1 Introduction
Nowadays, cancer has become the number one global cause of death worldwide [1]. In addition, the number of new cancer cases is expected to increase by about 70% over the next two decades [2]. Thus, finding safe and effective methods of treatment is critical. Therefore, the new era in cancer research calls for stronger working relationships between chemistry and biology for the discovery of novel anticancer agents. In fact, structural diversity and fascinating frameworks of molecules have triggered the researchers to probe in this domain. Among them are polycyclic aromatic hydrocarbons, which form a large group of organic compounds containing two or more fused benzene rings arranged in various configurations [3,4]. Benzo[c]phenanthrene and its derivatives [5–7] are the smallest representatives of this group and are often formed by oxidative or C–C bond coupling of appropriate stilbene precursors. In addition, the tetracyclic basic cores represent an attractive objective for further research in various branches of chemistry. Therefore, the preparation of this type of derivatives is of much interest, especially if functional groups are grafted in appropriate positions to complete the total synthesis of the target molecules. In this context, we have already described the preparation of the tetracyclic ketone 1 (Fig. 1) and demonstrated its importance as a building block for the synthesis of biologically active compounds [8].
Chemical structure of ketone 1.
On the other hand, a large number of reports suggest that the presence of hydroxyl groups in a molecule plays a key role in its biological applications. Remarkably, the most intriguing biological property for such compounds is their cytotoxic potential against various cancer cell lines [9–13]. This fact is the underlying strategy for the synthesis of compounds having the hydroxyl group in contemporary medicinal chemistry. To the best of our knowledge, diol frameworks are widely found in many natural products and biologically active molecules [14]. In recent years, the 1,3-diol system has attracted scientists to study the synthesis, as well as the chemical and biological properties of these compounds in regular life [15–17]. Despite the recent interesting findings on this class of compounds, the chemical literature lacks a comprehensive summary on the synthetic methodologies and biological activities of aromatic compounds containing hydroxylated units.
Thus, to assess the role of the hydroxyl groups in the cytotoxic effect, the purpose of the present study was the synthesis of two novel polycyclic alcohols based on a benzo[c]phenanthrene skeleton. The first approach includes the reduction of the tetracyclic ketone to produce a new secondary alcohol. The second approach includes a Claisen condensation followed by a reduction procedure to generate the novel polyaromatic 1,3-diol. Therefore, we assume that the hydroxyl groups attached to the aliphatic chain in the newly molecules may enhance their biological activity.
2 Results and discussion
2.1 Chemistry
