1 Introduction
Out of various heavy metals, copper is the third-most abundant metal found in the human body and plays an important role in the physiological process [1–3]. Low concentration of Cu2+ is required for the living system for growth and development. It also acts as a catalytic cofactor for different types of metalloenzymes [4–6]. In spite of its biological applications, copper is also widely used in domestic purposes and industries because of its high thermal/electrical conductivity, stability, and alloy formation ability with other metals [7–9]. In spite of its importance, high-dose copper can exhibit toxicity and severe neurodegenerative diseases such as prion disease, Alzheimer disease, hypoglycemia, dyslexia, Wilson disease, etc., through the formation of reactive oxygen species (ROS) [10–14]. Moreover, copper acts as a remarkable environmental pollutant because of its use in industrial, household, and agricultural processes [15,16]. Accordingly, the World Health Organization (WHO) has suggested that the acceptable concentration of copper should be of 31.5 μM in the drinking water [17]. Owing to the Janus-faced properties of copper in the living system, a fast, convenient, cheap, and reliable method for detection Cu2+ ions in drinking water and biological systems is important and challenging. In this regard, optical detections (via colorimetric or fluorescence changes) among the other detection techniques are more convenient because of their simplicity, high sensitivity, and finally ability of ‘naked-eye’ detection of cations or anions [18]. For these reasons, several non–carbohydrate-based colorimetric or fluorometric sensors have been reported for Cu2+ detection [19–28], but most of them suffer from the following disadvantages: poor water solubility, expensive starting materials, longer response time, interference by other cations or anions, and finally toxicity for the cell. In this context, colorimetric and fluorometric sensors based on biologically benign carbohydrates would be more advantageous for the presence of –OH groups, and oxygen atoms in the sugar moiety are very suitable for cation binding and for increasing the water solubility [29–31]. Generally, the carbohydrate-based fluorometric or colorimetric sensors have been designed with the incorporation of a fluorophore or chromophore. Various forms of carbohydrates, such as open chain, pyranose, and furanose, were used for copper ion detection. The pyranose form of sugar is considered a more suitable ring system to accommodate axial substituents than cyclohexane because anomeric effects of sugar favor the axial orientation of an electronegative substituent at the anomeric position. In spite of that, the axial lone pair of the ring oxygen is less open to 1, 3-diaxial repulsions than the axial hydrogen atom of cyclohexanes [32,33]. This unique phenomenon found in carbohydrates is useful in the detection of ions selectively.
To date, no review articles addressing carbohydrate-based fluorescent and colorimetric sensors for Cu2+ ions have been reported. The main purpose of this review is to fill in the gap between carbohydrate-based and non–carbohydrate-based Cu2+ sensors. In the present review, we have tried to summarize all the articles published to date for colorimetric/fluorimetric detection of Cu2+ ions by carbohydrate-based (monosaccharide) sensors.
2 Carbohydrate-based triazole-linked sensors
The sensing and complexing capability of 1,2,3-triazole moiety toward cations and anions is remarkable [34]. In addition, the use of the triazole group as a linker has been applied in various fields, including drug design, bioconjugation, and materials chemistry [35,36]. In view of this, Hseih et al. [37] reported bis-triazolyl sugar-aza-crown (SAC) ether fluorescent sensor 1 (Fig. 1) for selective detection of Cu2+ and Hg2+, where triazole groups provide suitable binding sites and anthracene moieties act as the signaling units. Two anthracenetriazolymethyl molecules containing sensor 1 exhibited dual signaling behaviors for Cu2+ and Hg2+ in methanol. Fluorescent intensity of 1 was examined in different solvents, namely, methanol, dichloromethane, and acetonitrile. Sensor 1 exhibited the highest emission in methanol solvent. The fluorescence of 1 was quenched by Cu2+, Hg2+, Ni2+, and Co2+ ions over the several metal ions. But, more effectively, fluorescence was quenched by Cu2+ and Hg2+ ions because of reverse photoinduced electron transfer (PET) from the excited anthracene units to triazole moieties [38–40]. The fluorescence intensity of 1 was quenched effectively 82% for Hg2+ and 92% for Cu2+. From the fluorescence titration experiment, the binding constant (Ka) and the detection limit of 1 for Cu2+ were calculated and found to be 4 × 105 M−1 and 1.39 × 10−6 M, respectively. The 1:1 stoichiometry of the 1·Cu2+ complex was obtained from a Job plot. To establish the binding mode of 1 with Cu2+ ions, 1H NMR titration was carried out in the presence of Hg2+ ion because of the paramagnetic nature of copper. From the 1H NMR titration experiment, they concluded that a significant conformational change of the SAC ring occurred after copper complex formation.
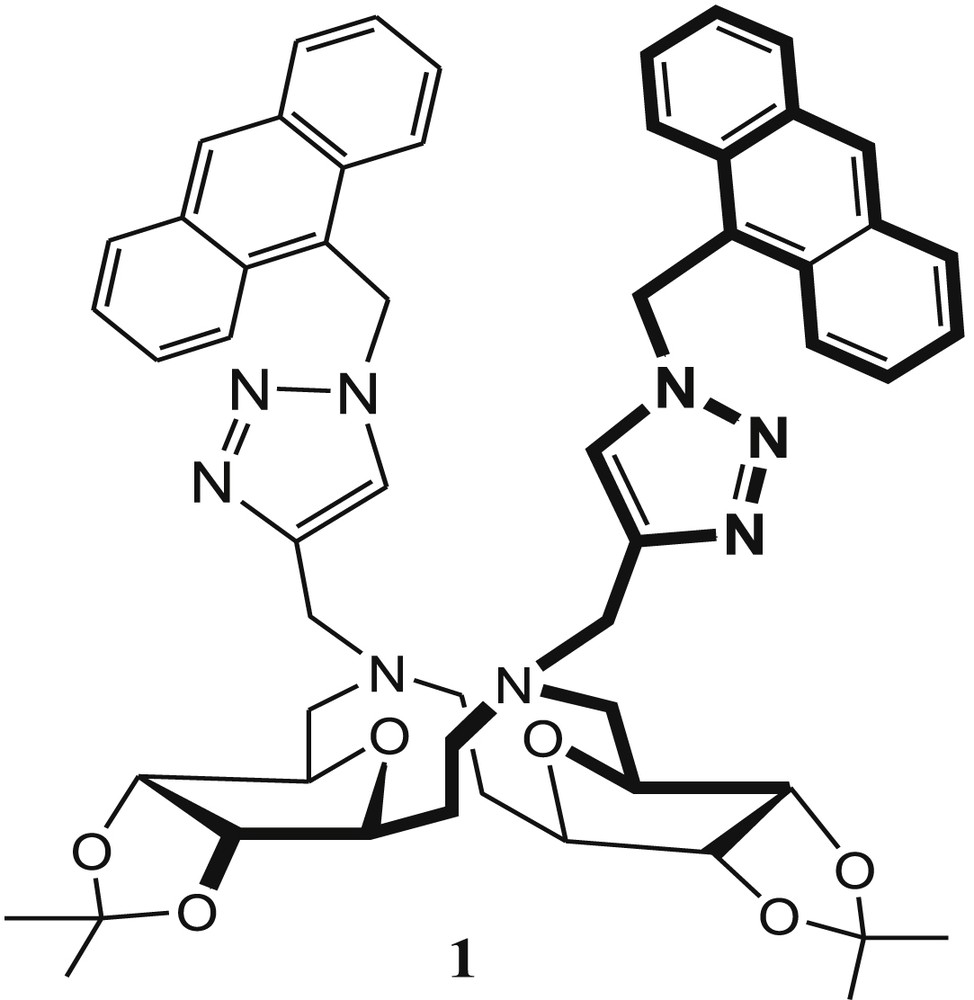
Sugar-aza-crown (SAC) ether 1.
