1 Introduction
Recently, magnetic nanoparticles (MNPs) have been used as a new alternative to porous materials for supporting catalytic transformations [1–7]. Their simple magnetically driven separation from a liquid-phase reaction makes catalyst recovery and recycling much easier than by cross-flow filtration and centrifugation. Additionally, the MNP-supported catalysts also show high dispersion and reactivity with a high degree of chemical stability. By utilizing these advantages of magnetic nanoparticles over other supporting materials, various catalysts and ligands have been immobilized on these nanoparticles [8–12].
The selective oxidation of primary and secondary alcohols to the corresponding carbonyl compounds is one of the most challenging reactions in organic chemistry [13], because aldehydes and ketones are important intermediates in the synthesis of other organic compounds and they are utilized particularly in the manufacture of medicines, flavors, fragrances and aniline dyes [14]. Traditionally, oxidation of alcohols has been performed with a stoichiometric amount of metal oxidants, such as Cr(VI) salts [15], permanganate [16] and bromate [17] reagents. However, these reagents show poor atom efficiency and generate a large amount of environmentally ill-disposed heavy-metal waste. Therefore, catalytic oxidation methods, which employ a variety of transition metals such as Cu (I) [18], Ni (II) [19], Co (II) [20], Pd (II) and manganese oxides [21–24] using molecular oxygen [25,26], H2O2 [27] and TBHP [28,29] as oxidizing agents are well explored. Vanadium based catalytic systems also have shown potential for alcohol oxidation, in some cases proving effective for substrates where palladium catalysts display limited activity [30–35]. However, the major disadvantage of metal-based catalyst systems is their separation from the reaction solution by classical methods; they may possibly leave toxic traces of heavy metals in the products. Also, the reuse of the catalysts is difficult. These drawbacks can be overcome by immobilization of these catalysts on MNPs.
2 Experimental
2.1 General procedure for the oxidation of alcohols to carbonyl compounds
The alcohol (1 mmol) was added to a mixture of TBHP (1 mmol) and VO(ephedrine)2@MNPs (50 mg) in PEG (1 mL), and then the mixture was refluxed at 80 °C for the time specified. The progress was monitored by TLC (EtOAc/n-hexane, 1/2). After completion of the reaction, the catalyst was separated from the product by an external magnet (within 5 s), and the mixture was washed with EtOAc (2×5 mL) and decanted. The decanted mixture was washed with 30% NaOH (5 mL) and the organic layer was dried over anhydrous Na2SO4. The evaporation of EtOAc under reduced pressure gave the pure products in 85–98% yields.
3 Results and discussions
In continuation of our studies on the synthesis and application of magnetic nanocatalysts [36–39], herein, we report the catalytic properties of VO(ephedrine)2@MNPs in the selective oxidation of alcohols to carbonyl compounds using TBHP as an oxidant. VO(ephedrine)2@MNPs were synthesized according to our recently reported protocol for the preparation of the chiral oxo-vanadium (+)-pseudoephedrine complex supported on magnetic nanoparticles [38]. The process of the preparation VO(ephedrine)2@MNPs shown in Scheme 1(See the Supporting information for experimental details). The characterization of the catalyst was carried out by FT-IR, SEM, TGA and EDX techniques.

The preparation of VO(ephedrine)2@MNPs
The explanation of characterization techniques has been provided in the Supporting information.