In our continuing effort to explore the application of benzo[c]phenanthrene derivatives as key intermediates for the synthesis of cytotoxic drugs, we emphasize the synthesis of two new alcohols based on a benzo[c]phenanthrene moiety. The synthetic route to the target derivatives 2 and 3 is depicted in Scheme 1.
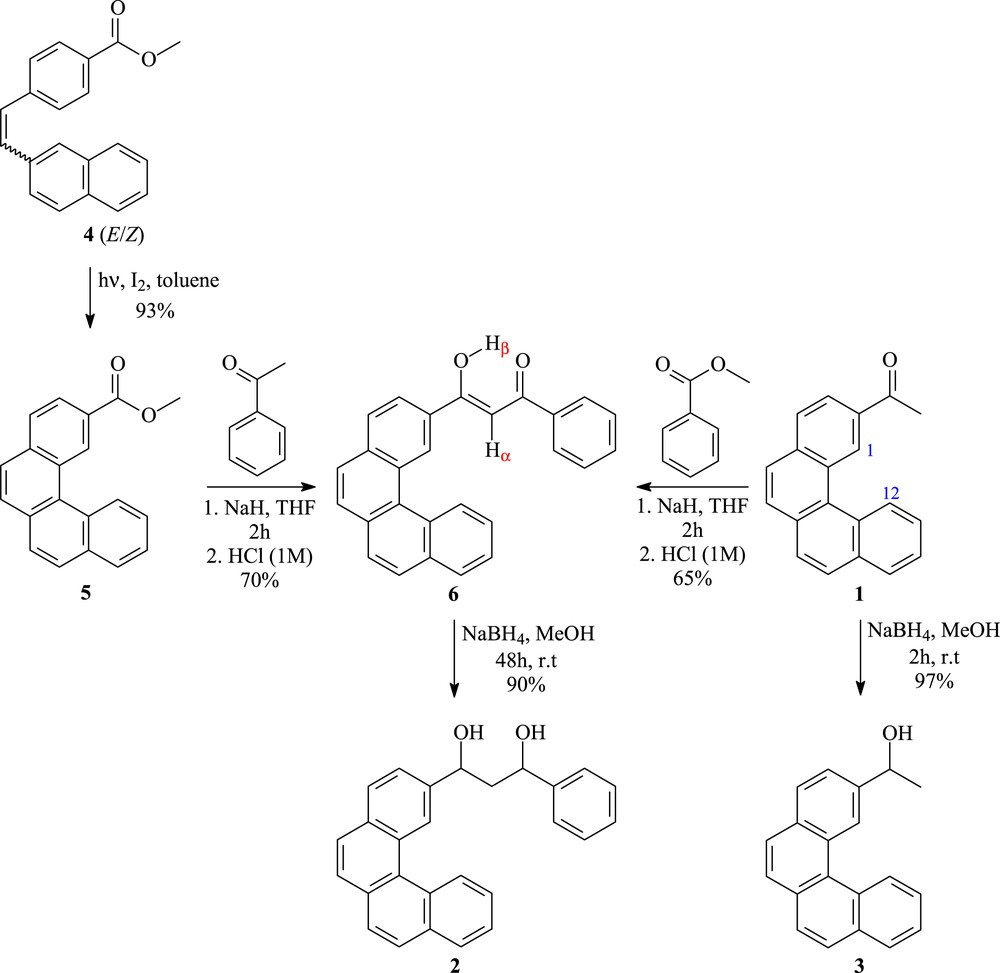
Synthetic pathway leading to alcohols 2 and 3.
First of all, we focused on a versatile and useful method for reduction of a carbonyl group of the known tetracyclic ketone 1 [8]. The reaction was carried out in anhydrous methanol, by using NaBH4 [18], leading to alcohol 3 with almost quantitative chemical yield, after 2 h of agitation at room temperature. On the other hand, olefin 4 [19] underwent oxidative cyclization [20,21] to give the benzo[c]phenanthrene skeleton 5. In fact, photolysis of 4 was performed, using a 500 W high-pressure Hg-vapor lamp, on a 250 mg scale per run in a 1-L reactor for about 2 h to build up the methyl benzo[c]phenanthrene-2-carboxylate (5) in 93% yield, after purification by column chromatography (Scheme 1).
As our continuing efforts toward the construction in high yields of new biologically active polyaromatic compounds, in this context, we wish to report a simple, practical, and efficient approach to prepare new diol framework (Scheme 1). Although, various alternative approaches have also been reported [22–24], reduction of 1,3-diketones to the corresponding diols has been little investigated. With respect to these considerations, we are thinking to use benzo[c]phenanthrene derivatives with ester or ketone functionality, which are promising candidates for the process route to prepare the new compound 6 via Claisen condensation [25] as a key point in this work. Thereby, under anhydrous conditions, we carried out the reaction in THF by mixing methyl benzo[c]phenanthrene-2-carboxylate (5) and the commercially available acetophenone in the presence of sodium hydride, as a suitable base. The reaction proceeded smoothly, and the aqueous workup with aqueous HCl (1 M) followed by silica gel column chromatography gave the expected new condensation product 6 with 70% yield. We next repeated this protocol by reacting 2-acetylbenzo[c]phenanthrene (1) with the commercially available methyl benzoate (Scheme 1). The reaction conditions were the same as before and the desired product 6 was isolated with a good yield. Importantly, the singlet resonating at 17.04 ppm in the 1H nuclear magnetic resonance (NMR) spectrum, which corresponds to the hydroxyl proton (H-β), confirmed the enol form for compound 6. Finally, the reduction step is also highly desirable. In this context, our protocol is based on the use of sodium borohydride, which has previously given a good result in the synthesis of alcohol 3. The operational simplicity of NaBH4 reduction makes experiments easy. As a result, polycyclic aromatic hydrocarbon system 6 was easily converted into the corresponding diol 2 with an excellent chemical yield (Scheme 1). Therefore, the ready availability of reactants and their robust and cost-effective employment, as well as the high yields make this procedure particularly appealing for the preparation of new type of compounds, which may provide a new hope for fight against cancer.
