1 Introduction
Acetophenone and its derivatives are widespread materials in organic synthesis because they can serve as intermediate compounds used in cosmetics and perfumes. Furthermore, they are used as flavouring agents. This group of compounds belongs to ketones because they have an acetyl group on the benzene ring.
This group of organic compounds is also studied by electrochemical methods according to their complexation ability towards metal ions [1,2]. Only a few works are presented in the literature regarding the electrooxidation of benzophenone and acetophenone derivatives in nonaqueous systems. Acetone is the most investigated ketone whose electrochemistry is examined extensively in aqueous solutions but studies are also carried out in nonaqueous solvents. Iodide ion is a good mediator because the formation of iodine during its anodic oxidation is able to react with aliphatic ketones on their α carbon atoms. These properties are widely applied in synthetic reactions accomplished in alcohols [3–5]. The final product is the corresponding ester substituting iodide ion by the nucleophilic attack of alcohol molecules or alkoxide ions. The Baeyer–Villiger electrooxidation of ketones uses a high concentration of oxygen and their electrocatalytic reactions can result in the formation of esters and lactones, which were successful in ionic liquids [6] on activated carbon fibre anode. This procedure resulted in case of acetophenones the corresponding methyl esters. There are acetophenone derivatives having moieties that can be electropolymerized easily, like the corresponding pyrrole [7] and thiophene [8].
In this study, acetophenone derivatives with different types of substituents in the para position and benzophenone were investigated with a platinum and glassy carbon electrode under ambient conditions.
2 Experimental section
The chemicals and solvent used in the experiments were of analytical grade and tetrabutylammonium perchlorate (TBuClO4) was the supporting electrolyte in solutions prepared with dry acetonitrile (water content around 10−3 M). In the aqueous solutions, KCl served as supporting electrolyte. Platinum and glassy carbon disc electrodes (1 mm in diameter) were used as the working electrodes in the electrochemical cell. The counter electrode was a platinum wire and a silver wire was the reference, whereas in the aqueous solutions saturated calomel served as a reference electrode. All experiments were carried out with a potentiostat (Dropsens, Spain). Polishing of the working electrodes was accomplished with a polishing cloth by using 1, 0.3 and 0.05 μm alumina powder. Then, they were thoroughly washed with tap and deionized water. Before all measurements in acetonitrile the traces of water were removed by rinsing the electrodes with dry acetone.
3 Results and discussion
3.1 Voltammetric experiments with a platinum electrode
The selected acetophenones and benzophenone were examined by cyclic voltammetry between 0 and 4 V with 0.1 V/s scan rate in acetonitrile. The results for the platinum electrode are presented in Fig. 1. The voltammograms of the different compounds are very similar, and they have a small peak around 3 V except for 4′-nitroacetophenone and benzophenone. It can be attributed to the oxidation of methyl group present in the acetyl group. Formation of the intermediate methyl radicals is followed by their dimerization resulting in the final product 1,4-diphenyl-1,4-butanedione containing the corresponding substituent in the para position in the benzene ring. The absence of this anodic peak in case of 4′-nitroacetophenone is because of the strong electron-withdrawing effect of the nitro group. Consequently, it reacts at higher potentials. Significant currents flow only at potentials close to 4 V as a result of the oxidation of solvent and subsequent oxidation of the substrate on the carbonyl oxygen. Benzophenone does not have this peak because it contains an additional aryl moiety instead of the methyl group. The voltammograms are very different in case of 4′-hydroxyacetophenone as compared with other derivatives, which have an additional anodic peak at 2 V, and the heights of the subsequent scans are almost the same indicating the lack of passivation. It has a phenol moiety and the acetyl group is present in the para position. It suggests that this para-substituted phenol derivative does not foul the surface of the platinum electrode in acetonitrile. This is because radical formed due to the electrochemical oxidation contained the unpaired electron in the para position, which is the most stable form. The final products can dissolve and diffuse into the bulk.
