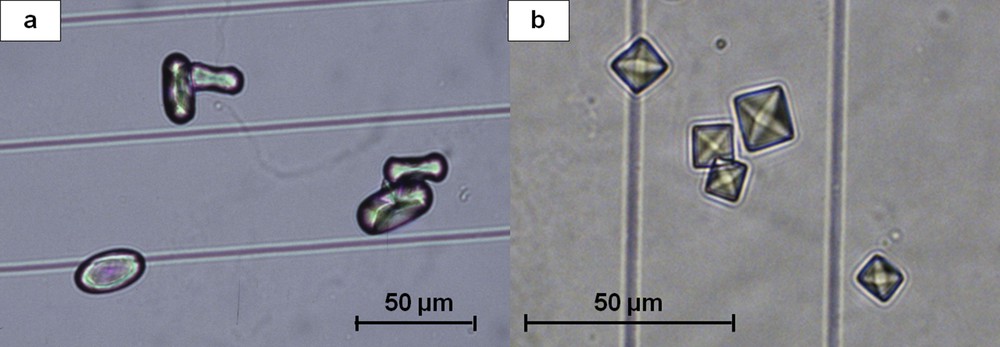
(a) Typical oval crystals of whewellite with a depressed core. (b) Bipyramidal (octahedral) crystals of weddellite.
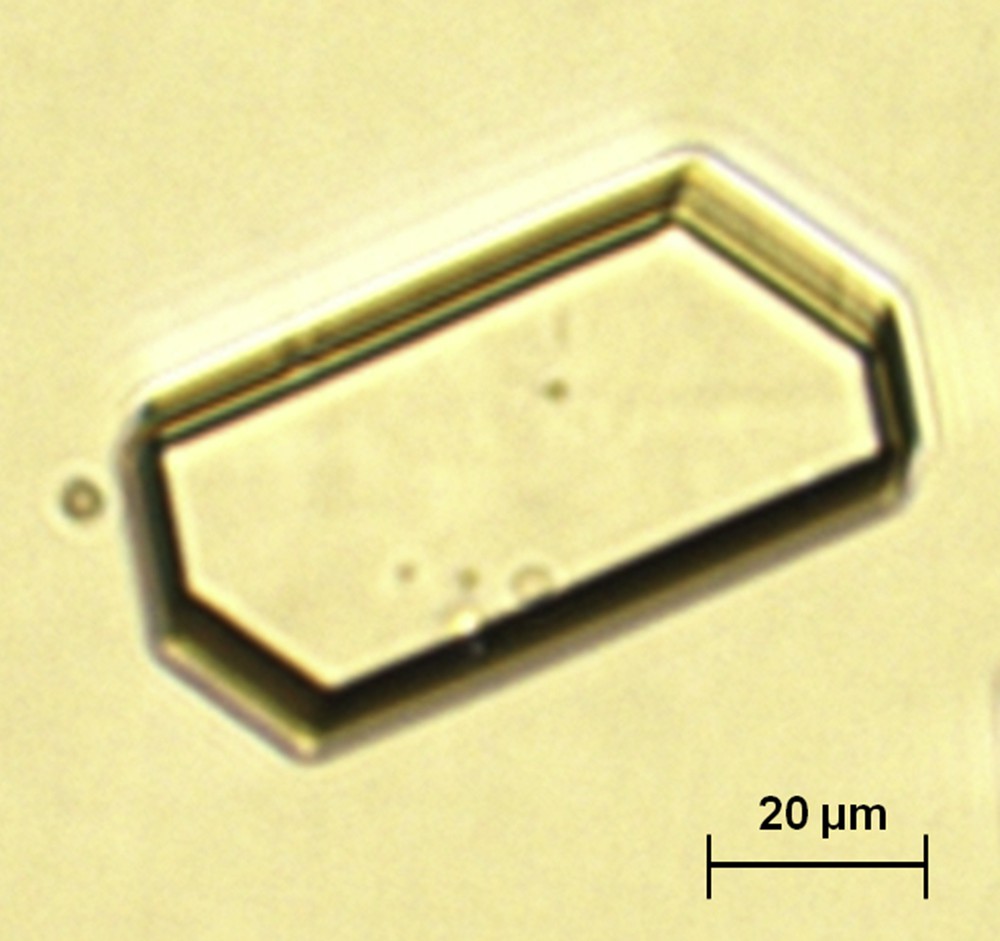
Hexagonal crystal of caoxite (calcium oxalate trihydrate).
Simultaneous determination of calcium and oxalate concentrations and search for crystalluria in 21,220 urine samples from calcium stone formers allowed to define threshold values for the risk of CaOx crystal formation [18]. Thus, the concentration of oxalate ions associated with the formation of CaOx crystals, determined as the inflexion of the curve plotting the percentage of urine samples exhibiting the presence of CaOx crystals versus the concentration of oxalate, was 0.3 mmol/l (Fig. 3).
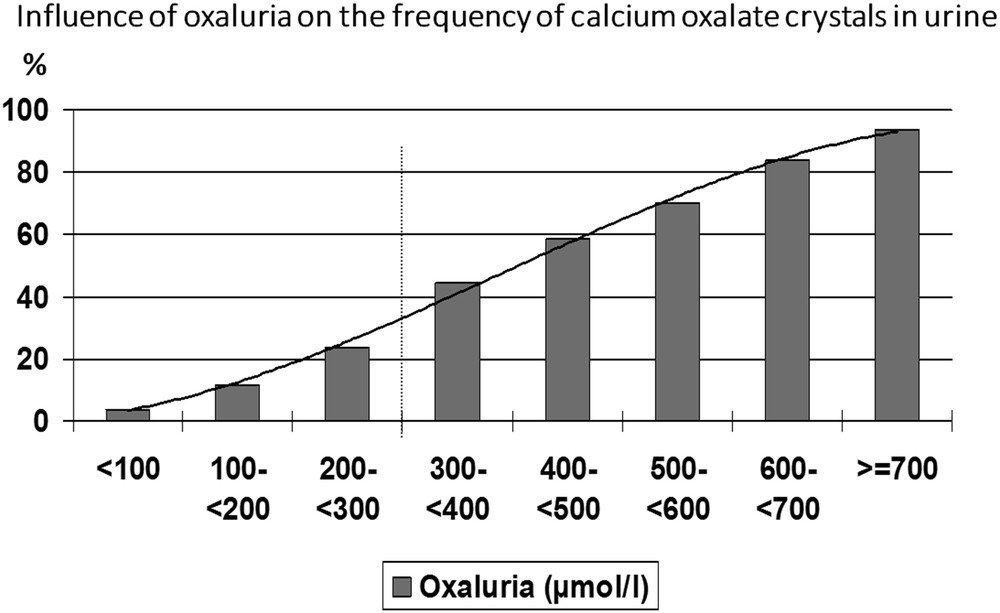
Influence of oxalate concentration on the occurrence of CaOx crystalluria.
The concentration of calcium ions associated with the formation of CaOx crystals, determined as the inflexion of the curve plotting the percentage of urine samples exhibiting the presence of CaOx crystals versus the concentration of calcium, was 3.8 mmol/l (Fig. 4).