2.2 Cytotoxic activity
The study of the cytotoxic activity of the new derivatives 2 and 3 was carried out in vitro against the human epidermoid carcinoma epithelial cell Hep-2 and human colon carcinoma cell Caco-2 using the MTT assay method [26]. In fact, the results of the tested compounds expressed as IC50 values (concentration required to inhibit tumor cell proliferation by 50%) are presented in Table 1 (values are expressed as means ± standard deviation of three experiments). As it can be seen, diol 2 showed the strongest cytotoxicity against Caco-2 cell line with IC50 of 0.6 μg/mL. On the other hand, the effect of this compound against Hep-2 cancer cell was slightly lower than that against Caco-2 cell (IC50 = 0.7 μg/mL). As proposed for the tested product, hydroxyl groups attached to the aliphatic chain in the molecule may improve their cytotoxic property. Nevertheless, alcohol 3 displayed weak activity against two cells (Table 1).
Cytotoxicity IC50 values for compounds 2 and 3 against Hep-2 and Caco-2 cancer cells.
| Entry | Concentrations (μg/mL) | IC50 (μg/mL) | |||
| 10 | 5 | 2.5 | 1.25 | ||
| Against Hep-2 cancer cell | |||||
| 2 | 95.48 ± 0.56 | 87.56 ± 3.05 | 78.62 ± 1.77 | 73.51 ± 2.11 | 0.7 |
| 3 | 53.57 ± 3.03 | 47.75 ± 2.85 | 44.30 ± 2.50 | 30.12 ± 3.45 | 8 |
| Against Caco-2 cancer cell | |||||
| 2 | 98.11 ± 1.11 | 95.16 ± 3.02 | 90.72 ± 2.27 | 85.66 ± 2.67 | 0.6 |
| 3 | 40.01 ± 2.13 | 30.66 ± 1.95 | 24.70 ± 1.80 | 20.33 ± 2.76 | >10 |
The structure–activity relationship between alcohols 2 and 3 is a very good choice to show that a diol side chain results in an increase in cytotoxicity as compared with a terminal alcohol side chain [27]. As we all know, if the proton donor and proton acceptor belong to the same molecules, the system will form an intramolecular hydrogen bond (IHB). In fact, the formation of IHB is used by medicinal chemists to modulate biological and chemical properties of interest [28]. Along with this concept, it should be emphasized that IHB in the 1,3-diol system 2 appears to be an important structural requirement to observe their excellent effect.
3 Conclusions
Our approach can be considered as one of the favored methods, because the ultimate goal of the current research was to develop economically and viable reactions for the synthesis of new biological polycyclic aromatic alcohols. Simple experimental workup, shorter reaction time, easily available reagents, and excellent yields are the advantages of the proposed method. Moreover, the use of a tetracyclic basic core with ketone or ester functionality can serve as a key intermediate for the synthesis of novel cytotoxic drugs. As a result, the chemical reduction of 3-(benzo[c]phenanthren-2-yl)-3-hydroxy-1-phenylprop-2-en-1-one, available via the Claisen condensation, affords the novel 1,3-diol in excellent yield and purity. In fact, whose application as a cytotoxic agent has been well confirmed. Extension of this approach to the synthesis of new biologically active polycyclic aromatic systems is currently under active study in our laboratory.