The same group has reported another SAC ether–based cavitand 2 (Fig. 2) for selective recognition of Cu2+ in methanol [41]. In the presence of the Cu2+ ions, the fluorescence intensity of 2 was enhanced via the PET process upon complexation of the nitrogen atoms by the metal ion. These results indicate that Cu2+ is recognized by either the triazole moieties or two linker nitrogen atoms of the sensor 2. The fluorescence quenching was observed after addition of 10 equiv of Hg2+ to the solution of the 2·Cu2+ complex, which was explained by the presence of two different recognition sites in SAC ether 2. The actual binding mechanism for both ions is not reported in the article. Fluorescence titration indicates that the fluorescence intensity of 2 increased with increasing concentration of Cu2+ ions. In addition to the Cu2+-sensing property of 2, the recognition ability of 2 with anions was also investigated. This experiment indicated that the fluorescence intensity of 2 was not affected in the presence of anions such as F−, Cl−, Br−, and I−. The association constant (Ka) of 2 for Cu2+ was found to be 2.5 × 104 M−1 from the Stern–Volmer plot, and the detection limit was estimated to be 1.39 × 10−6 M in MeOH from fluorescence titration. The Job plot indicated the formation of the 1:1 complex between 2 and Cu2+ ions.
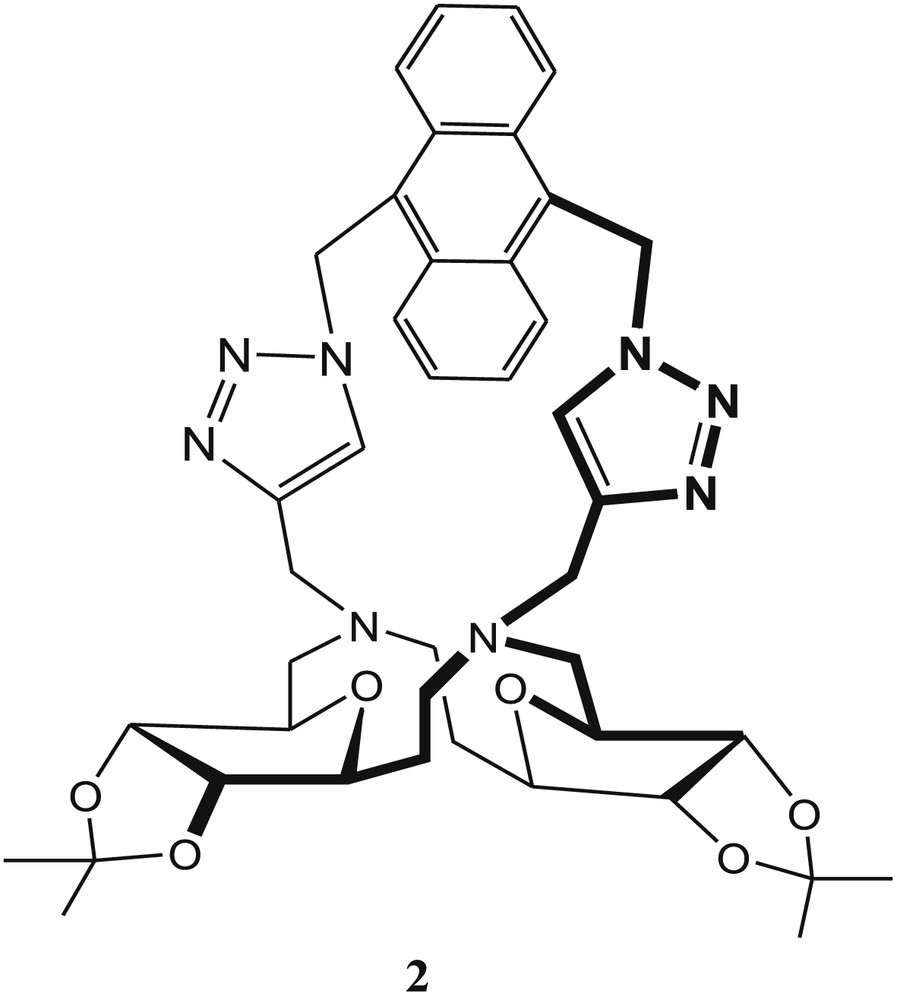
Sugar-aza-crown (SAC) ether–based cavitand 2.
The triazole-linked ribosyl-based ester ending “on-off” fluorescence sensor 3 (Fig. 3) exhibits selectivity toward Cu2+ and Ni2+ in methanol [42]. To increase the water solubility, the ester end groups of 3 have been hydrolyzed and completely water-soluble fluorescent sensor 4 has been synthesized (Fig. 3), which showed high selectivity toward Cu2+ and Hg2+ ions in water medium [43], whereas the control compound 5 (Fig. 3) exhibited poor selectivity toward metal ions. Huang et al. [42] claimed that the ribosyl functionalities of sensor 4 act as a scaffold to bring two triazole groups on the same side and enhanced the quenching effect in the presence of Cu2+ or Hg2+. The association constant (Ka) of 4·Cu2+ was calculated as 2.15 × 105 M−1 from fluorescence titration spectroscopy. In the Job plot, maximum fluorescence emission was noticed when the mole fraction of Cu2+ was 0.5, which suggested the formation of a 1:1 metal–ligand complex. The limit of detection of 4 for the analyte Cu2+ was calculated as 0.89 μM.
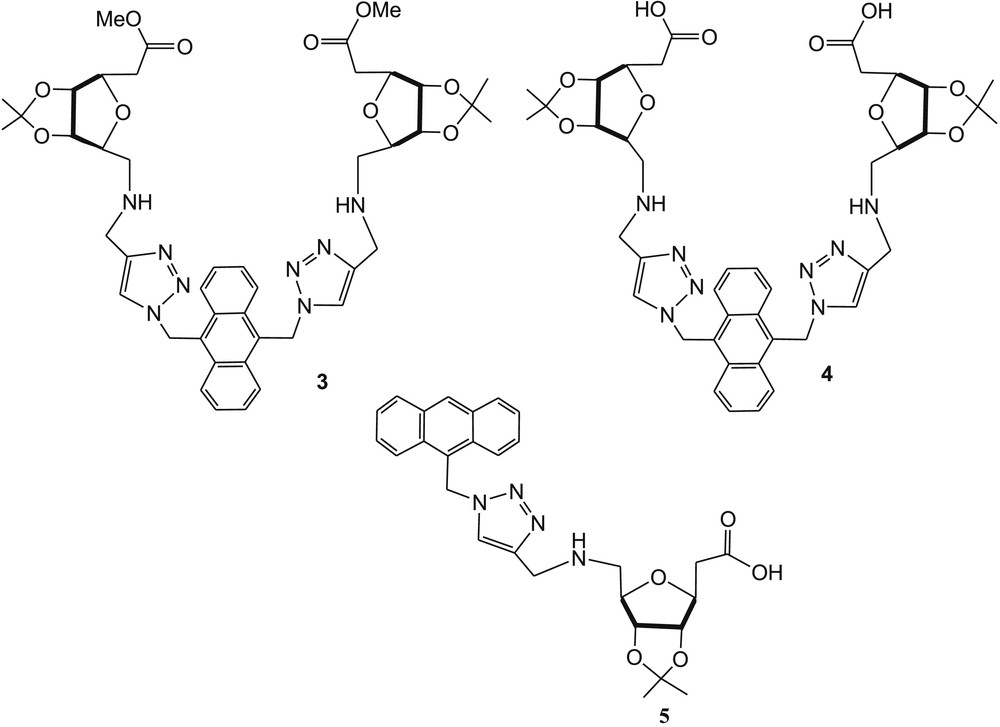
Ribosyl-based triazolyl fluorescent sensors 3, 4, and reference compound 5.
Zhang et al. [44] reported a triazole-linked glucosyl anthraquinone 6 (Fig. 4) for selective detection of Cu2+ ions. The photophysical properties of 6 were scrutinized with absorbance and fluorescence studies in the presence of several metal ions. In the absorption spectra, remarkable blue shift (Δλ = 70 nm) and intensity enhancement were noticed after addition of Cu2+ in the sensor solution, whereas the fluorescence of 6 quenched effectively in the presence of Cu2+ under the same experimental condition. The Job plot experiment of the 6·Cu2+ complex was carried out by fluorescence spectroscopy, which exhibited a metal-ligand binding ratio of 1:2. The association constant (log Ka) of the 6·Cu2+ complex was obtained from fluorescence titration spectrometry and found to be 5.64. The optical selectivity of 6 toward Cu2+ was explained through intramolecular charge transfer (ICT) [45] and anthraquinone → Cu2+ μ-cation interaction [46] and/or paramagnetic nature of Cu2+ [47,48]. 1H NMR spectra of 6 in the presence of various concentrations of Cu2+ suggested that the triazole moiety and ether oxygen of anthraquinone directly participated in the binding event (Fig. 4).