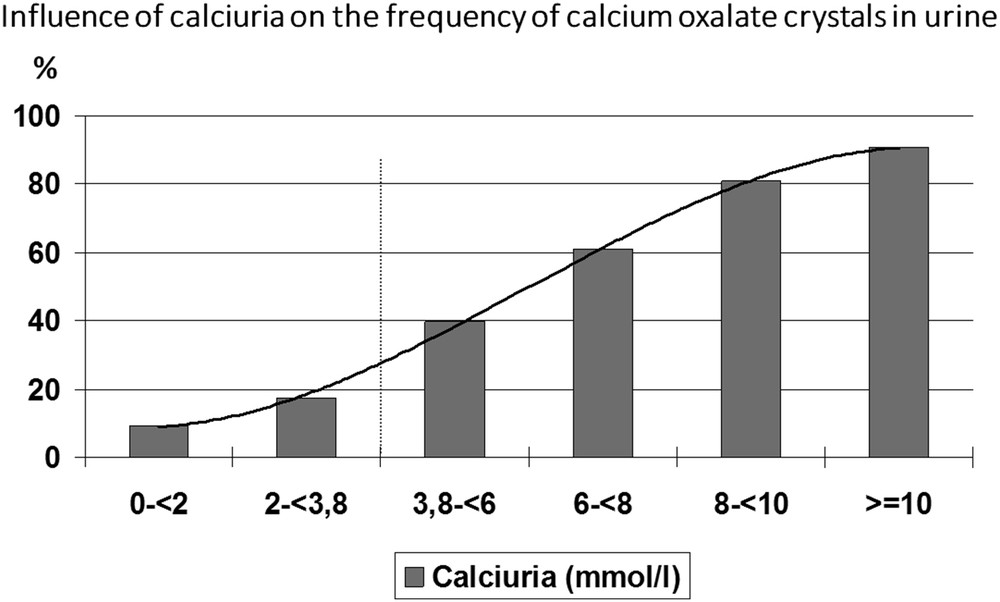
Influence of calcium concentration on the occurrence of CaOx crystalluria.
3.1.1.1 CaOx crystalline species
In idiopathic CaOx nephrolithiasis, predominance of COM (whewellite) crystals is associated with an increased concentration of oxalate [18], whereas predominance of COD (weddellite) crystals is mostly associated with hypercalciuria [18,19]. COM crystalluria was found in 66% of urine samples with a high oxalate concentration (>0.3 mmol/l) facing a relatively low calcium concentration (Ca < 2 mmol/l), whereas COD crystals were found in 99% of samples with both a high calcium concentration (>3.8 mmol/l) and a normal or relatively low oxalate concentration.
In fact, whewellite is preponderantly formed when the molar ratio of Ca/Ox (rCaOx) is <5, that is, in urine with a high Ox concentration facing a relatively low Ca concentration. On the contrary, weddellite is almost exclusively formed when rCaOx is >14, that is, in urine of high Ca concentration facing a normal or slightly augmented Ox concentration. In the interval estimate, both forms are formed in variable proportions (Fig. 5).
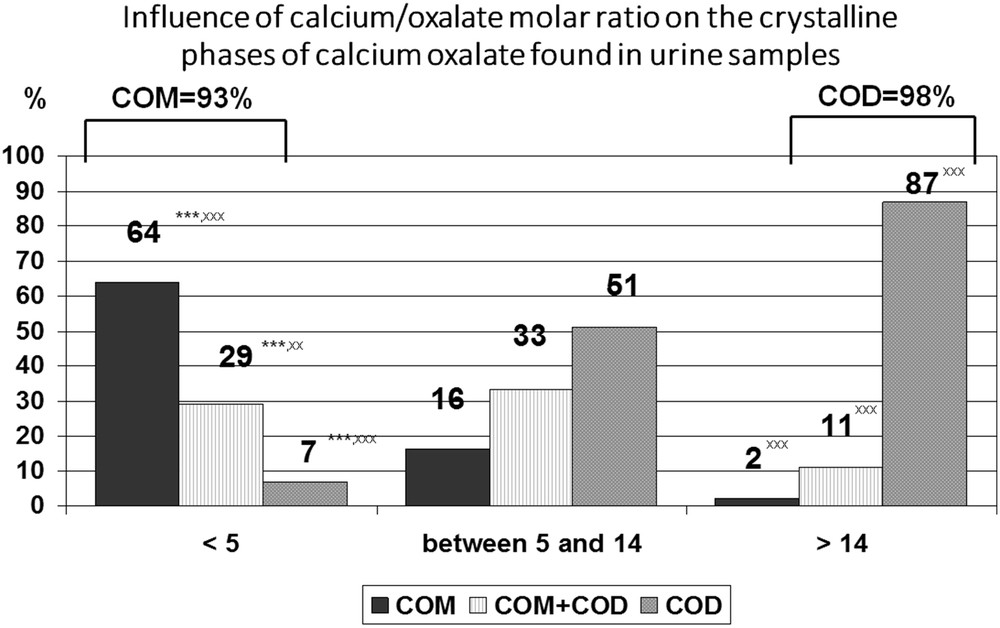
Distribution of crystal phases of calcium oxalate according to the calcium/oxalate molar ratio in urine samples. Data collected from 6869 urine samples that contained calcium oxalate crystals. ∗∗∗P < 0.00001 vs rCaOx > 14. xxP < 0.001, xxxP < 0.00001 vs 5 ≤ rCaOx < 14.
3.1.1.2 Influence of pH on CaOx crystalluria
Although crystallization of CaOx is poorly dependent on urine pH [20], we observed in our whole series of urine samples from stone formers that a urine pH beyond 6.5 is associated with a lower frequency of COD crystalluria, the calcium-dependent form of CaOx, probably because of a higher citrate activity [21]. Indeed, citric acid is a tricarboxylic acid with three ionizable acid functions, of which the pKa are, respectively, 3.13, 4.76, and 6.40. Consequently, the pH level is able to influence ionization of citrate, making citrate3− in neutral or alkaline urine more efficient for complexing calcium ions than do citrate2− in more acidic urine. Moreover, in alkaline urine, citrate is able to form larger amounts of [CaCitPO4]4− ions which are soluble, thus reducing significantly urine supersaturation as suggested by Rodgers et al. [22]. As a result, a significant decrease in COD crystalluria is observed in the pH range >7.0 (Fig. 6). It was often reported that calcium stone formers excrete less citrate than healthy subjects, thus explaining, at least in part, the formation of calculi. In our experience, COD crystalluria peaked in the pH range 5–6.5. In this pH range, the mean concentration of citrate in the presence of COD crystalluria was 2.39 mmol/l, an obviously normal value [23–25] and did not change significantly for higher pH values. By contrast, urine calcium concentration was lower in alkaline urine (P < 0.00001) in comparison with more acidic samples, in association with a lower prevalence of COD crystalluria (Fig. 7). As shown in Fig. 8, this decrease was associated with a significant decrease in the calcium/creatinine molar ratio in alkaline urine samples with and without crystals as well. These data suggest that calcium excretion is reduced in alkaline urine. Of note, the higher level of creatinine in patients with COD crystalluria (Cr+) for all values of urine pH suggests that Cr+ is, at least in part, associated with a lower diuresis or with an insufficient water intake before the bedtime. Thus, a moderate alkalization of urine could benefit CaOx stone formers by reducing the risk of COD crystallization, especially in patients with heavy hypercalciuria.