4 Experimental section
4.1 Material and physical measurements
All of the chemicals used in this work were purchased from commercial sources and used without further purification. Solvents were carefully dried and freshly distilled according to the standard procedures. All reactions were carried out under an argon atmosphere, if not otherwise specified, and were monitored by thin-layer chromatography (TLC) Merck 60 F254 silica gel plates (layer thickness 0.25 mm). Spots on the TLC plates were visualized using UV light at 254 nm. Column chromatography was performed with silica gel (70–230 mesh) using a cyclohexane and ethyl acetate mixture as eluents. Photochemical reaction was carried out by using a photoreactor equipped with a 500 W high-pressure mercury-vapor lamp in a 1.5-L water-cooled quartz immersion. NMR spectra were recorded using a Bruker AC 300 instrument in CDCl3 [300 MHz (1H NMR) and 75 MHz (13C NMR)]. All chemical shifts were reported as δ values (ppm) relative to internal tetramethylsilane. Melting points were determined on an Electrothermal 9002 apparatus and were reported uncorrected. Time-of-flight mass spectroscopy (TOF MS ES+) was carried out on a Micromass, UK and Manchester.
4.2 Synthesis of 1-(benzo[c]phenanthren-2-yl)ethanol (3)
NaBH4 (0.74 mmol; 2 equiv.) was dissolved in 5 mL of anhydrous methanol and the suspension was cooled to 0 °C. After several minutes, 100 mg of 2-acetylbenzo-[c]phenanthrene 1 (0.37 mmol; 1 equiv.) was added to the NaBH4 solution in methanol. The resulting solution was stirred at room temperature for 2 h until the transformation proceeded efficiently (TLC). After completion of the reduction, the reaction mixture was acidified with HCl (1 M). Then the product was extracted with dichloromethane (30 mL × 3), dried over MgSO4, filtered off, and the solvent was evaporated in vacuum using a rotary vacuum evaporator. The desired alcohol was obtained as white needles in 97% yield after purification by SiO2 chromatography (90:10 up to 70:30 cyclohexane–EtOAc eluent); mp = 113–115 °C; 1H NMR (300 MHz, CDCl3): δ = 1.68 (d, J = 6.6 Hz, 3H, CH3), 2.12 (s, 1H, OH), 5.19 (q, J = 6.6 Hz, 1H), 7.63–7.75 (m, 3H), 7.80–7.93 (m, 4H), 8.00 (d, J = 8.4 Hz, 1H), 8.04 (d, J = 7.8 Hz, 1H), 9.14 (s, 1H, H-1), 9.14 (d, J = 7.8 Hz, 1H, H-12); 13C NMR (75 MHz, CDCl3): δ = 24.90 (–CH3), 70.42 (–COH), 123.06 (CH), 123.89 (CH), 125.34 (CH), 125.72 (CH), 126.26 (CH), 126.35 (CH), 126.67 (CH), 126.93 (C), 127.02 (CH), 127.25 (CH), 128.10 (CH), 128.39 (CH), 129.83 (C), 129.89 (C), 130.77 (C), 132.49 (C), 133.09 (C), 143.08 (C); GC/MS: m/z = 272; HRMS (MALDI-TOF) Calcd for (C20H15O): [M − H]−: 271.1222. Found: 271.1215.
4.3 Synthesis of methyl benzo[c]phenanthrene-2-carboxylate (5)
To a solution of the olefin 4 (250 mg; 0.86 mmol) in toluene was added iodine (1.1 equiv.). The solution was degassed for 15–30 min and the irradiation was performed using a photoreactor and a high-pressure Hg-vapor lamp (500 W). Progress of the reaction was monitored by TLC. After completion, the solvent was removed under reduced pressure. The crude residue was purified by SiO2 chromatography (98:02 cyclohexane–EtOAc eluent). Methyl benzo[c]phenanthrene-2-carboxylate 5 was obtained as a brown solid in 93% yield; mp = 82–84 °C; 1H NMR (300 MHz, CDCl3): δ = 4.03 (s, 3H, CH3), 7.65–7.70 (m, 1H), 7.74–7.80 (m, 1H), 7.82 (d, J = 8.7 Hz, 1H), 7.91–7.95 (m, 3H), 8.04 (d, J = 8.1 Hz, 2H), 8.20–8.24 (m, 1H), 9.08 (d, J = 8.4 Hz, 1H, H-12), 9.86 (s, 1H, H-1); 13C NMR (75 MHz, CDCl3): δ = 52.47 (CH3), 125.65 (CH), 126.35 (CH), 126.66 (CH), 126.85 (CH), 126.96 (CH), 127.46 (C), 127.92 (CH), 128.01 (C), 128.17 (CH) 128.71 (2CH), 129.39 (CH), 129.59 (C), 130.09 (C), 130.62 (CH), 131.24 (C), 133.71 (C), 135.99 (C), 167.68 (CO); HRMS (MALDI-TOF) Calcd for C20H14O2 [M + H]+: 287.0933. Found: 287.1023.