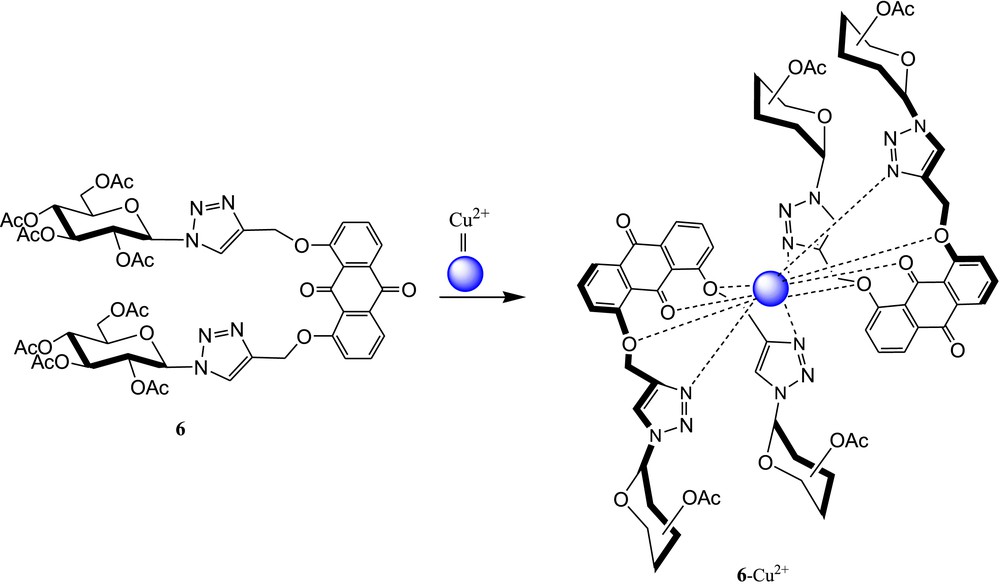
Triazole-linked glucosyl anthraquinone 6 and possible binding mode of the 6·Cu2+ complex.
Triazole-based ferrocene-carbohydrate bioconjugates 7 and 8 (Fig. 5) have been developed by Thakur et al. [49] for selective recognition of Cu2+ in CH3CN/H2O (1:4, v/v). The complexation properties of 7 and 8 with Cu2+ ions in CH3CN have been confirmed by electrochemistry and UV-vis spectroscopic measurements. After incremental addition of Cu2+ (0–1 equiv) to both sensor solutions, a new and a weak low-energy absorption band at λ = 630 nm appeared for both cases. This spectral shift explained the color change from yellow to dark green. The color change from yellow to dark green occurred by oxidation of the ferrocene moiety upon complexation with Cu2+ ions [50]. The 1:1 stoichiometries of the copper complexes were established from Job plot experiments using UV-vis titration data. The metal–ligand binding ratio (1:1) was further supported by the high-resolution mass spectra (HRMS) experiment of the complex.
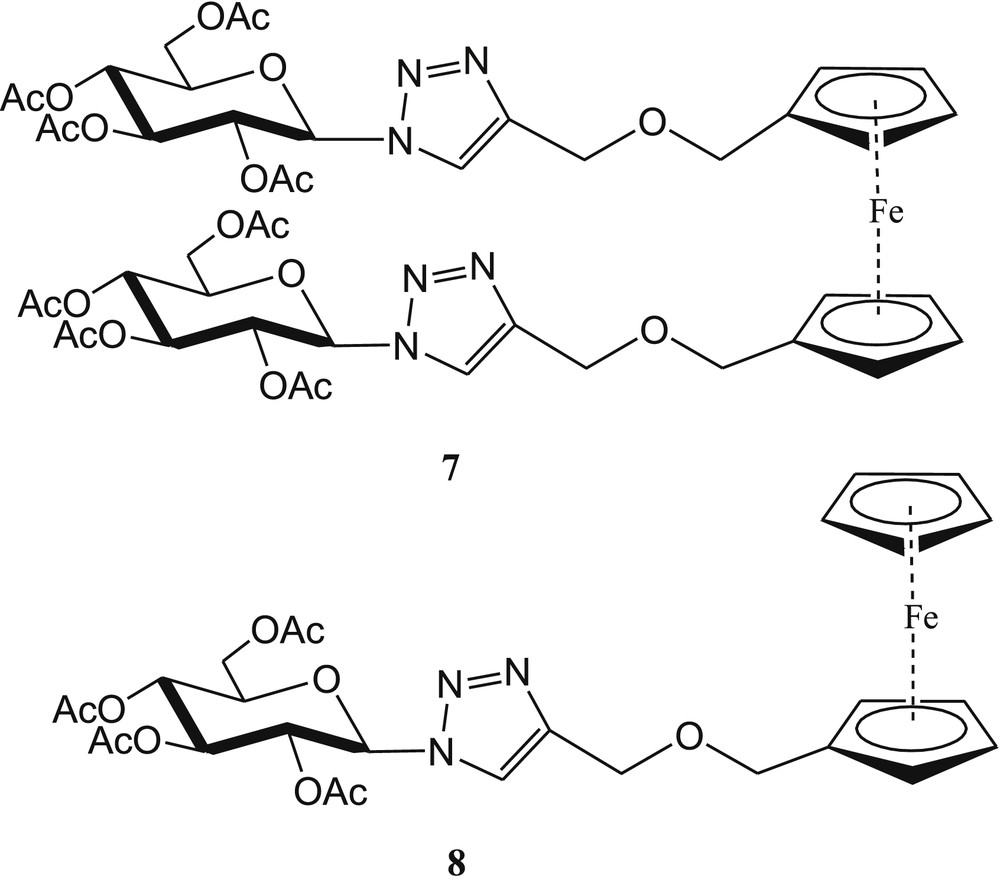
Triazole-linked monoferrocene-carbohydrate and diferrocene-carbohydrate conjugates 7 and 8.
Kushwaha et al. [51] developed the bis-triazolyl-linked glycosyl fluorescent sensors 9 and 10 (Fig. 6) from 1,2:5,6-di-O-isopropylidene-α-d-glucofuranose for selective detection of Cu2+ ions. The fluorescence intensity of 9 and 10 was reduced significantly in the presence of Cu2+ ions in acetonitrile solvent. The fluorescence quenching efficiencies [(I0–I)/I0]×100 of 9 and 10 were found to be 70% and 68%, respectively, after addition of 10 equiv of Cu2+ ions. The Cu2+-induced fluorescence quenching was explained by PET from coumarin to bound Cu2+ [52,53].
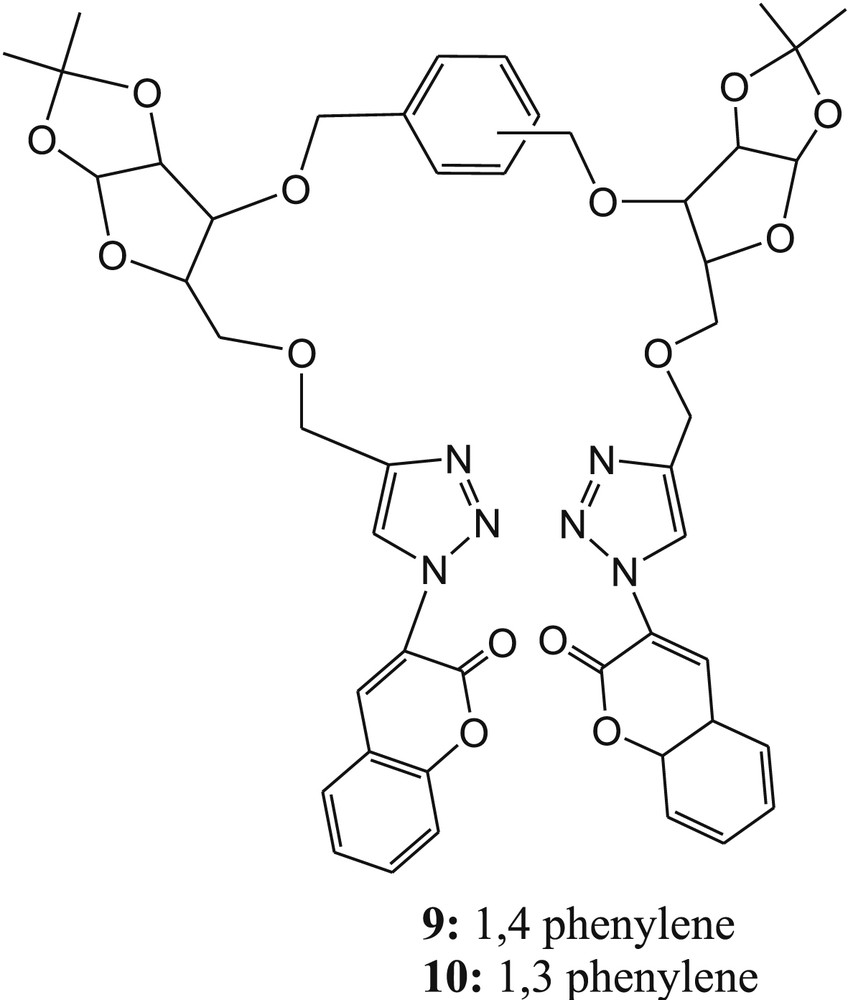
Bis-triazolyl-linked glycosyl fluorescent sensors 9 and 10.