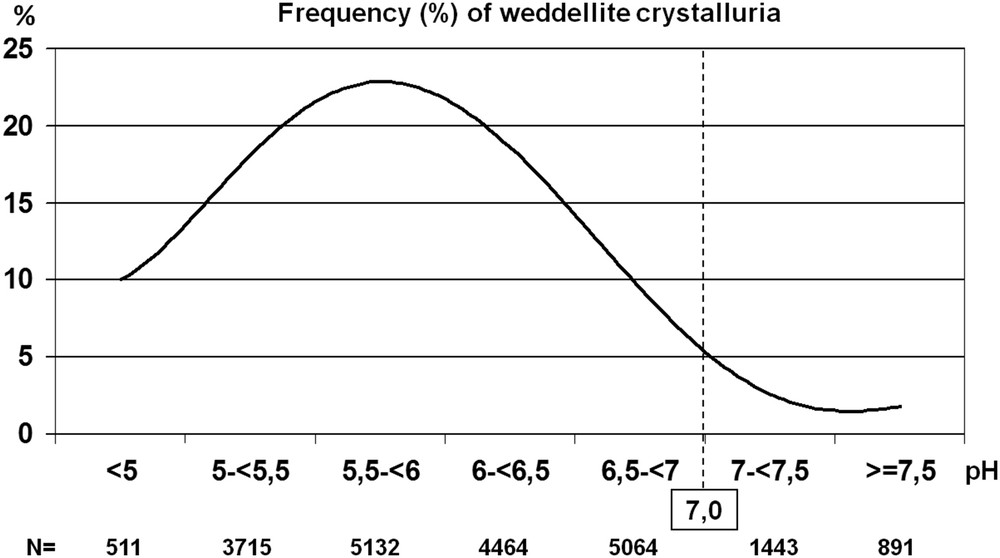
Influence of urine pH on the occurrence of CaOx crystalluria (whole series of 21,220 urine samples). The percentage of urine samples containing COD crystals was significantly lower (P < 0.00001) in acidic urine (pH < 5) and in alkaline urine (pH ≥ 7) as compared with urine in the intermediate range of pH value, i.e., between 5.5 and 6.5.
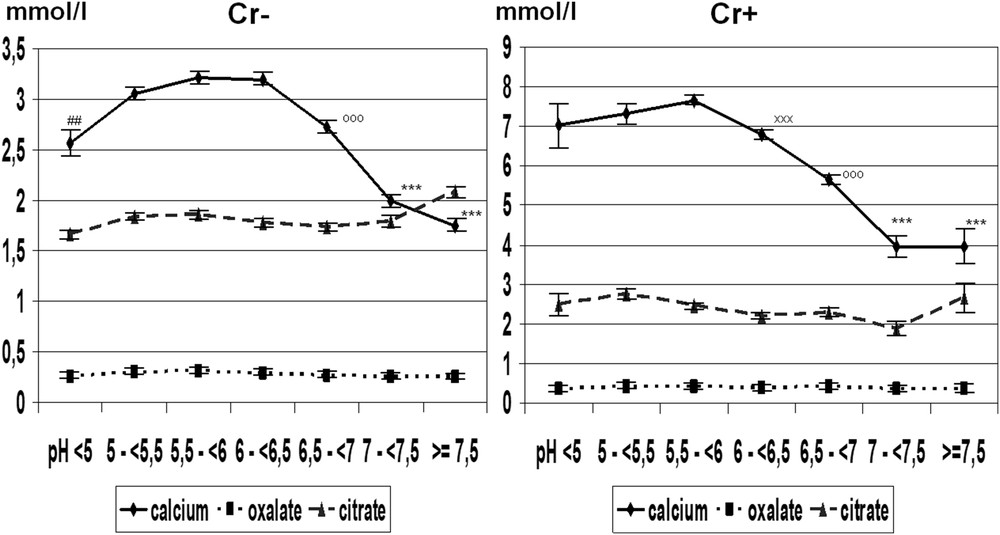
Mean concentrations of calcium, oxalate, and citrate in urine samples with (Cr+, n = 3109) and without (Cr−, n = 18,111) COD crystals in relation to urine pH. We observe that urine calcium concentration is 2–3 times higher in Cr+ than in Cr− urine samples. Values are expressed as the mean ± SEM.
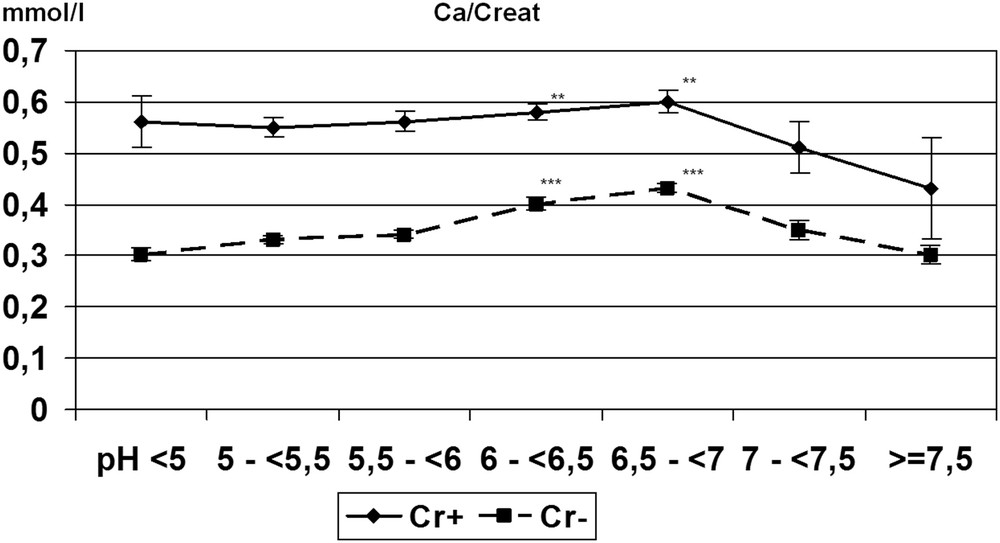
Change in the calcium/creatinine (Ca/Creat) molar ratio in relation to urine pH. Although the Ca/Creat ratio was nearly two times higher in urine samples with (Cr+, n = 3109) than without (Cr− COD , n = 18,111) COD crystals and slightly increased with pH up to pH 7, this ratio markedly decreases beyond pH 7 in both cases. Values are expressed as the mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.00001 vs pH < 6 or pH ≥ 7.
3.1.1.3 Crystal morphology
Morphology of weddellite crystals depends on the level of calciuria. Usually, COD crystals are octahedral presenting as two flat pyramids joined at their bases (Fig. 1). However, as calcium concentration increases, the interface between pyramids enlarges, leading to COD crystals with a dodecahedral aspect (Fig. 9). The presence of dodecahedral COD crystals (i.e., bipyramidal with a thick zone between the two pyramids) is indicative of heavy hypercalciuria, usually over 7 mmol/l. As shown in Fig. 10, the frequency of dodecahedral COD crystals increases with increasing urine calcium concentration.

Two morphological aspects of dodecahedral crystals of weddellite: (a) polarizing microscopy; (b) light microscopy.