4.4 Synthesis of 3-(benzo[c]phenanthren-2-yl)-3-hydroxy-1-phenylprop-2-en-1-one (6)
Under an argon atmosphere, a dry 25-mL three-necked flask equipped with a three-way valve was charged with NaH (1.4 mmol; 4 equiv.). The mineral oil was removed by washing with anhydrous hexane allowing NaH to settle and removing the supernatant by a syringe. After drying by heating under vacuum followed by being stirred at room temperature, 5 mL of anhydrous THF was added and the suspension was heated to reflux. Then 10 mL of a THF solution containing ketone (0.35 mmol; 1 equiv.) and ester (0.35 mmol; 1 equiv.) was successively added dropwise to the aforementioned mixture. The solution was allowed to reflux for 2 h, then cooled to room temperature, and treated with 1 M HCl. Thereafter, the aqueous layer was extracted three times with 20 mL of dichloromethane and the combined organic layers were washed with brine, dried over MgSO4, and concentrated by rotary evaporation. Finally, the crude product was purified by silica gel column chromatography using cyclohexane–EtOAc (95:05) as eluent to give the desired product 6 as yellow needles in 65% yield from 1; 70% yield from 5; mp = 144–146 °C; 1H NMR (300 MHz, CDCl3): δ = 7.03 (s, 1H, Hvinyl), 7.49–7.61 (m, 3H), 7.68 (t, J = 9 Hz, 1H), 7.75–7.84 (m, 2H), 7.90 (s, 2H), 7.93 (d, J = 9 Hz, 1H), 8.02–8.06 (m, 4H), 8.13 (d, J = 9 Hz, 1H), 9.10 (d, J = 9 Hz, 1H, H-12), 9.77 (s, 1H, H-1), 17.04 (s, 1H, OH); 13C NMR (75 MHz, CDCl3): δ = 93.06 (CH), 122.99 (CH), 125.86 (CH), 126.18 (CH), 126.23 (CH), 126.38 (CH), 126.72 (2CH), 127.30 (CH), 127.52 (C), 127.55 (CH), 127.63 (CH), 128.19 (CH), 128.23 (2CH), 128.36 (CH), 128.75 (CH), 129.32 (C), 129.59 (C), 130.82 (C), 131.95 (CH), 132.28 (C), 133.24 (C), 135.19 (C), 135.21 (C), 185.11 (C–OH), 185.41 (CO); HRMS (MALDI-TOF) Calcd for C27H17O2 [M − H]−: 373.1229. Found: 373.1219.