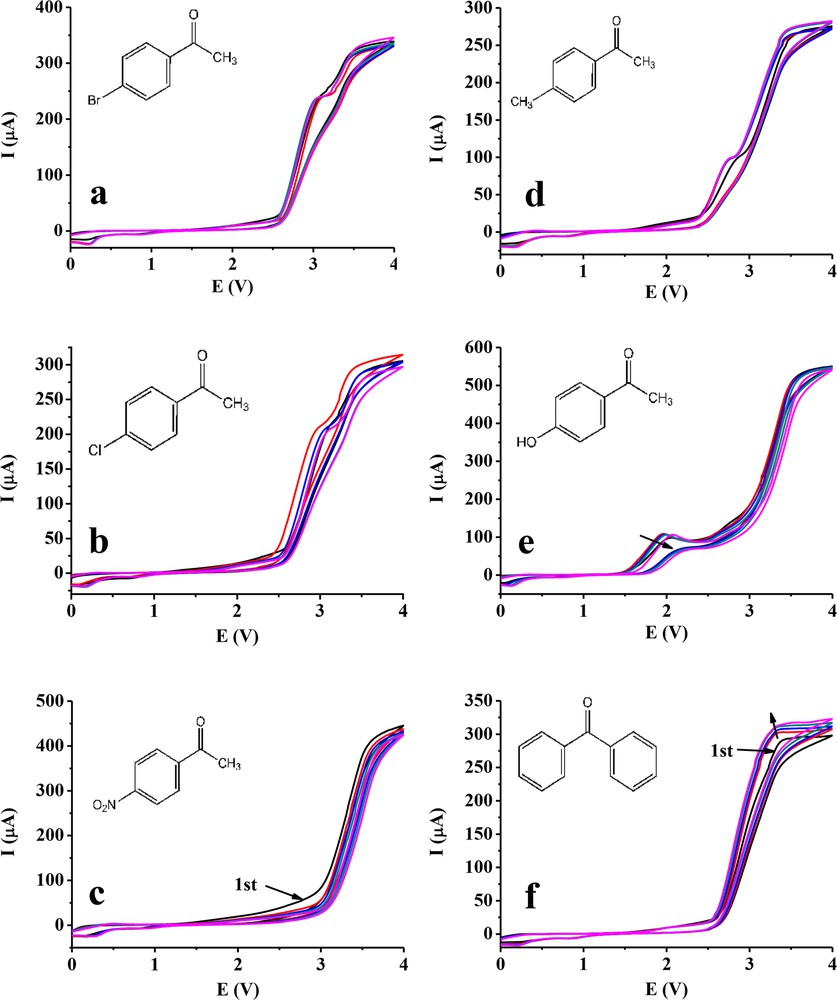
Repeated cyclic voltammograms of 4′-bromo- (a), 4′-chloro- (b), 4′-nitro- (c), 4′-methyl- (d), 4′-hydroxyacetophenone (e) and benzophenone (f) in acetonitrile with a platinum electrode (scan rate 0.1 V/s, c = 10 mM, supporting electrolyte 50 mM TBuClO4).
3.2 Investigation of acetophenones and benzophenone with a glassy carbon electrode
The electrochemical behaviour of the outlined compounds was also investigated with a glassy carbon electrode under the same conditions as in the case of the platinum electrode (Fig. 2). The remarkable difference as compared with the platinum electrode is the appearance of a more enhanced oxidation peak between 2.5 and 3 V during the first scans of each compounds except for 4′-nitroacetophenone. The curves have similar shape to that recorded with the platinum electrode, indicating also that this derivative does not show redox character in this voltage range. The small peak around 3 V can be attributed to the solvent, which was verified in a separate experiment (see below). The voltammograms of 4′-hydroxyacetophenone differ also significantly from the other ones as it contains also the oxidation peak of phenolic moiety at 1.8 V in the first scan. This acetophenone derivative can be oxidized through its two functional groups (hydroxy and methyl) scanning between 0 and 4 V; therefore, chain polymers can form and then adsorb on the surface. The subsequent voltammograms contain smaller peaks but after the second scan they do not decrease as it can be observed generally in the surface fouling reactions. It suggests that the polymer formed has a loose structure elevating the diffusion of the monomers to the electrode surface. Because the hydroxy group is an activating substituent the oxidation peak attributable to the methyl group appeared around 2.6 V. This is also characteristic for 4′-methylacetophenone-containing methyl group being also an activating substituent. This oxidation peak showed up close to 3 V by the halogen derivatives because they have electron-withdrawing character.