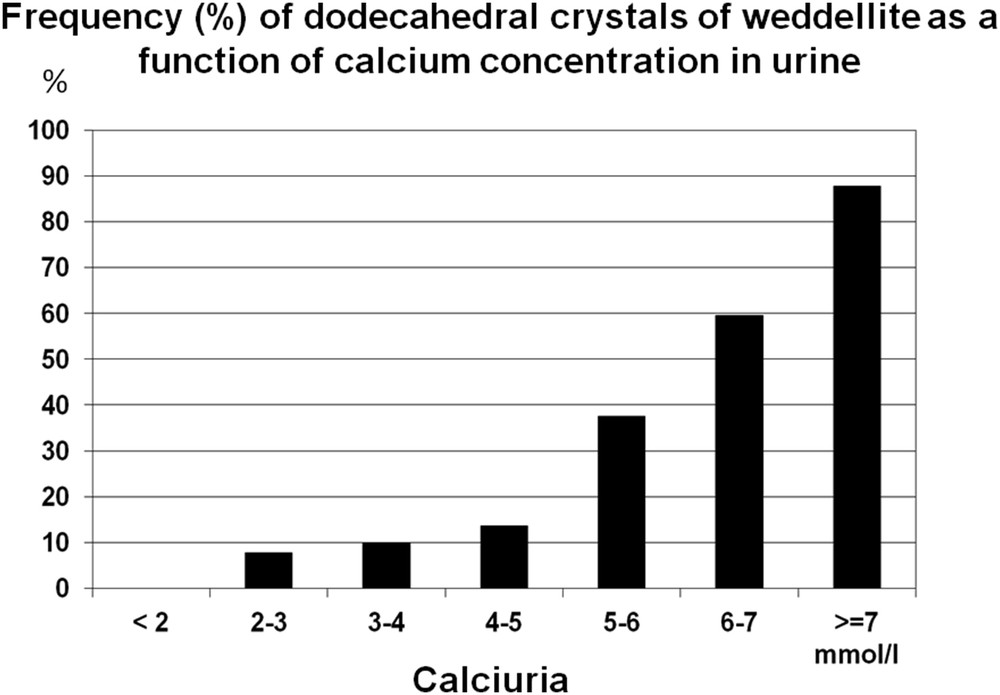
Occurrence (%) of dodecahedral crystals of weddellite as a function of calcium concentration in urine.
As shown in Fig. 1, COM crystals present as oval forms with a depressed part in the center of the crystals. It is the common form of COM in urine. However, in some cases, COM crystals may present as narrow hexagons or lozenges, which suggest an ethylene glycol poisoning [10].
3.1.1.4 Quantitative aspect
An abundant amount of pure COM crystals (>200/mm3) is highly suggestive of primary hyperoxaluria type 1 (PH1) and in our experience was found in virtually all untreated patients with primary hyperoxaluria, although the disease may be associated with lower counts of COM crystals, especially when calcium concentration is very low (<1 mmol/l).
3.1.2 Calcium phosphates
Most CaP crystals are made of calcium orthophosphates, mainly amorphous carbonated CaP and/or carbapatite, and present at light microscopy as small granulations giving no polarization (Fig. 11). Carbapatite crystals were mostly found in hypercalciuric urines with a normal pH (5.8–6.5), whereas amorphous carbonated CaP was observed in urines with a high pH (>6.6) and normal or near-normal calcium and phosphate concentrations [26].

Agglomerate of small grains and irregular translucent plaque of amorphous carbonated calcium phosphate.
Brushite crystals, made of calcium and hydrogenophosphate ions, present as sticks, which often form large aggregates (Fig. 12). Brushite crystals were mainly found in urines with both marked hypercalciuria ± hyperphosphaturia, and a pH of nearly 6.4. In addition, unlike other CaP crystals, brushite is often associated with a decreased citrate concentration and consequently a high calcium/citrate molar ratio (average 8.28 vs 3.99 for other CaP crystals, P < 0.0001). Also, the presence of brushite crystals or aggregates >500/mm3, a very uncommon finding, should prompt the search for primary hyperparathyroidism [27].

(a) Rod-shaped crystals of brushite. (b) Aggregate of brushite crystals.
All types of CaP crystals are pH-dependent and form in urines whose pH is commonly >6.4 (Fig. 13).
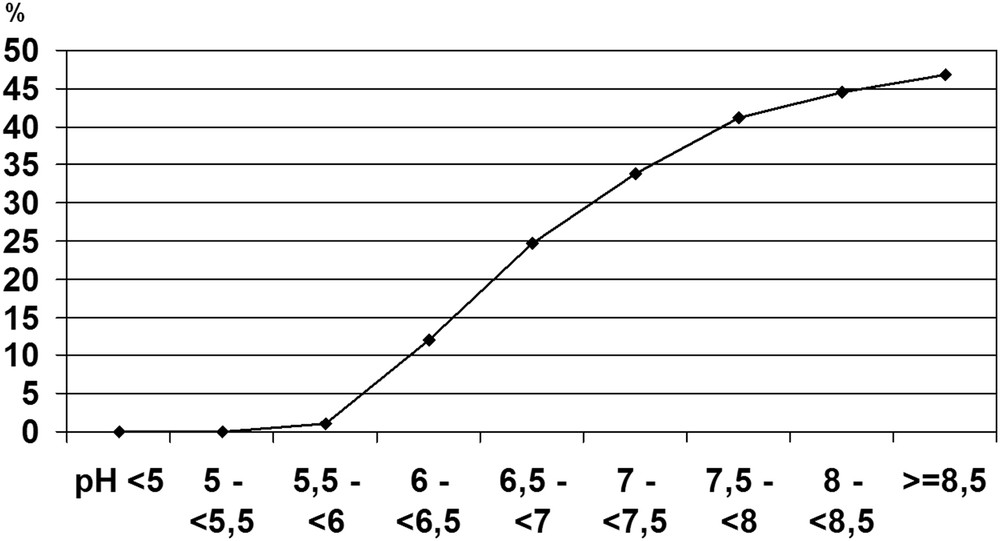
Influence of urine pH on calcium phosphate crystallization (n = 2800 urine samples).
3.1.3 Uric acid
UA crystals form in acidic urine. UA dihydrate is the commonest form. It has the lowest crystallization pH (5.25) and presents with various morphologies, typically as hexagonal or diamond-shaped crystals, characteristically having a polychrome aspect in polarized light (Fig. 14). Crystalluria made of UA dihydrate is suggestive of defective renal ammoniagenesis as observed in the metabolic syndrome or type 2 diabetes [28]. Anhydrous UA and UA monohydrate are less frequently observed and also form in acidic urine, whereas amorphous UA (Fig. 15) precipitates are found in urine with a high UA concentration in moderately acidic pH.
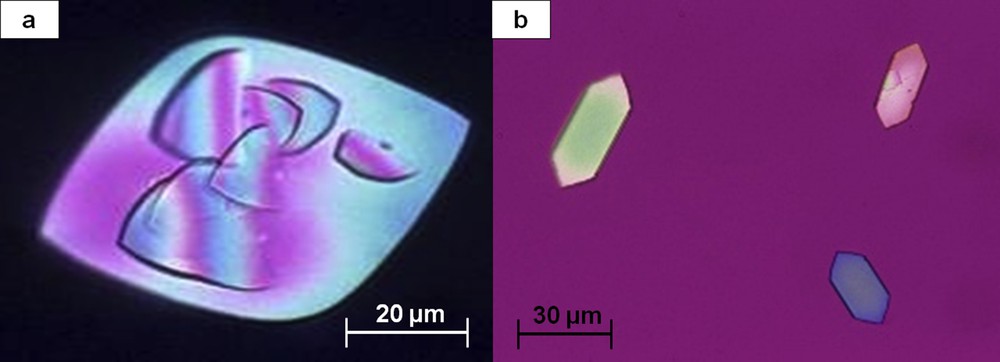
Two morphological aspects of uric acid dihydrate crystals: (a) polychromatic diamond-shaped crystal seen at polarizing microscopy. Note the initiation of new crystals at the surface of the initial crystal; (b) polychromatic hexagonal crystals.