In order to optimize the reaction conditions, the oxidation reaction of benzyl alcohol to benzaldehyde was selected as a model reaction, and various parameters including solvent, temperature and the amount of catalyst were optimized to develop the scope of this reaction further (Table 1). We have conducted the reaction in different solvents such as CH2Cl2, CH3CN, H2O and PEG in the presence of 50 mg of the catalyst and 1 mmol of TBHP at room temperature (Table 1, entries 1–4), as shown in Table 1, in PEG, benzaldehyde was produced in 100% yield after 15 h. Therefore, PEG was chosen as the best reaction solvent. When the reaction was carried out at 80 °C, the rate of reaction was increased and the reaction was completed after 3 h (Table 1, entry 5). To optimize the amounts of catalyst, different amounts of VO(ephedrine)2@MNPs (0, 30 and 40 mg) were used in the oxidation of benzyl alcohol using TBHP (1 mmol) in PEG at 80 °C. In the absence of a catalyst, the reaction was incomplete even after 24 h (Table 1, entry 6). When 30 and 40 mg of catalyst were used, the reaction times were prolonged to 8 and 7 h under same reaction conditions respectively (Table 1, entries 7 and 8). It should be noted that the use of Fe3O4 MNPs instead of VO(ephedrine)2@MNPs gives a lower yield of benzaldehyde even after prolonging the reaction time (Table 1, entry 9). As a result, the optimized reaction conditions were identified as using 50 mg of VO(ephedrine)2@MNPs in PEG as a solvent at 80 °C.
The selective oxidation of benzyl alcohol to benzaldehyde using TBHP under different conditions.
| Entry | Solvent | T (°C) | Catalyst (mg) | Time (h) | Conversion (%) |
| 1 | CH2Cl2 | rt | VO(ephedrine)2@MNPs (50) | 24 | No Reaction |
| 2 | CH3CN | rt | VO(ephedrine)2@MNPs (50) | 24 | No Reaction |
| 3 | H2O | rt | VO(ephedrine)2@MNPs (50) | 24 | No Reaction |
| 4 | PEG | rt | VO(ephedrine)2)@MNPs (50) | 15 | 100 |
| 5 | PEG | 80 | VO(ephedrine)2@MNPs (50) | 3 | 100a |
| 6 | PEG | 80 | None | 24 | 20 |
| 7 | PEG | 80 | VO(ephedrine)2@MNPs (30) | 8 | 100 |
| 8 | PEG | 80 | VO(ephedrine)2@MNPs (40) | 7 | 100 |
| 9 | PEG | 80 | Fe3O4 MNPs (50) | 15 | 50 |
a The bold letters represent the most effective reaction conditions.
In order to generalize the scope of the reaction, a series of alcohols was subjected to oxidation under the optimized reaction conditions, the results are presented in Table 2. As shown in Table 2, various types of primary benzylic alcohols, including those with both electron-withdrawing and electron-donating groups, were selectively converted to the corresponding carbonyl compounds in excellent yields under optimal reaction conditions (Table 2, entries 1–6). Interestingly, 2-methyl benzyl alcohol as a model for hindered primary benzylic alcohols was also successfully oxidized to its corresponding carbonyl compound, although a longer reaction time was required (Table 2, entry 3). The primary benzylic alcohols with electron-withdrawing groups show less reactivity and their oxidation requires some more of TBHP (4 mmol) (Table 2, entries 4–6). The oxidation of 2-phenyl ethanol as a model for primary aliphatic alcohols was satisfactorily subjected as well (Table 2, entry 8). In general, alcohols containing heterocyclic moiety are highly challenging substrates for oxidation in most transition-metal catalyst systems because they have a tendency to bind to transition metals and can act as catalyst deactivators. Another important aspect of this method is the successful oxidation of furfuryl alcohol and 3-pyridinemethanol to give the expected aldehydes (Table 2, entries 9 and 10). Furthermore, benzylic and cyclic secondary alcohols could be effectively oxidized using the present method to give excellent yields of the corresponding carbonyl products (Table 2, entries 11–14).