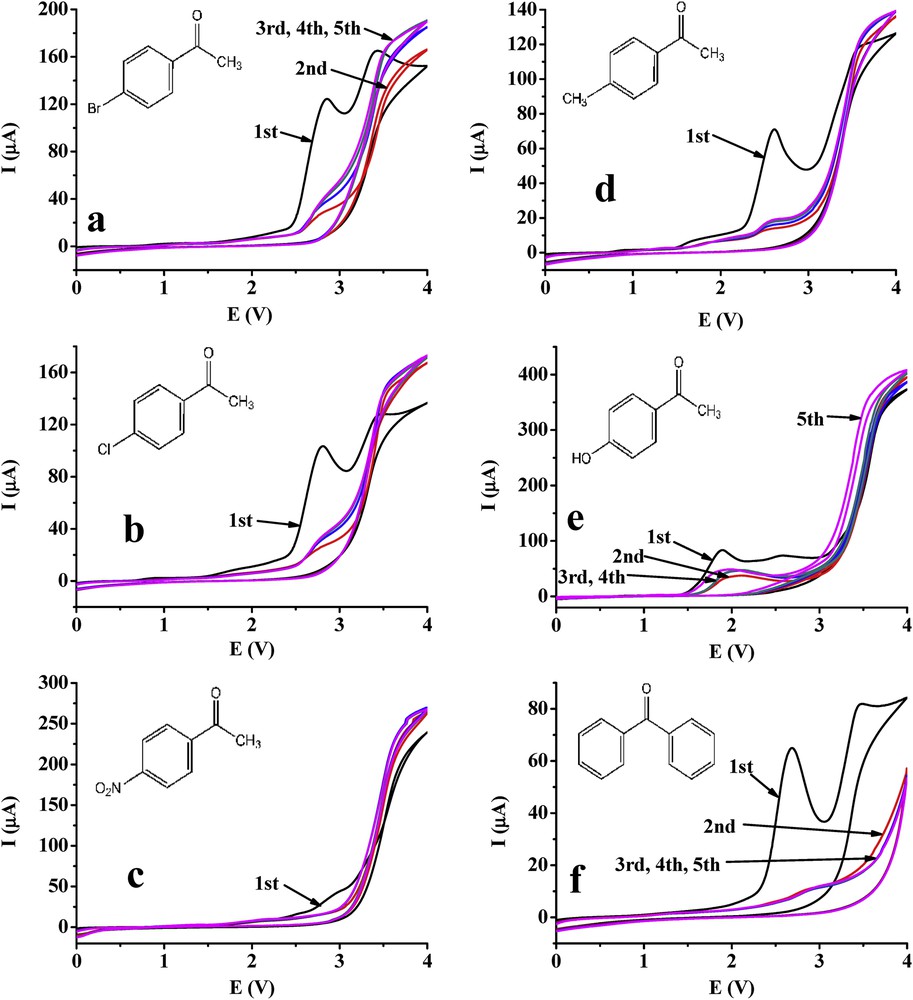
Repeated cyclic voltammograms of 4′-bromo- (a), 4′-chloro- (b), 4′-nitro- (c), 4′-methyl- (d), 4′-hydroxyacetophenone (e) and benzophenone (f) in acetonitrile with a glassy carbon electrode (scan rate 0.1 V/s, c = 10 mM, supporting electrolyte 50 mM TBuClO4).
Further experiments were performed with 10 mM acetone in acetonitrile using 0–4 V potential range with the aim to get information whether the methyl group is oxidized. An oxidation peak appeared around 2.75 V, which is similar to that of acetophenones reinforcing the earlier hypothesis.
Interestingly, the oxidation peak above 2.5 V appeared also approximately in the same potential interval by benzophenone as in the case of acetophenones. In spite of that this compound does not have methyl group, only two phenyl groups. It suggests that benzophenone reacts on its benzene ring while an aryl radical forms. This radical can dimerize and then oxidize on the other phenyl group building up polymer chains. It is also clearly seen from the figure that the currents measured in the next scans are significantly lower indicating the deactivation of the glassy carbon surface. Moreover, this finding suggests that the examined acetophenones can be oxidized also on their benzoyl moieties on the glassy carbon electrode, resulting in polymeric products.
The voltammograms of acetophenones and benzophenone suggest that these compounds foul the surface of the glassy carbon electrode. However, looking at the ranges between 3 and 4 V where oxidation of solvent molecules occur, the currents do not decrease by repeating the scans indicating the lack of serious passivation. To get more insight into the processes on its surface a cycle was taken between 0 and 4 V with acetonitrile and then, a cyclic voltammogram in the solutions of the selected compounds. These scans showed high similarity to the second and subsequent cycles of acetophenones and benzophenone obtained previously (see Fig. 2). Two reasons can be responsible for these observations. Acetonitrile electrooxidation at higher potentials deactivates the glassy carbon electrode making the surface unsuitable for the further electrochemical reaction of acetophenones and benzophenone. This observation is supported by an earlier study of Staley et al. [9] with quinones in acetonitrile on the glassy carbon electrode due to the presence of surface oxides. These surface sites provide appropriate possibilities to build hydrogen bonds between substrate, products and the surface. This deactivation process could also be observed by repeating the scans only to 3 V where the first oxidation peak appeared in case of each compound.