Multiple polarized grains of amorphous uric acid.
Anhydrous UA presents as large polygonal crystals with a monochrome aspect in polarized light.
As shown in Fig. 16, UA dihydrate forms mainly in very acidic urine, whereas amorphous UA mainly depends on the concentration of urate ions in urine.
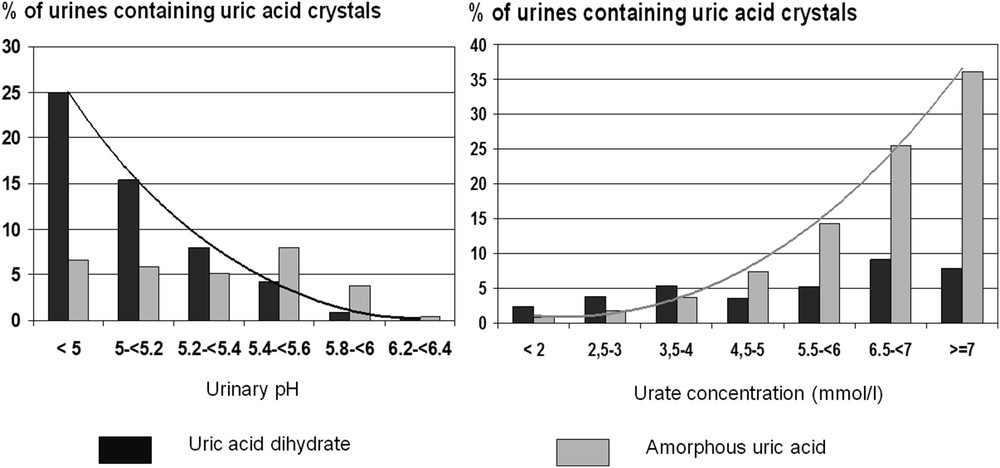
Respective influence of urine pH (left) and uric acid concentration (right) on the formation of uric acid dihydrate crystals and amorphous uric acid precipitates.
3.1.4 Urate salts
The most frequent urate form is anhydrous ammonium hydrogen urate. Its crystals form in alkaline urine or in the pH range of 6.3–7, depending on the associated metabolic factors, and they exhibit various morphologies (Fig. 17).

Various forms of ammonium hydrogen urate crystals.
Unlike ammonium urate crystals, crystals made of urate salts such as sodium hydrogen urate, potassium quadriurate, calcium hydrogen urate, or magnesium hydrogen urate are very uncommon. All of these species are observed in neutral or alkaline urine.
3.1.5 Struvite
Crystals of magnesium ammonium phosphate (struvite) form in strongly alkaline urine. Struvite crystals typically have a “coffin-lid” aspect (Fig. 18), but they also present as other morphologies (Fig. 19). The presence of struvite crystals, even in small amounts, in a markedly alkaline urine (pH > 7) is indicative of urinary tract infection (UTI) with urease-producing microorganisms, usually Proteus mirabilis or certain strands of Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, or less frequently, Corynebacterium urealyticum, responsible for encrusted cystitis and/or pyelitis [29]. Finding of struvite crystals even in the absence of overt UTI should prompt careful bacteriological urine analysis to identify the responsible microorganism, including culture on special media if a usual urease-producing microorganism is not found in routine analysis.
Typical coffin-shaped crystal of struvite.
Large crystal of struvite seen by polarizing microscopy.
3.2 Infrequent types of urinary crystals
Other crystals are much less frequently found in urine. They reflect the presence of rare hereditary stone diseases or the renal elimination of drugs.
3.2.1 Hereditary stone diseases
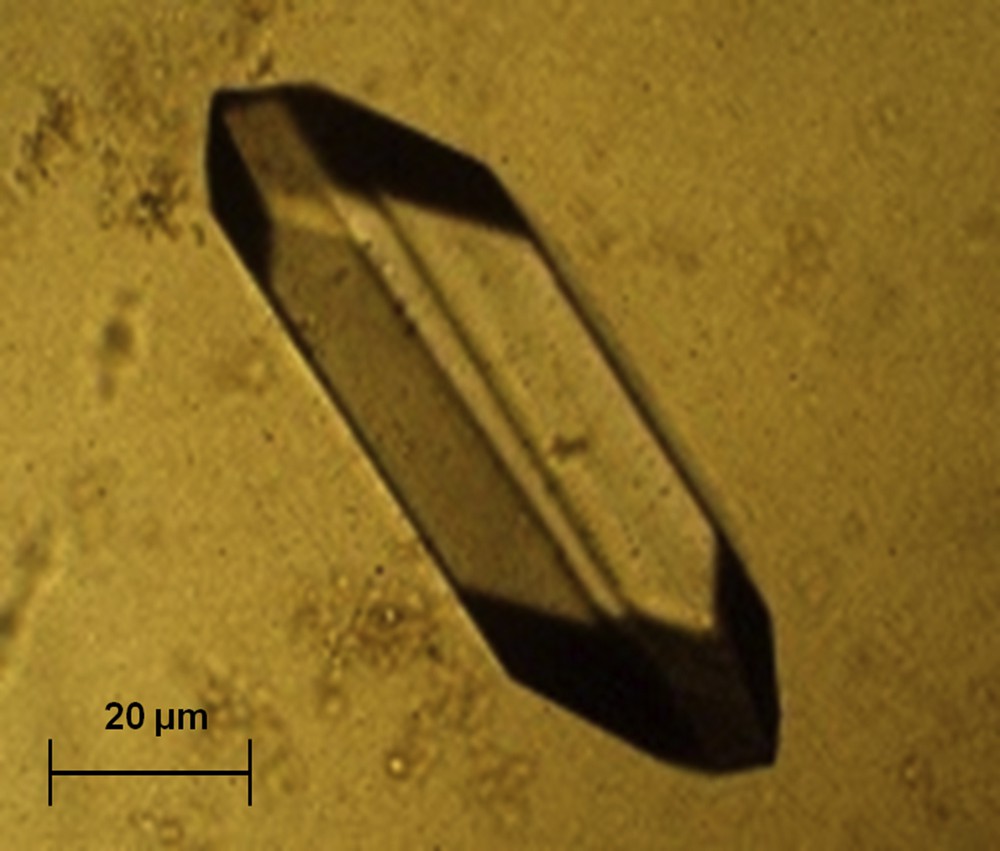
Cystine crystals present as hexagonal slides often forming macles of large dimensions (Fig. 20). They are pathognomonic of proximal tubular defects because of mutations of the genes coding for membrane transporters of dibasic amino acids [30].

Typical crystals of cystine.