VO(ephedrine)2@MNPs (50 mg) catalyzed selective oxidation of alcohols (1 mmol) to carbonyl compoundsa using TBHP (1–4 mmol) as oxidant in PEG as solvent at 80 °C.
| Entry | Alcohol | Time (h) | TBHP (mmol) | Producta | Yield (%)b |
| 1 | Image 2 | 3 | 1 | Image 3 | 98 |
| 2 | Image 4 | 2 | 1 | Image 5 | 96 |
| 3 | Image 6 | 47 | 1 | Image 7 | 97 |
| 4 | Image 8 | 4:20 | 4 | Image 9 | 95 |
| 5 | Image 10 | 6 | 4 | Image 11 | 91 |
| 6 | Image 12 | 7 | 4 | Image 13 | 98 |
| 7 | Image 14 | 10 | 4 | Image 15 | 98 |
| 8 | Image 16 | 55 | 1 | Image 17 | 90 |
| 9 | Image 18 | 50 | 1 | Image 19 | 85 |
| 10 | Image 20 | 15 | 4 | Image 21 | 92 |
| 11 | Image 22 | 4:50 | 4 | Image 23 | 94 |
| 12 | Image 24 | 3:15 | 4 | Image 25 | 96 |
| 13 | Image 26 | 7:15 | 4 | Image 27 | 93 |
| 14 | Image 28 | 12 | 4 | Image 29 | 90 |
a All products are known and were characterized by IR and 1H NMR as compared with those reported in the literature [25,26].
b Isolated yield.
On the basis of a previously reported mechanism for the oxidation of alcohols using peroxides (H2O2 or TBHP) in the presence of vanadium based catalysts, one explanation for this process is that the mechanism involves radical intermediates [30]. Another explanation is that the oxidation process involves the reaction of the TBHP with VO(ephedrine)2@MNPs to form an active peroxidovanadium (V) intermediate, which subsequently oxidizes the substrate molecule and returns to the original state [31,32]. In order to conduct mechanistic investigation, the oxidation reaction of benzyl alcohol using tBuOOH as an oxidant and VO(ephedrine)2@MNPs as a catalyst was carried out in the presence of 2,2′-azobis(isobutyronitrile) as a radical trap. It was observed that addition of 2,2′-azobis(isobutyronitrile) has no significant effect on the yield of the product and reaction time. This observation suggests that the mechanism probably involves the TBHP activation by vanadium (V) centers forming a peroxy intermediate (Scheme 2). However, at this time the exact mechanism of the reaction is not clear and the actual role of this catalyst should be further studied in detail.
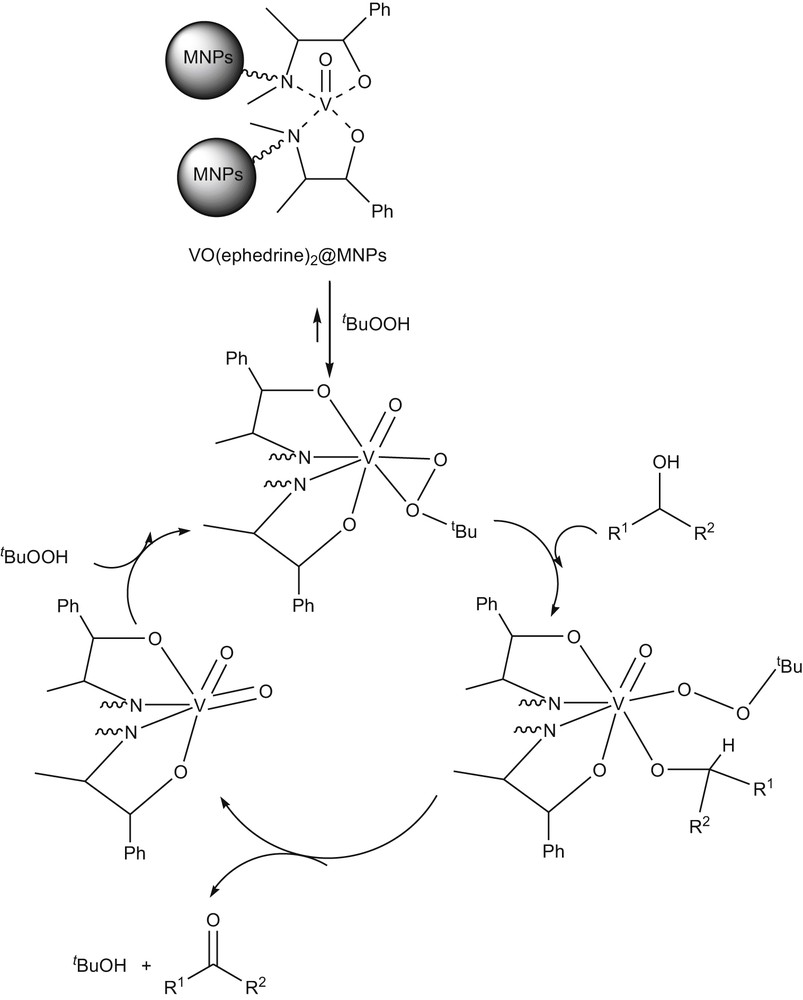
Proposed mechanism.