The formation of the 1:1 complex was confirmed in both cases by Job plot experiments. Furthermore, the binding constants for the complexes (9·Cu2+ and 10·Cu2+) were calculated from fluorescence titration data using the Benesi-Hildebrand plot and estimated to be 3.34 × 103 M−1 for 9·Cu2+ and 5.93 × 103 M−1 for 10·Cu2+. The detection limits of the sensors 9 and 10 for Cu2+ were calculated to be 6.99 μM and 7.30 μM, respectively, from emission titration experiments. The binding mode of each sensor with Cu2+ was established from density functional theory (DFT) calculations. The theoretical observation suggested that the optimized structure of sensor 9 exhibited trans-configuration and 10 existed cis-configuration of triazolocoumarin arms with respect to phenylene ring (Fig. 6). The optimized structures of both complexes (9·Cu2+ and 10·Cu2+) suggested that N2 of triazole and oxygen of coumarin carbonyl take part in the binding mechanism (Fig. 7).
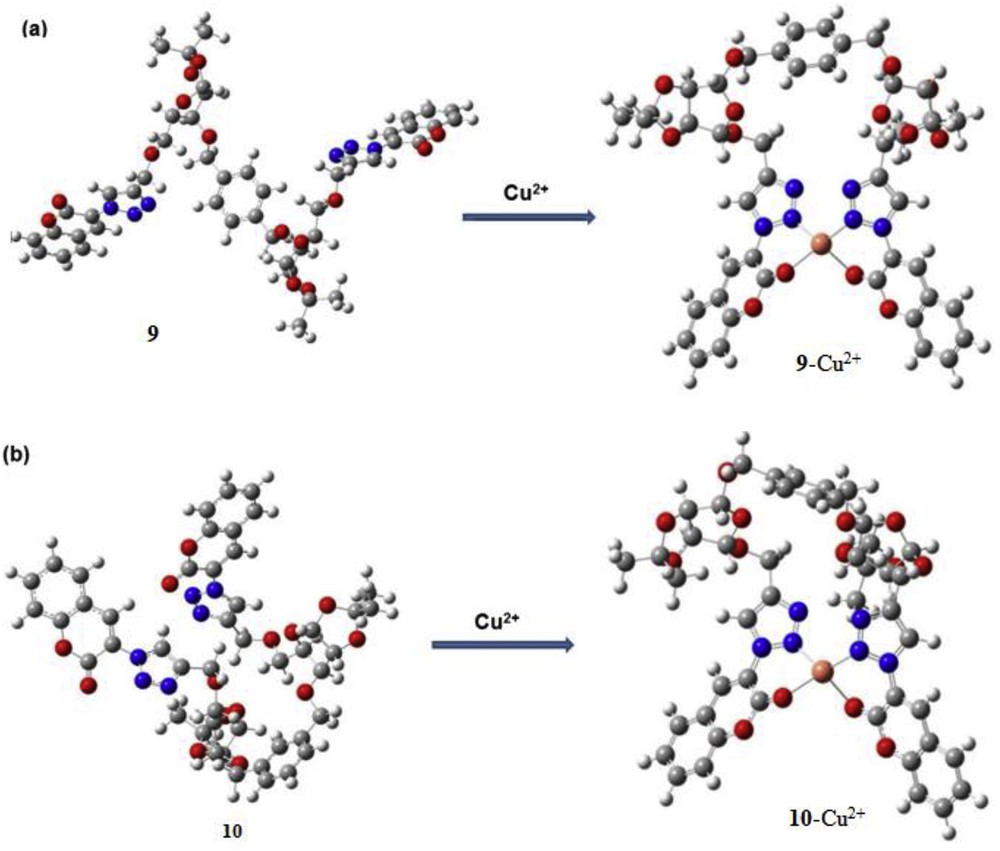
DFT-optimized structures of (a) 9 and 9·Cu2+; (b) 10 and 10·Cu2+ complexes. DFT, density functional theory.
Recently, we [54] reported a new and simple triazole-linked glucofuranose derivative 11 (Fig. 8a) for selective colorimetric detection of Cu2+ ions in an aqueous medium (CH3CN/phosphate buffer; 1/4, v/v, pH 7.4). The UV-vis absorption and color were remarkably changed in the presence of Cu2+ ions over various cations and anions. The binding mode of 11 with Cu2+ was established by the synthesized model compound 12 (Fig. 8b). The absorption of 12 was unaffected toward cations and anions, suggesting the involvement of aromatic –OH in the binding mechanism. Sensor 11 exhibited the naked-eye color change from yellow to colorless in the presence of Cu2+ ions, which was attributed by the hypsochromic shift of 46 nm of 11 [55]. The metal–ligand binding ratio (1:1) was established from the Job plot using absorption titration data. The 1:1 stoichiometry of the 11·Cu2+ complex was further supported by electrospray ionization mass spectrometry (ESI-MS) spectrometry of the complex.
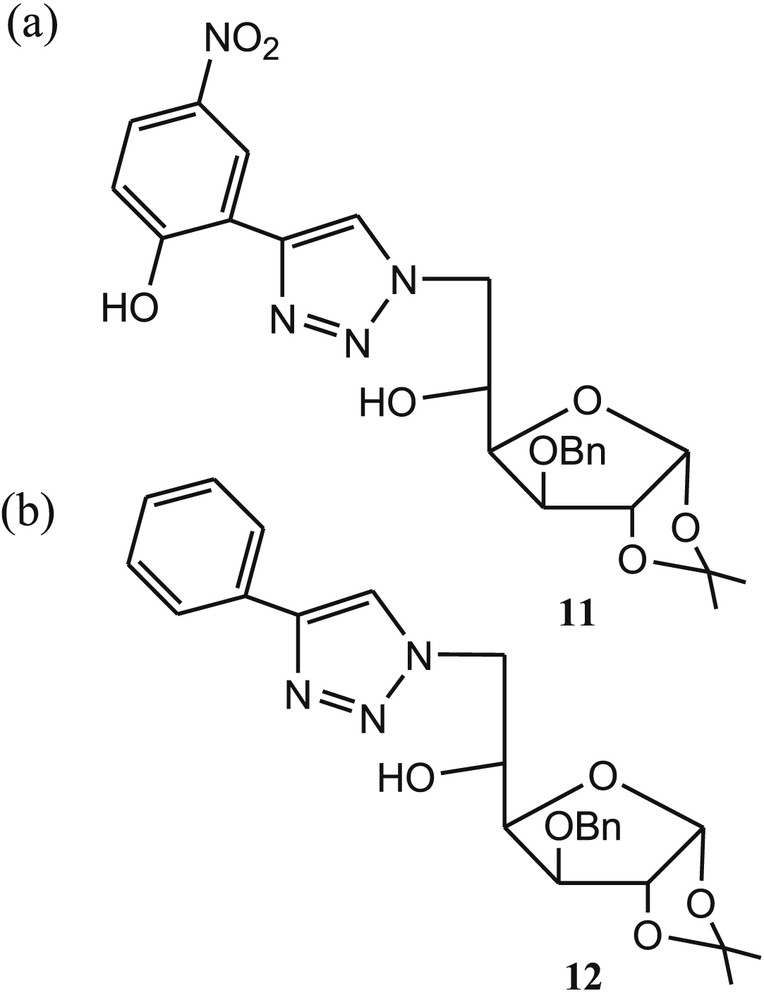
Structures of glucofuranose colorimetric sensor 11 and (b) model compound 12.