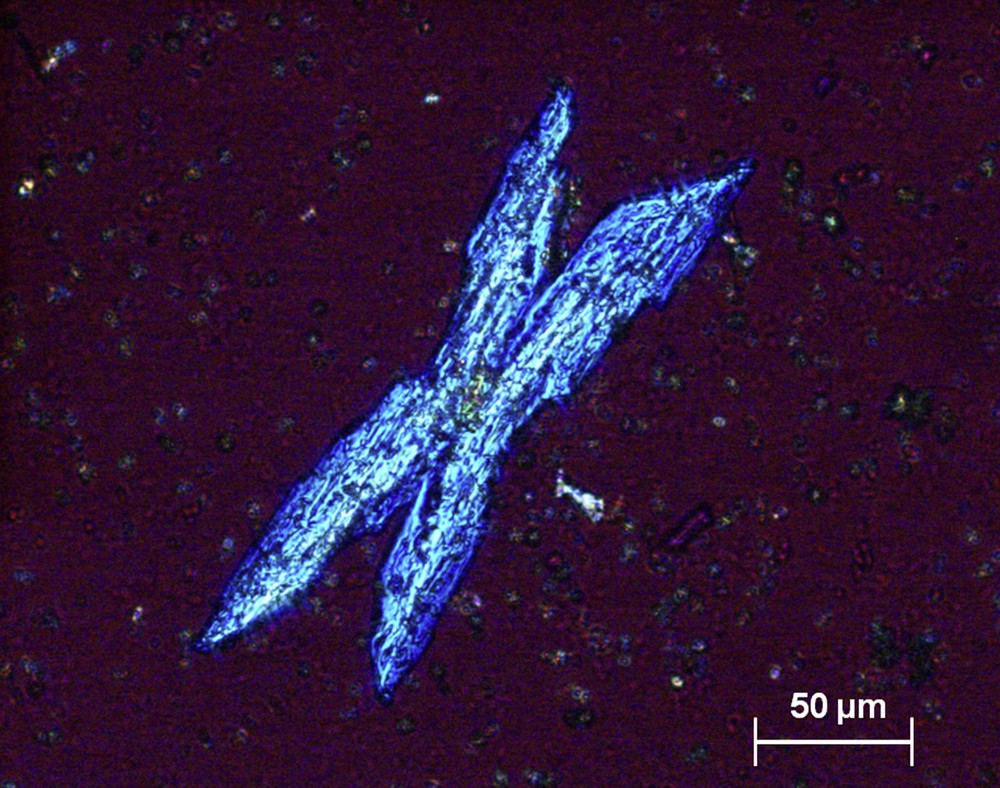
2,8-Dihydroxyadenine crystals present as small round crystals (about 5 μm in diameter) showing a characteristic central black cross (“Maltese cross” aspect) at polarizing microscopy (Fig. 21). They are found in patients with an adenine phosphoribosyltransferase deficiency [31,32] and may be responsible for progressive interstitial crystal infiltration leading to end-stage renal failure [33–35].

Small spherical crystals of dihydroxyadenine: (a) light microscopy; (b) polarizing microscopy. Note the typical aspect under polarized light showing that all crystals exhibit a Maltese cross.
Xanthine crystals present as refringent granulations or sticks without a characteristic aspect, and Fourier transform infrared analysis is required for accurate identification. Xanthine crystals are observed in two different pathological conditions: (i) inherited xanthine dehydrogenase deficiency, and (ii) long-term treatment with allopurinol in patients with hereditary hypoxanthine guanine phosphoribosyltransferase deficiency.
Tyrosine, leucine, or potassium orotate crystals are very infrequent. They reflect very rare metabolic diseases.
3.2.2 Drug-induced crystallurias
A few drugs, mainly antimicrobial or antiviral agents, may crystallize in urine when used at high dose for several days and, sometimes, for long periods [36]. They may be responsible for stone formation or sometimes heavy tubular precipitation and acute anuric renal failure [37–43].
In a patient presenting with acute oliguric renal failure of no obvious cause, immediate search for crystalluria in the few passed urine may allow proper diagnosis by identifying characteristic drug crystals. Rapid institution of appropriate therapeutic measures such as modulation of urinary pH and hydration often obtained recovery of renal function even in patients with major renal impairment [44].
Several sulfonamides may induce crystalluria (Fig. 22). N-acetylsulfamethoxazole chlorhydrate, the main metabolite of sulfamethoxazole largely used for the treatment of UTIs in combination with trimethoprim, forms crystals in acidic urine. Its crystals with a losangic form (Fig. 22a) resemble UA dihydrate, and those with a hexagonal or oval form may be mistaken with cystine or whewellite crystals. N-acetylsulfadiazine, the main metabolite of sulfadiazine used in the treatment of cerebral toxoplasmosis, also crystallizes in acidic urine (Fig. 22b), whereas crystals of N-acetylsulfaguanidine, a metabolite of sulfaguanidine used as intestinal antiseptic, are very infrequently observed.
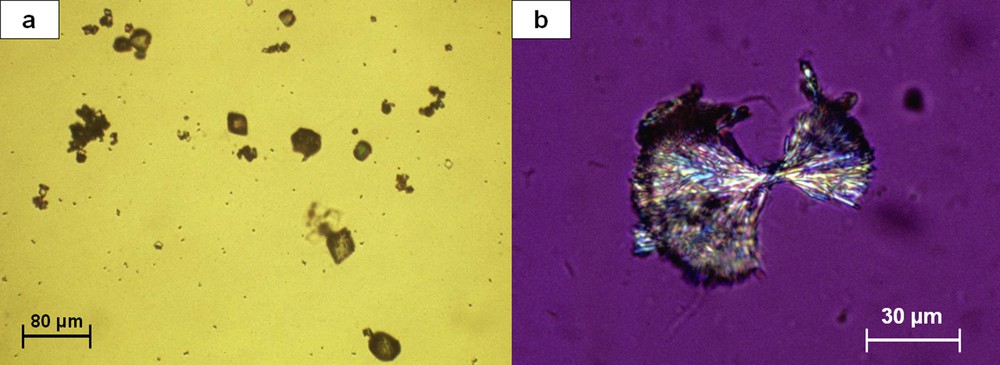
(a) Crystals of N-acetylsulfamethoxazole hydrochloride resembling uric acid dehydrate crystals (light microscopy). (b) Aggregated crystals of N-acetylsulfadiazine (polarizing microscopy).
Occasionally, other antibacterial agents such as fluoroquinolones, aminopenicillines (particularly high-dose amoxicillin), or ceftriaxone may induce heavy crystalluria and intratubular precipitation with acute renal failure (Fig. 23) [45,46].

Isolated or aggregated large needle-shaped crystals of amoxycillin trihydrate (polarizing microscopy).