As could be seen in the voltammograms of 4′-hydroxyacetophenone, the oxidation peak of phenolic hydroxy group appeared around 2 V. For obtaining more information concerning the electrochemistry of the phenolic moiety, the cyclic voltammetric experiments were repeated between 0 and 2.2 V with the glassy carbon electrode (Fig. 3). The peak currents, which are attributed to this phenol moiety, decreased slowly by repeating the scans. Previously, when the switching potential was 4 V the decreasing tendency of this peak was more significant. It indicates that the electrode becomes deactivated only at higher potentials because of the adsorption of products and solvent electrooxidation.
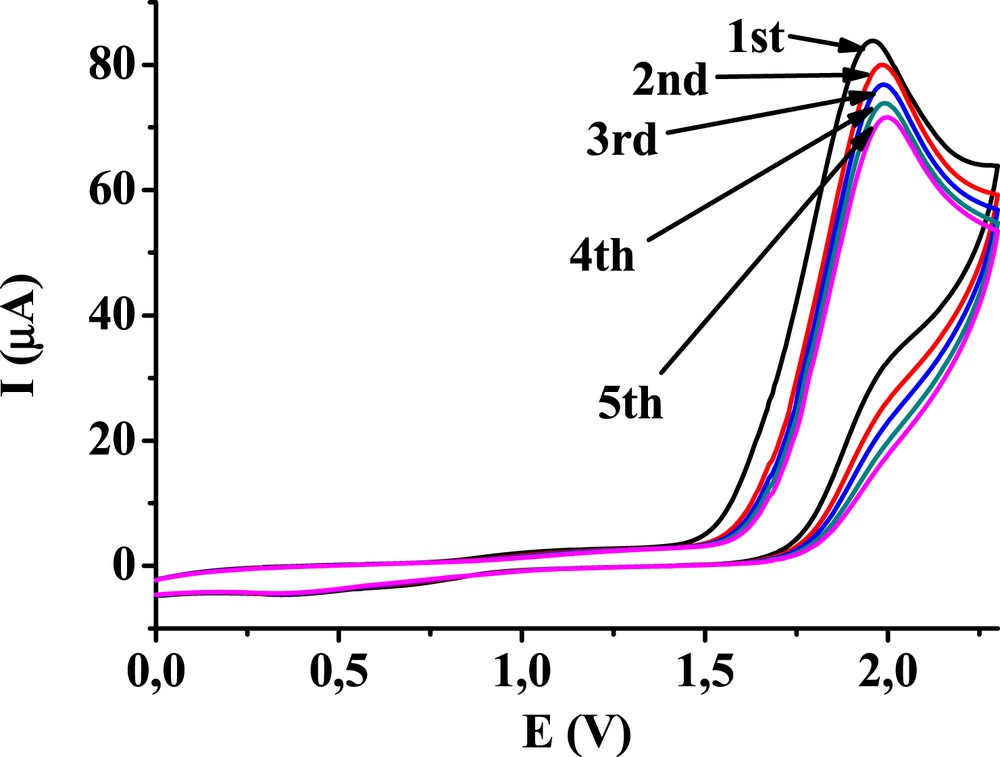
Complementary voltammograms of 4′-hydroxyacetophenone (c = 10 mM) in acetonitrile with a glassy carbon electrode (supporting electrolyte 50 mM TBuClO4).
3.3 Studies using 1,4-dihydroxybenzene as redox probe
Complementary studies were carried out with the glassy carbon electrode using a redox probe 1,4-dihydroxybenzene between 0 and 1 V to get more information about the deactivation process. Previously, this electrode showed passivation and more insights were needed to understand the surface processes. The 5 mM concentration aqueous solution of the redox probe was used and the obtained data are collected in Table 1. Five cyclic voltammograms were recorded in acetonitrile solutions of the studied compounds between 0 and 4 V and finally a scan with the redox probe in its aqueous solution, respectively. For comparison, the data obtained after taking five cycles in the acetonitrile solvent are also presented. It can be seen in Table 1 that the anodic peak currents diminished to ∼90% of the value measured with the glassy carbon electrode without cycling in any acetonitrile solution. Remarkably, the anodic peak potentials were smaller and cathodic peak potentials were higher. Two voltammograms are depicted in Fig. 4 for 4′-chloroacetophenone in aqueous solution of 1,4-dihydroxybenzene. The curves clearly show that the anodic peaks are shifted to less positive and the cathodic peaks shifted to less negative potentials as compared with bare carbon. These findings suggest that substrate adsorbs readily on the surface of pretreated glassy carbon. The more oxidized surface and the presence of adsorbed products of acetophenone derivatives and benzophenone electrooxidation offer appropriate surface sites for adsorption of the substrate and the product 1,4-benzoquinone because of hydrogen bonds. The higher cathodic currents indicate that the increased number of adsorbed 1,4-benzoquinone is capable to reduce in less negative potential regions. This increase in the current could also be observed in case of the electrode with modified surface in acetonitrile but pretreatment in solutions of the acetophenones and benzophenone contributed to an additional small enhancement.