In addition, the reversible binding mode of 11 with Cu2+ was established by ethylenediaminetetraacetic acid (EDTA) experiment. The limit of detection and binding constant of 11 for Cu2+ ions were found to be 3.50 μM and 4.2 × 105 M−1, respectively. Finally, the binding mode was supported by fourier-transform infrared spectroscopy (FTIR), DFT, and time-dependent density functional theory (TDDFT) calculations and concluded that triazole moiety and aromatic hydroxyl group of 11 are coordinated with Cu2+ ions after deprotonation of aromatic –OH group (Fig. 9a). The DFT-optimized structure of the 11·Cu2+ complex is shown in Fig. 9b.
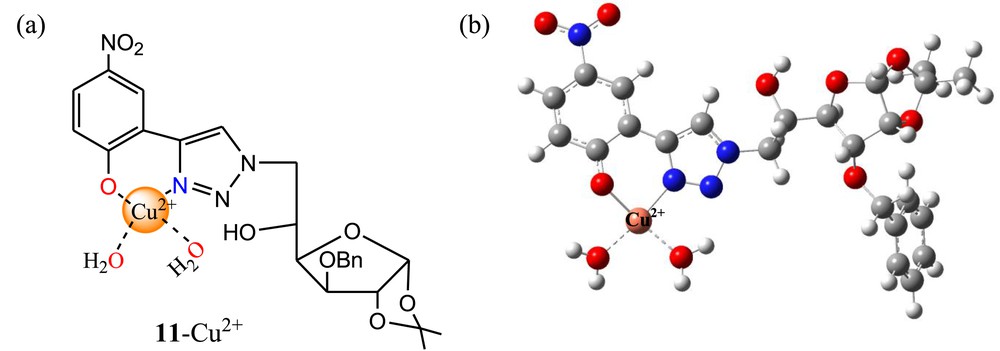
(a) Proposed binding mode of 11 with Cu2+; (b) DFT-optimized structure of the 11·Cu2+ complex. DFT, density functional theory.
3 Carbohydrate-based imino conjugate as sensors
The presence of imine functionality in metal sensors is highly in demand and acceptable owing to its effective role for cation sensing and easy synthesis [56–58]. The literature survey revealed that absorption and fluorescence studies of galactosyl-based naphthylimine derivative 13 (Fig. 10) exhibited selectivity toward Cu2+ ions in N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES) buffer at pH 7.2–7.4 [59]. The absorption and fluorescence spectral changes of 13 were observed in the presence of both ions Cu2+ and Zn2+ in MeOH system. Absorbance versus the [M2+/13] mole ratio clearly suggested the formation of a 1:1 complex in case of Zn2+ and of a 1:2 complex for Cu2+ ions. The same binding ratios were obtained from fluorescence studies. However, the fluorescence studies of 13 in HEPES buffer at pH 7.2–7.4 exhibited altogether different results. The fluorescence intensity of 13 was gradually increased in the presence of Cu2+ only. The fluorescence was further quenched at higher and lower pH, namely, 6.0 and 5. To establish the function of imine and carbohydrate moiety of 13 for selective fluorogenic recognition of Cu2+, Singhal et al. [59] have synthesized another three molecular systems 14, 15, and 16 (Fig. 10). Fluorescence titration of 14 with Cu2+ in buffer did not affect the fluorescence of 14, which clearly indicated that imine moiety of 13 is involved in the formation of the complex. Further titrations of 15 and 16 with Cu2+ resulted in an increase of fluorescence by 2.7 times and 2.3 times, respectively, which was attributed to the presence of imine and naphthylic –OH moieties in both compounds. The maximum increase of fluorescence intensity of 13 with Cu2+ as compared with 14, 15, and 16 revealed the C2–OH of 13 to be also involved in the formation of the complex. The sensor 13 showed switch-on fluorescence property in the presence of Cu2+ ions. The 1:2 stoichiometry of the 13·Cu2+ complex was further established by Q-TOF ES MS titration of 13 and 15 with Cu2+ ions (Fig. 11). The quadrupole time-of-flight electrospray mass spectrometry (Q-TOF ES MS) titration of ligands with various concentrations of Cu2+ (0–0.2 equiv) suggested the existence of 1:1, 2:1, and 2:2 ligand metal species in the solution (Fig. 11]. The binding constants of sensor 13 with Cu2+ were calculated as 50,500 ± 1000 M−1 and 50,500 ± 700 M−1 from absorption and fluorescence titration experiments, respectively.
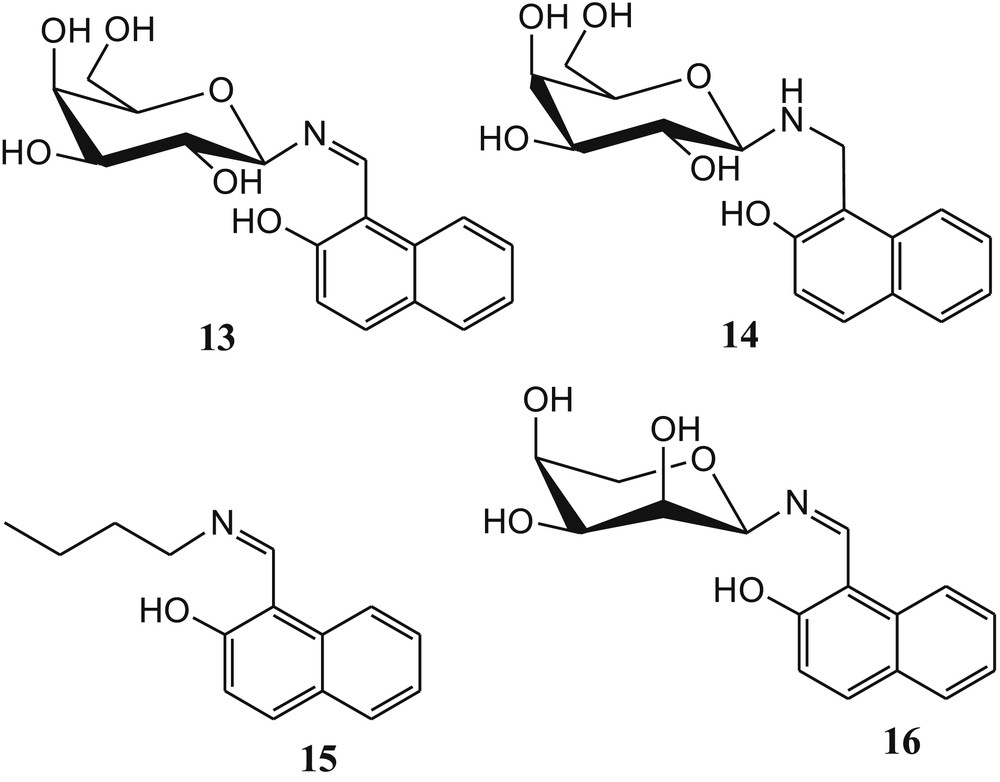
Structures of sugar-based sensor 13 and control compounds 14–16.
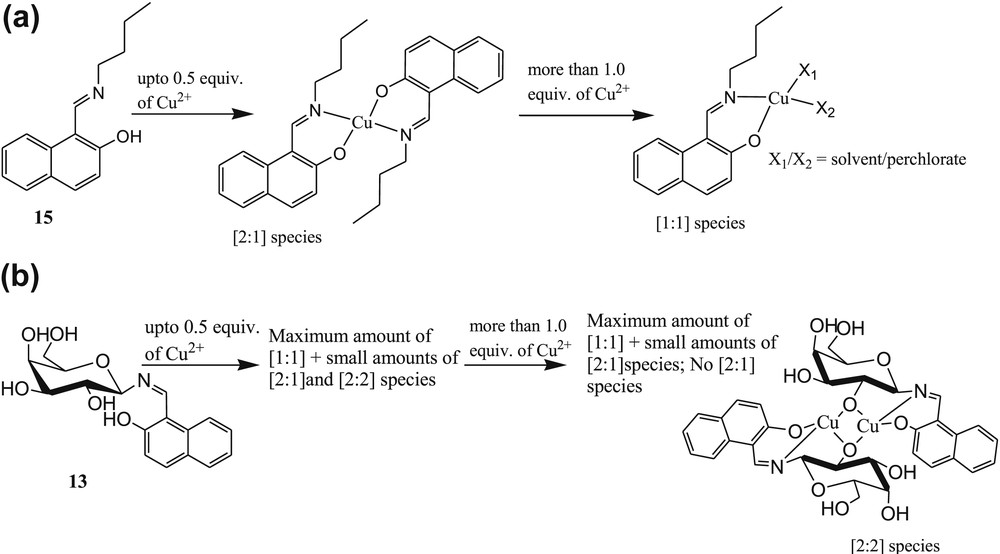
Existence of species based on the Q-TOF ES MS experiment: titration of (a) 15 and (b) 13 with Cu2+ ions.