For practical purposes, the ability to easily recover and recycle the catalyst is highly desirable. The reusability of this magnetic nanocatalyst was examined using benzyl alcohol as a model substrate. After the first use of the catalyst in the oxidation of benzyl alcohol to give benzaldehyde, the catalyst was rapidly separated by an external magnet and was washed thoroughly with EtOAC. It was reused for subsequent experiments under similar reaction conditions. As shown in Fig. 1, the catalyst can be recycled up to six runs without significant loss of activity.

The recycling experiment of VO(ephedrine)2@MNPs (50 mg) in oxidation of benzyl alcohol using TBHP in PEG at 80 °C for 3 h.
We have compared the efficiency of VO(ephedrine)2@MNPs with VO(ephedrine)2 (the homogenous analogue) and other reported heterogeneous vanadium based catalysts in the oxidation of benzyl alcohol (Table 3). It was observed that the reaction proceeds more rapidly with a homogenous analogue than VO(ephedrine)2@MNPs, instead, a higher yield has been obtained by VO(ephedrine)2@MNPs, suggesting the importance of magnetic separation (Table 3, entries 1 and 2). The characteristic aspects of the described method in comparison with other previously reported catalysts are: (1) the product separation and catalyst recycling are easier and simpler with the assistance of an external magnet and (2) the used solvent (PEG) is green.
Comparison of efficiency of various supported vanadium based catalysts in the oxidation of benzyl alcohol.
| Entry | Catalyst (mg) | Conditions | Time (h) | Yield (%) | Ref. |
| 1 | VO(ephedrine)2@MNPs (2 mol %) | TBHP (1 mmol), PEG, 80 °C | 3 | 98 | This work |
| 2 | VO(ephedrine)2 (2 mol %) | TBHP (1 equiv), PEG, 80 °C | 1.5 | 95 | This work |
| 3 | VO(Schiff base)@nano-starch (5 mol %) | TBHP (1.5 equiv), MeCN, 65 °C | 1 | 92 | 32 |
| 4 | VO(acac)2@Polyaniline (2.3 mol %) | O2, toluene, 100 °C | 9 | 98 | 33 |
| 5 | VO(Schiff base)@ nano silica (20 mol %) | H2O2 (1.2 equiv), MeOH, reflux | 4 | 95 | 34 |
| 6 | VO(Schiff base)@PMOs (2 mol %) | TBHP (1.5 mmol), MeCN, 65 °C | 3 | 90 | 35 |
4 Conclusion
VO(ephedrine)2@MNPs as a magnetically separable and efficient nanocatalyst were applied for the selective oxidation of alcohols to aldehydes and ketones using TBHP as an oxidant in good to high yields in PEG as a green solvent at 80 °C. The product separation and catalyst recycling are easier and simpler with the assistance of an external magnet. The catalyst can be recycled and reused six times with little loss of activity.
Acknowledgements
We are grateful to the University of Kurdistan Research Councils for partial support of this work.