Measured parameters of cyclic voltammograms of 5 mM 1,4-dihydroxybenzene in its aqueous solution with a bare electrode and after cycling in the corresponding acetonitrile solution (acf, acetophenone).
| Epa/V | Ipa/μA | Epc/V | Ipc/μA | |
| Bare electrode | 0.51 | 22.4 | 0.08 | −10.08 |
| Acetonitrile | 0.49 | 18.443 | 0.1 | −10.821 |
| Benzophenone | 0.46 | 21.5 | 0.116 | −12.5 |
| 4-NO2-acf | 0.484 | 19.7 | 0.1 | −11.367 |
| 4-Cl-acf | 0.437 | 20.474 | 0.137 | −11.987 |
| 4-Br-acf | 0.45 | 20.075 | 0.118 | −11.946 |
| 4-CH3-acf | 0.457 | 18.9 | 0.116 | −11.029 |
| 4-OH-acf | 0.45 | 18.717 | 0.127 | −11.853 |
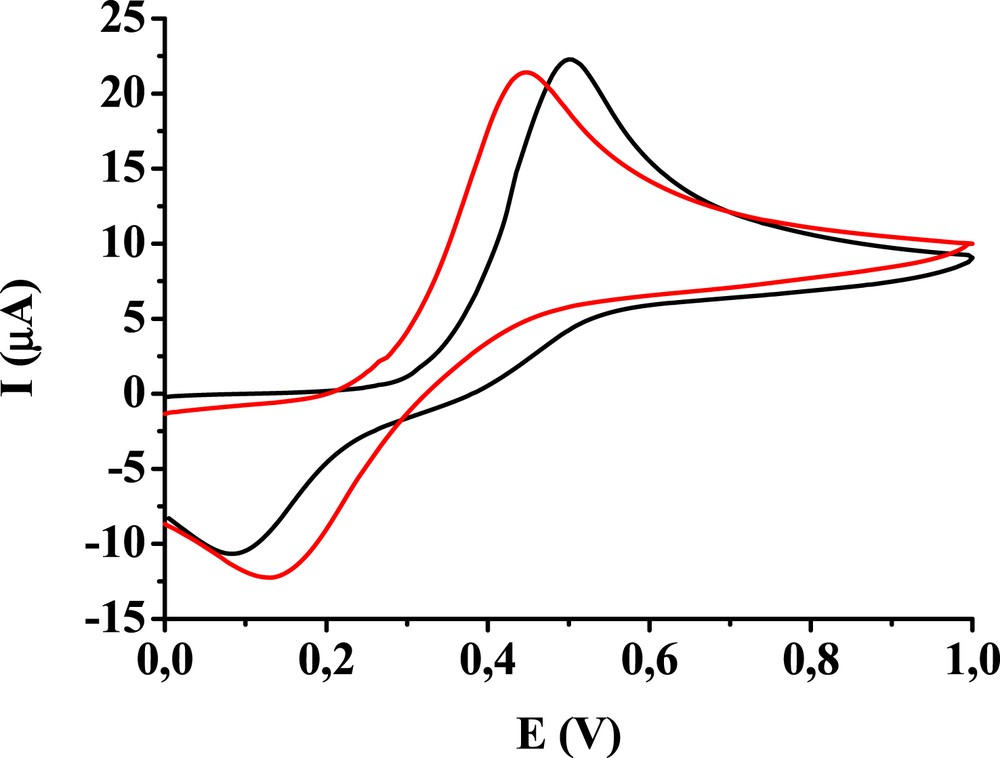
Cyclic voltammograms in aqueous solution of 5 mM 1,4-dihydroxybenzene with bare glassy carbon electrode (black curve) and with glassy carbon after five cycles taken in acetonitrile solution of 10 mM 4′-chloroacetophenone (red curve).
4 Conclusions
The electrochemical oxidation of benzophenone and acetophenone derivatives showed that their reactions at a platinum and glassy carbon electrode are remarkably different, as the studied compounds react at significantly lower potentials on the latter one. The oxidized forms of quinones give higher cathodic currents on the pretreated glassy carbon in the acetonitrile solutions of substrates. Therefore, the glassy carbon electrode modified in this way might be useful for a more sensitive detection of quinones.
Acknowledgements
Financial support of the GINOP 2.3.2-15-2016-00022 grant is highly appreciated.