Ma et al. [60] developed a solvent-controlled fluorescence sensor 17 (Fig. 12) based on imine-linked sugar-rhodamine for selective detection of Cu2+ by the spirolactam ring–opening reaction in a 20% acetonitrile-water medium. The acetonitrile-water solution of sensor 17 is colorless and nonfluorescent. In the presence of Cu2+, the aqueous solution (CH3CN/H2O, v/v, 1/4) of sensor 17 displayed a noticeable color change from colorless to pink along with enhancement of fluorescence intensity at room temperature. These phenomena were explained by the spirolactam ring opening and the formation of an acid derivative 19 (Fig. 12). The irreversible response of 17 to Cu2+ was established by EDTA experiments. The reaction mechanism (spirolactam ring opening) of sensor 17 with Cu2+ was confirmed by matrix-assisted laser desorption ionization (MALDI)-TOF mass spectroscopic analysis. The formation of compounds 18 and 19 after addition of Cu2+ in 17 was confirmed by HRMS spectrometry of the 17·Cu2+ complex. Finally, the detection limit of sensor 17 toward Cu2+ was evaluated to be 12 μg/L (1.88 × 10−7 M). Interestingly, Huang et al. [61] observed that the synthesized sensor 17 showed high fluorometric selectivity toward Hg2+ in pure water system.
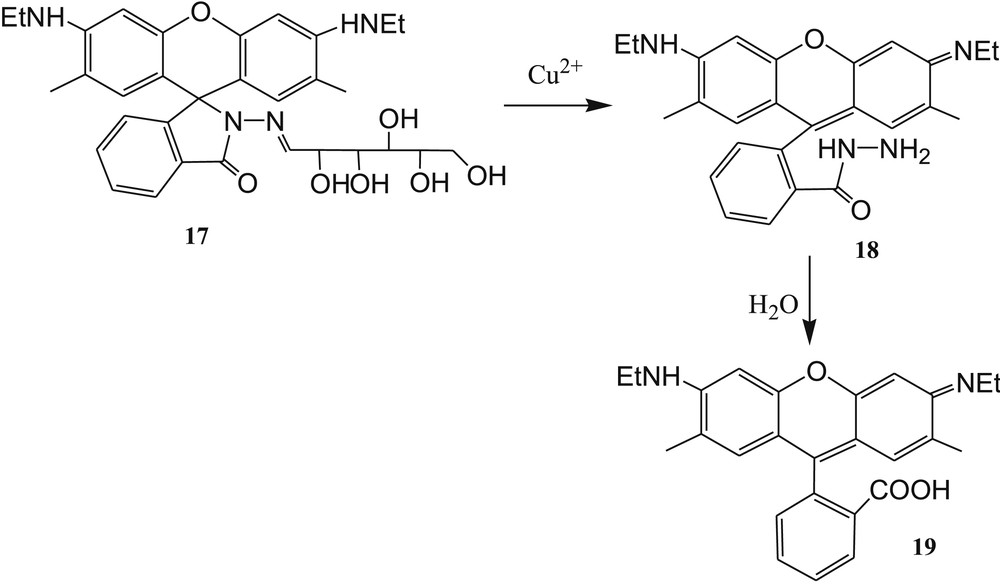
Imine-linked sugar-rhodamine 17 and proposed reaction mechanism of 17 with Cu2+.
Zhang et al. [62] have reported three colorimetric and “off-on” fluorescent sensors 20, 21, and 22 based on rhodamine B and carbohydrates (Fig. 13). The selectivity of sensors toward Cu2+ ions was established by the measurements of absorbance and fluorescence of 20, 21, and 22 in CH3CN-H2O (2/3, v/v) solution before and after addition of 5 equiv of Cu2+ ions. All the three sensors exhibited remarkable fluorescence and absorbance enhancements in the presence of Cu2+ over other metal ions and anions. The 1:1 binding mode of both complexes was supported by ESI mass spectra and infrared measurements [63,64]. In the presence of Cu2+, the spirolactam ring of sensors was opened and rhodamine B was formed, which was also established from ESI mass data. This phenomenon explained the significant enhancement of absorbance and fluorescence of sensors in the presence of Cu2+ ions. In addition, the detection limit for Cu2+ was calculated and found to be 1 ppm for all sensors. The proposed nonreversible binding mechanism of 20 through the formation of 23 and 24 is shown in Fig. 14 [65–67]. The sensors 21 and 22 exhibited similar binding mechanism with Cu2+ ions as illustrated in Fig. 14.
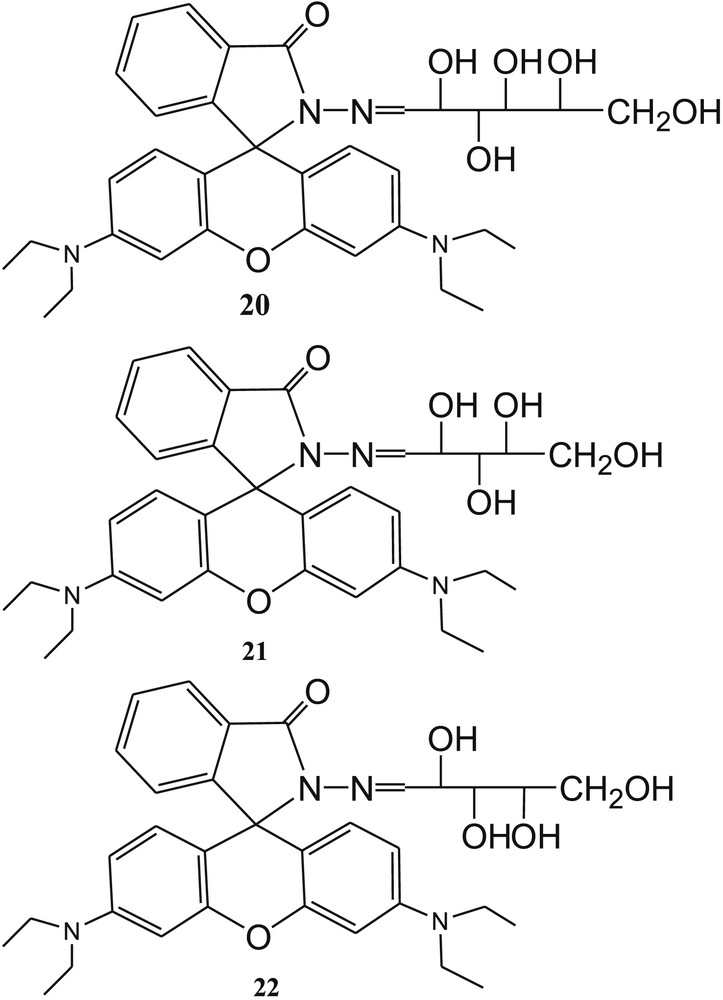
Structure of carbohydrate-based sensors 20, 21, and 22.
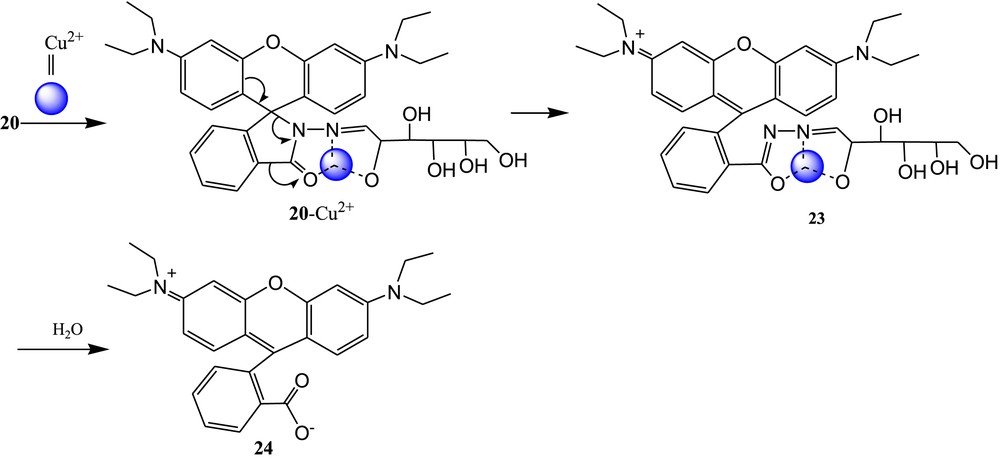
Proposed sensing mechanism of 20 with Cu2+.