4.5 Synthesis of 1-(benzo[c]phenanthren-2-yl)-3-phenylpropane-1,3-diol (2)
In an oven-dried 50 mL two-neck flask, a cold solution (0 °C) of enol 6 (0.06 mmol; 1 equiv.) in 3 mL of methanol was vigorously agitated under anhydrous conditions. NaBH4 (0.24 mmol; 4 equiv.) was then added in small portions to the above mixture. Afterward, the resulting solution was agitated at room temperature for 48 h. Progress of the reaction was continuously monitored by TLC until conversion of the maximum of the enol to the desired product. After completion, the reaction mixture was treated with HCl (1 M) and the aqueous layer was extracted with dichloromethane (30 mL × 3). This extract was dried over anhydrous MgSO4 and filtered. After removal of the solvent, the obtained crude product was filtered on a short silica gel column eluted with cyclohexane–EtOAc (80:20 up to 60:40). Diol 2 was isolated as a white solid in 90% yield, and characterized by FT-IR, NMR, and mass spectral analyses; mp = 86–88 °C; 1H NMR (300 MHz, CDCl3): δ = 2.04–2.16 (m, 1H), 2.28–2.40 (m, 1H), 3.34 (s, 1H, OH), 3.59 (s, 1H, OH), 4.97–5.07 (m, 1H), 5.23–5.29 (m, 1H), 7.28–7.39 (m, 5H), 7.56–7.72 (m, 3H), 7.78–7.91 (m, 4H), 7.95–8.03 (m, 2H), 9.10 (d, J = 9 Hz, 1H, H-12), 9.13 (s, 1H, H-1); 13C NMR (75 MHz, CDCl3): δ = 46.13 (CH), 47.36 (CH), 71.36–71.75 (CH), 74.49–74.92 (CH), 123.24 (CH), 124.18 (CH), 124.40 (CH), 125.16 (CH), 125.22 (CH), 125.37 (CH), 125.78 (CH), 126.33 (CH), 126.67 (CH), 127.05 (CH), 127.22 (CH), 127.27 (CH), 128.04 (CH), 128.09 (CH), 128.34 (CH), 128.42 (CH), 129.78 (C), 129.85 (C), 130.74 (C), 132.43 (C), 132.60 (C), 133.06 (C), 141.48 (C), 143.68 (C); IR: (νOH) = 3325.61 cm−1; ESI-MS: m/z = 377.1554 [M − H]−; HRMS (MALDI-TOF) Calcd for C27H21O2 [M − H]−: 377.1541. Found: 377.1535.
4.6 Measurement of the cytotoxic activity by MTT assay
The in vitro test for estimating the cytotoxic activity of the hydroxylated products was performed in Dulbecco's modified Eagle medium. At 85–90% confluence, Hep-2 and Caco-2 cells were harvested using 0.25% trypsin/EDTA solution and subcultured into 96-well plates. The MTT colorimetric assay is commonly used to determine mitochondrial reductive function and hence is a good indicator of cell death or inhibition of growth. After incubation of cells with a range of concentrations of each compound, the MTT assay in combination with cell viability of controls containing no compound can be used to obtain an IC50 value. This is the concentration of compound where 50% of cells are viable.
Briefly, 96-well flat bottom cell culture plates were seeded with Caco-2 and Hep-2 cell lines, respectively, at a density of 1 × 105 cells per well. When a partial monolayer was formed, the supernatant was flicked off, the monolayer washed once with medium, and 100 μL of different doses (10, 5, 2.5, and 1.25 μg/mL) of tested products were added to the cell in the microtiter plates. After 24 h, the medium was washed gently with 0.01 mL MTT reagent (Invitrogen) prepared in 5.0 mg/mL of phosphate buffered saline per well. Plates were incubated for 4 h at 37 °C in 5% CO2, and 0.1 mL of DMSO was added. At the end of the experiment and after incubation overnight at 37 °C, the absorbance was measured at 550 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Thermo scientific Multiskan FC) and was compared with the control cultures without compounds. Results were estimated from three independent experiments and each experiment was performed in triplicate. Percent cytotoxicity was calculated using the following equation:
| %Cytotoxicity = 100 − [(absorbanceoftreated sample)/(control absorbance)] × 100 |
Acknowledgments
The authors are grateful to the DGRS (Direction Générale de la Recherche Scientifique) of the Tunisian Ministry of Higher Education and Scientific Research and the ISSAT Mahdia (Institut Supérieur des Sciences Appliquées et de Technologie de Mahdia, Unité d'analyses et procédés appliqués à l'Environnement, Sidi Massoud, 5111 Mahdia, Tunisia).