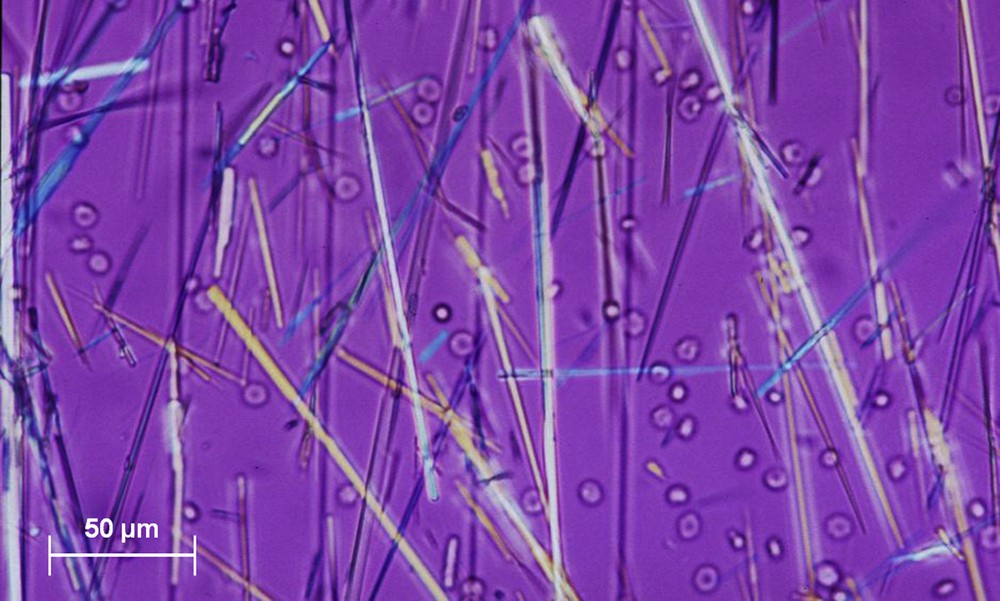
Antiviral agents currently are a common cause of crystalluria, especially indinavir and now atazanavir. These antiproteases, widely used in combination therapy in AIDS patients, form very large bundles of drug needles (150–250 μm or more) [37,47]. Of note, although indinavir crystalluria is often abundant with large aggregates, atazanavir crystals are commonly dispersed and poorly aggregated (Fig. 24). Aciclovir, used for the treatment of herpes virus infections, also forms crystals with an aspect of long, thin needles that may form aggregates (Fig. 25) [48].

Crystals of antiprotease drugs used to treat HIV patients. (a) and (b) Aggregates of indinavir monohydrate crystals. (c) and (d) Needle-shaped crystals of atazanavir. The crystals are commonly associated with white cells (arrow).
Needle-shaped crystals of aciclovir (polarizing microscopy).
Felbamate, an anti-seizure drug, was also reported to crystallize in urine [49].
4 Crystalluria and follow-up of stone formers
Both qualitative and quantitative aspects of crystalluria studies may be clinically relevant for the follow-up of stone formers.
4.1 CaOx crystalluria
4.1.1 Calcium concentration
The validity of a value of 3.8 mmol/l for urinary calcium concentration as the threshold for the risk of calcium stone formation was confirmed in vivo in a prospective case–control study including 181 patients with severe, recurrent idiopathic calcium nephrolithiasis who were followed for at least 3 years (up to 20 years) with search for crystalluria at each visit and all of whom receiving the same therapeutic recommendations [7]: 72 patients experienced a new stone episode within a mean time of 2.4 years, whereas the other 109 had no recurrence during a follow-up of 6.9 years. In this study, ROC curve analysis determined the cutoff value of urinary calcium concentration indicative of the risk of stone recurrence at 3.78 mmol/l.
A similar threshold value was also evidenced by other groups. In a prospective incidence study by Curhan et al. [50] in the USA, the risk of formation of calcium stones increased significantly in patients of either gender when urinary calcium concentration was in excess of 3.75 mmol/l. In a prospective Italian study, the corresponding risk value was estimated at 3.63 mmol/l [51].
Thus, on the basis of such concordant data, a calcium concentration <3.8 mmol/l should be adopted as the recommended goal to reduce the risk of calcium stone formation or recurrence.
4.1.2 Correlation of crystalluria with activity of the lithogenic process: crystalluria index
Crystalluria is more frequently found in stone formers than among healthy people, although some crystals may transiently be found in urines of nonstone forming persons, especially during the hours after a meal.
As there is a necessary relationship between precipitation of urinary crystals and formation of stones, we reasoned that the risk of stone formation would increase with an increased frequency of crystalluria. By evaluating the presence or absence of crystals in first morning urine samples at each visit in 204 calcium stone formers prospectively followed at our institution for a median duration of 7 years (5–15 years), we observed sustained crystalluria to be indicative of persistent stone disease activity and highly predictive of the recurrence of stones. The presence of crystals in 50% or more of urine samples was associated with stone recurrence in 87% of cases, whereas stone recurrence was observed in only 9% of patients with less frequent crystalluria (Fig. 26). In another study, we found that around 90% of recurrent stone formers exhibited crystals in at least 50% of urine samples [52].
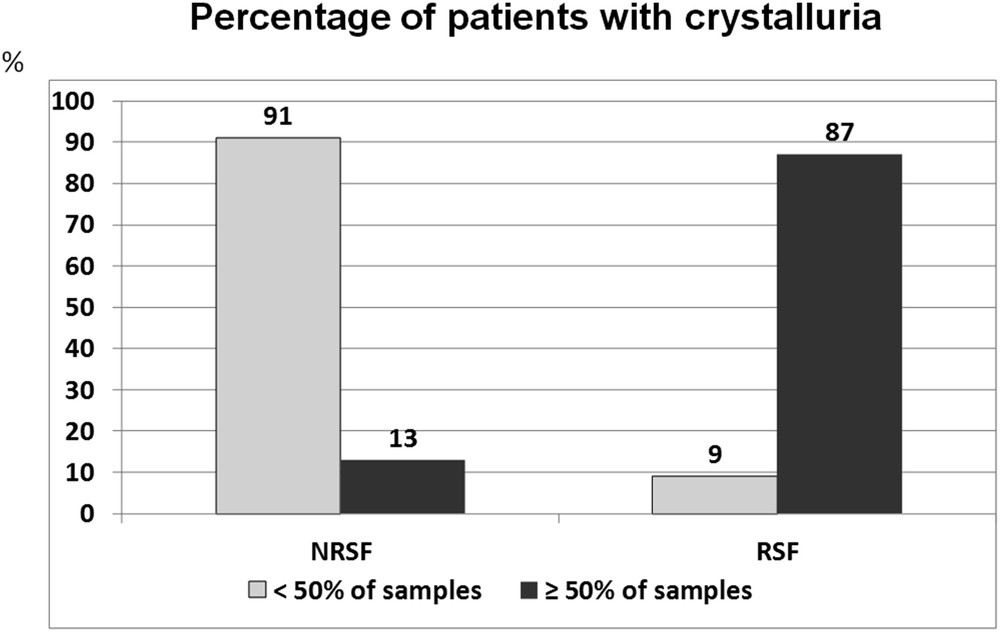
Relation between the occurrence of crystalluria in serial urine samples and the risk of stone recurrence in stone formers. NRSF = nonrecurrent stone formers, RSF = recurrent stone formers over a period of 7 years of follow-up.
Accordingly, we proposed a “crystalluria index” defined as the ratio of the number of urine samples containing crystals to the total number of examined samples in a given patient, with a crystalluria index ≥0.50 as the threshold value indicative of persistent lithogenic activity and risk of stone recurrence [7]. In clinical practice, the interval of time between two successive urine sample examinations should be about 6 months (or less in the case of an active nephrolithiasis) for an optimized follow-up of the patients. According to the ROC curve analysis, the cutoff value of a crystalluria index for the risk of developing stone recurrence was 0.50, and multivariate analysis by the Cox model identified a crystalluria index of 0.50 as the most powerful independent predicting parameter, with a hazard ratio of 16.8 [7].