Yin et al. [68] reported another sugar-rhodamine “turn-on” fluorescent sensor 25 (Fig. 15) for selective detection of Cu2+ in acetonitrile. In the presence of Cu2+ ions, the fluorescence intensity of 25 was greatly enhanced in acetonitrile medium, whereas the fluorescence quenching was observed in water–acetonitrile medium. This phenomenon was explained by decreasing the oxidation ability of Cu2+ ions in water system. Strong fluorescence emission of 25 after addition of Cu2+ in CH3CN was explained by spirolactam ring opening (Fig. 16). The irreversible nature of sensor 25 to Cu2+ was established by Cu2+ chelating agent, such as EDTA. The 1:2 stoichiometry of 25 with Cu2+ was calculated from Job plot experiments. However, the reference compound 26 (Fig. 15) exhibited 1:1 metal–ligand binding ratio, and response to Cu2+ was reversible. This result indicated that the sugar moiety in sensor 25 changed the reaction process. The reaction mechanism and the formation of intermediates 27, 27 + Cu2++CH3CN, and 28 (Fig. 16] were confirmed from MALDI-TOF mass spectral analysis of the 25·Cu2+ complex. Finally, the limit of detection of Cu2+ with sensor 25 in acetonitrile was calculated to be 0.15 μM.
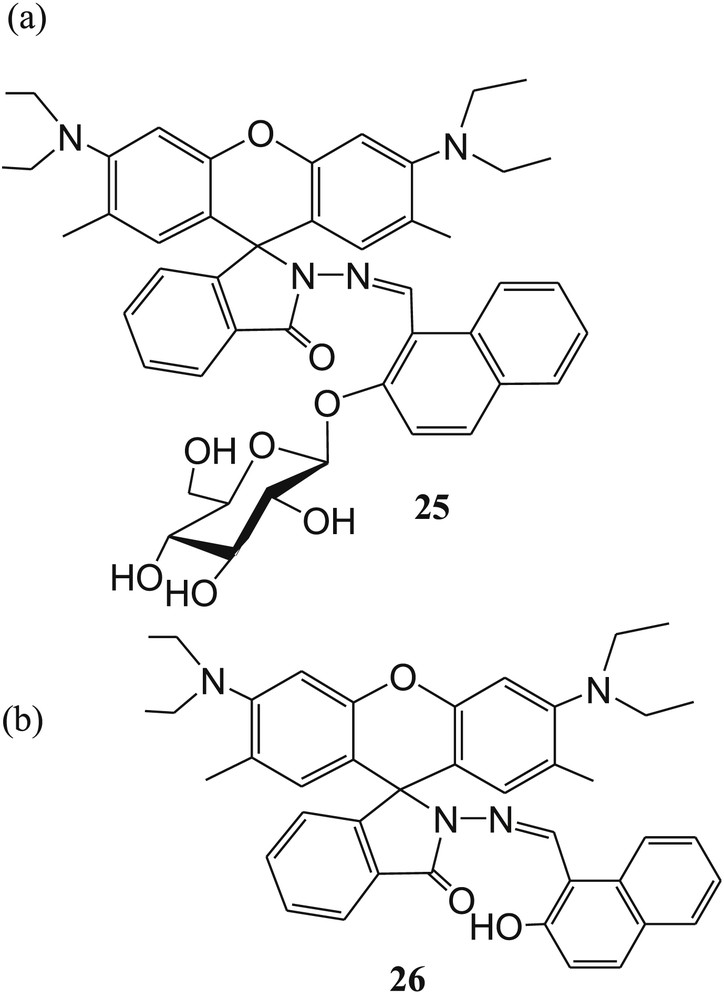
The structures of (a) sugar-rhodamine “turn-on” fluorescent sensor 25 and (b) reference compound 26.
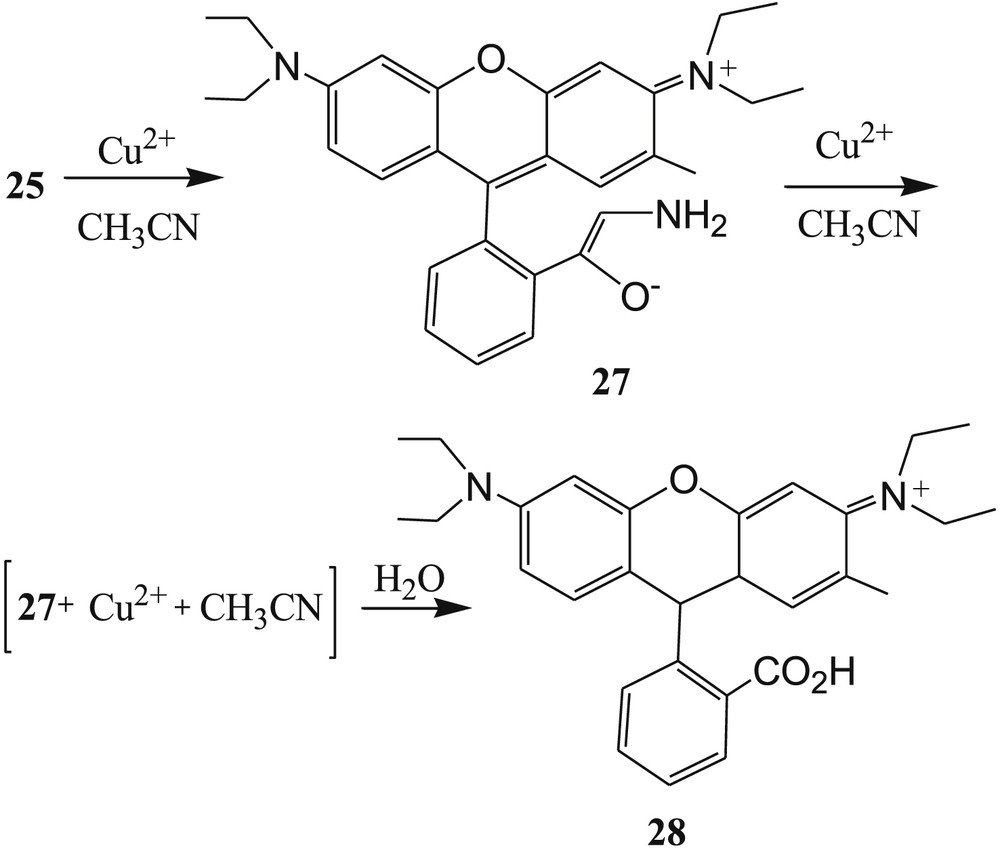
Proposed reaction mechanism of sensor 25 with Cu2+.
Mitra et al. [69] reported a diamino conjugate of glucosyl-cresol 29 (Fig. 17) for selective detection of Cu2+ ions in ethanol. The selectivity of 29 toward Cu2+ ions was established by absorbance and fluorometric color studies. By the addition of Cu2+ to the ethanolic solution of 29, the greenish fluorescent color of 29 turned to colorless. By the incremental addition of Cu2+ in the sensor solution, the absorption spectrum of 29 was shifted by 75 nm. In addition, the 29·Cu2+ complex was used for recognition of cysteine and histidine (Fig. 17). Among the all naturally occurring amino acids, only cysteine and histidine bring back the original color of the sensor 29 after addition of the aforementioned amino acids in the 29·Cu2+ complex solution. This result suggested that the displacement of Cu2+ from the 29·Cu2+ complex occurred in the presence of amino acids (cysteine and histidine). In the original article [69], maybe they have drawn a d-sugar and an l-sugar on the same molecule.