4.2 Quantitative aspects: GCV
Crystalluria abundance is usually evaluated by simply counting the number of crystals per cubic millimeter. It may be evaluated more precisely by determining their total volume per cubic millimeter. Such quantification is especially useful for the management of severe forms of nephrolithiasis.
Disappearance of crystalluria is the best indicator of well-controlled lithogenic activity. This result is generally reached in patients with common forms of calcium or UA nephrolithiasis. However, total disappearance of crystalluria is a difficult goal to achieve in genetic diseases, such as primary hyperoxaluria and cystinuria, which have a very active, permanent crystallization process. In these severe diseases, a partial decrease in the amount of crystals present in urine samples guided by the determination of GCV is a more attainable goal often sufficient to reduce stone formation.
Determination of the global volume occupied in urine by a given crystal species per volume unit provides the most precise estimation of the abundance of crystalluria.
The GCV (expressed as μm3/mm3) is calculated as the product of the number of crystals per cubic millimeter multiplied by the average size of crystals in micrometers and by a numeric factor taking into account the geometric form of crystals specific for each species [10].
Formulae proposed for the calculation of GCV of crystallurias of whewellite, weddellite, cystine, or dihydroxyadenine are presented in Figs. 27 and 28.
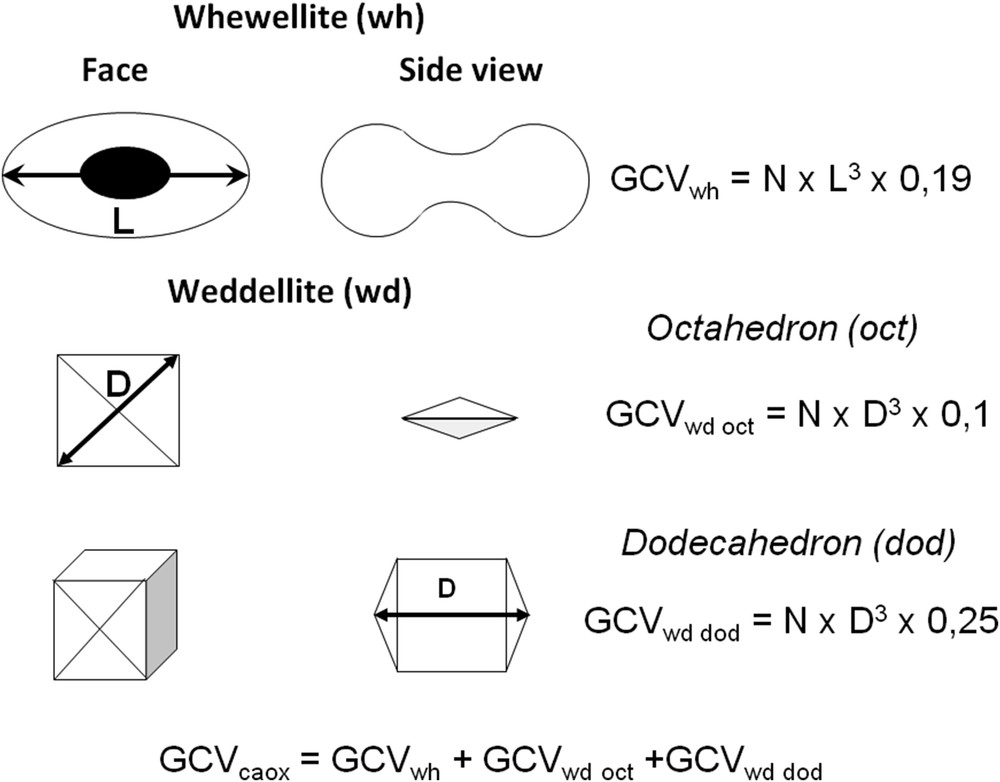
Crystal shapes of whewellite and weddellite used for the calculation of global crystal volume (GCV) of calcium oxalate crystalluria. N = number of crystals/mm3; L = average length (μm) of the whewellite crystals; D = average diagonal or length (μm) of weddellite crystals.

Formulae proposed for calculation of the global crystal volume (GCV) of cystine crystals and 2,8-dihydroxyadenine in urine. N = number of crystals/mm3.
By comparing clinical outcomes and variations in crystal volume in serial urine samples, we could determine the thresholds of GCV associated with stone recurrence in PH1 and cystinuria, thus defining the desirable goals to achieve.
In PH1 patients, a crystal volume <500 μm3/mm3 was associated with a lowered risk of tubular plugging with COM crystals in the days following combined liver–kidney transplantation, a crucial period when considerable amounts of CaOx stored in bones are excreted through the kidneys after restoration of renal function, with the risk of massive tubular obstruction and loss of the kidney transplant [53]. By closely monitoring CaOx crystal volume, early post-transplant care of PH1 children could be optimized [54,55] and long-term outcome was improved [56].
In cystinuric patients, measurement of cystine excretion is not recommended for the management of patients to prevent stone recurrence [57]. Crystalluria study may be clinically relevant [6,58]. The presence of >25 cystine crystals/mm3 is indicative of an active lithogenic process, although a lesser number of cystine crystals may be associated with stone formation, especially in the case of large crystals. Therefore, determination of the GCV of cystine appears as a better means to assess the risk of cystine stone formation during the follow-up of patients. Thus, a cystine crystal volume >3000 μm3/mm3 was shown to be predictive of next recurrence of cystine stones, whereas a stable lower value was associated with a lack of recurrence [6,59].
5 Conclusions
The search for crystalluria in stone former patients affords useful information as to the likely mechanisms of lithogenesis in common forms of calcium nephrolithiasis. Preponderance of COM or COD crystalline phases of CaOx orients toward a lithogenic condition predominantly associated with hyperoxaluria or hypercalciuria, respectively. The identification of peculiar chemical species such as struvite, cystine, dihydroxyadenine, or xanthine is of considerable value for the diagnosis of infection stones and of genetic diseases such as cystinuria and dihydroxyadeninuria, which require specific therapy. Determination of the GCV allows to assess the efficacy of therapeutic measures in severe genetic diseases such as primary hyperoxaluria and cystinuria. Crystalluria index was clearly shown as the best marker for predicting stone recurrence. In addition, other criteria such as the size of COD crystals or the presence of heterogeneous nucleation process may be relevant for identifying particular metabolic disorders in urine or the risk of recurrence in some patients [60].
In all types of renal stone disease, especially in the most severe forms, serial search for crystalluria appears as a valuable tool for identifying patients with active crystal formation and at risk of forming kidney stones, therefore allowing to timely institute or modify therapeutic measures to prevent stone recurrence.
Of note, crystalluria was rarely studied in normal subjects [1]. Thus, the interest of studying crystalluria to predict a first stone formation in healthy subjects was never assessed. However, it was recently found that high values of risk indices such as the Tiselius index, reflecting high urine concentration of lithogenic factors, were associated with a high occurrence of CaOx crystalluria in healthy subjects, suggesting that crystalluria study could be, in stone formers and in healthy subjects as well, an interesting tool for detecting a possible risk of stone formation [61]. These preliminary results require further investigations to be validated on a larger series of normal subjects.
Conflicts of interest
The authors have no conflicts of interest to declare.