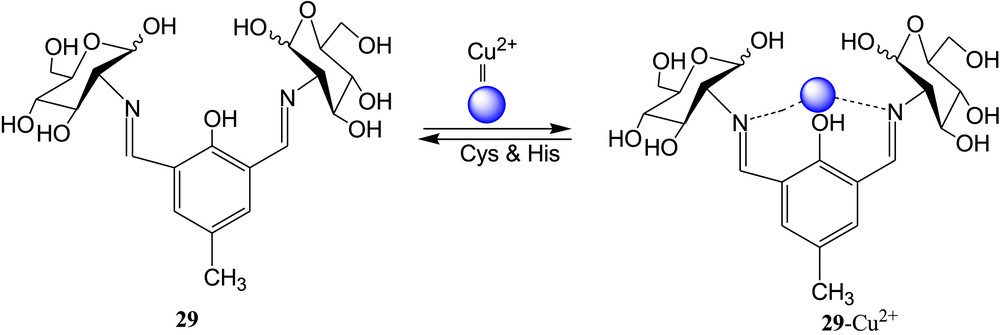
Diamino conjugate of glucosyl-cresol 29 and proposed binding mode.
The same group designed and synthesized another carbohydrate-based imino coumarin fluorescent sensor 30 (Fig. 18) for selective recognition of Cu2+ [70]. In the presence of Cu2+, the fluorescent sensor 30 exhibited 95% fluorescence quenching and absorption change in aqueous HEPES buffer at pH 7–10, even in the presence of several cations. The formation of a complex between 30 and Cu2+ was established by the changes observed in fluorescence, absorbance, ESI-MS, and 1H NMR titrations. The Job plot suggested 1:1 stoichiometry of the complex, but actually, metal–ligand binding ratio of 2:2 was found from the ESI-MS spectrum of the copper complex. The DFT calculations were used to establish the structural modes of the [Cu30]2 complex in both isomeric forms. DFT-optimized geometries of 30 and [Cu30]2 are shown in Fig. 19. Silica gel sheet coated with sensor 30 was used to detect the Cu2+ through fluorometric color change. The developed silica gel strips coated with sensor 30 were used to detect Cu2+ up to a minimum concentration 5±1 μM. The sensor 30 can detect the copper ion up to a minimum concentration of 110±16 nM in HEPES buffer. Tridentate binding nature of 30 (both isomeric forms) with Cu2+ was established by 1H NMR titration experiment and supported by DFT calculations. In addition, sensor 30 showed effective fluorescence responses in the presence of Cu2+ in living cells.
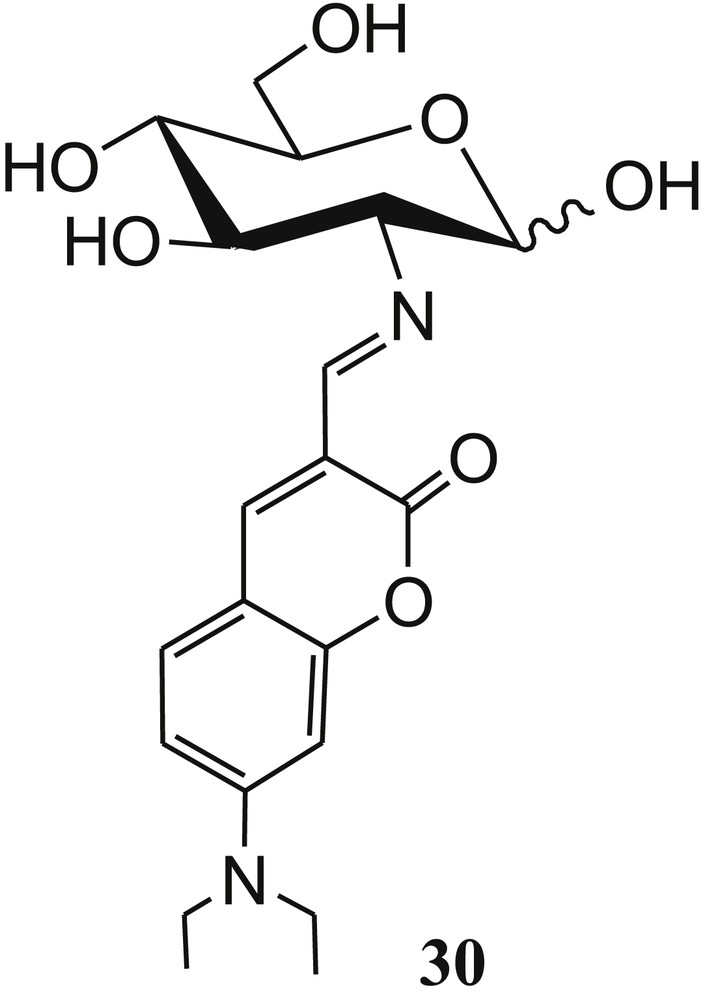
Carbohydrate-based coumarin derivative 30.
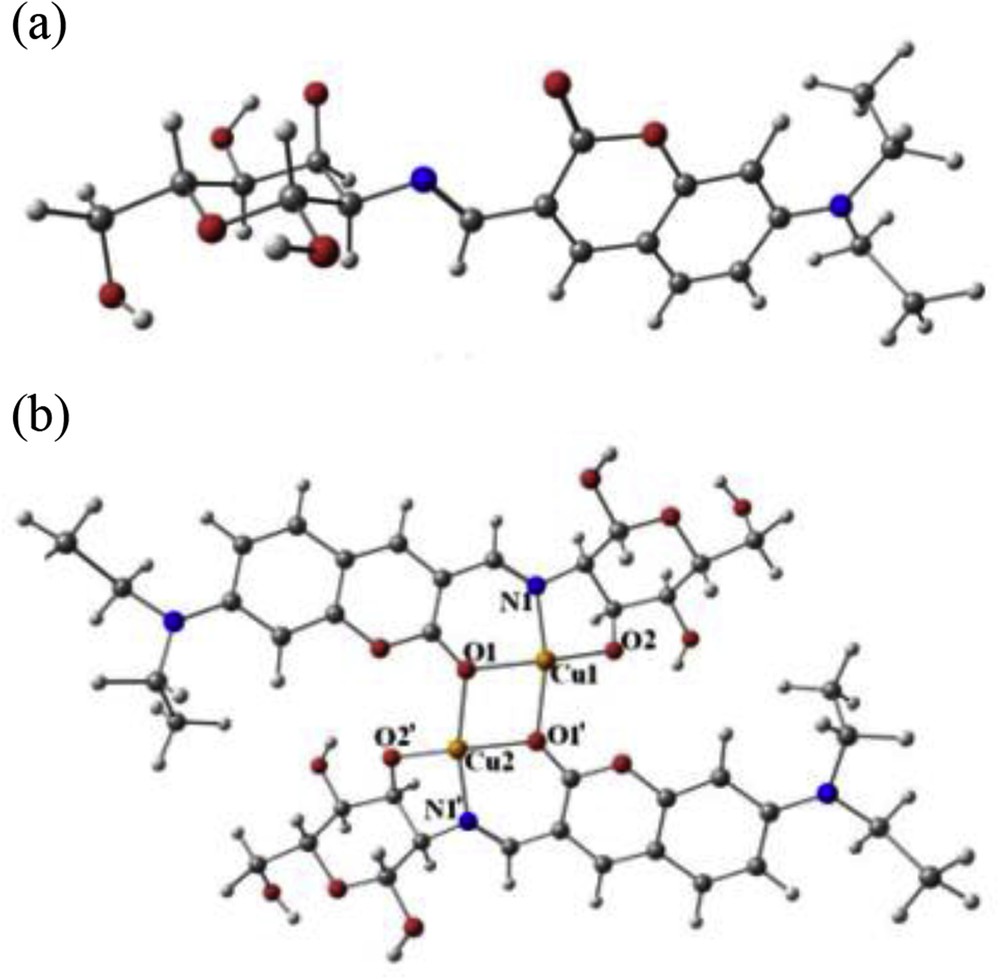
DFT calculated optimized geometries of (a) 30 and (b) 2[30-Cu2+] in methanol. DFT, density functional theory.
A simple and easy-to-prepare imine-linked glucofuranose derivative 31 (Fig. 20a) has been reported by our group [71] for colorimetric detection of Cu2+ in semiaqueous medium. The carbohydrate-based sensor 31 exhibited a remarkable color change from yellow to colorless upon binding with Cu2+ in 20% acetonitrile–water. This selective color change was attributed to the remarkable absorption shift. More amusingly, the color and absorption of the 31·Cu2+ complex could be restored by the addition of EDTA. The 1:1 stoichiometry of the 31·Cu2+ complex was established by Job plot experiments [21] and further confirmed by the ESI-MS data of the complex. Furthermore, in terms of sensitivity, the limit of detection of the sensor 31 for Cu2+ was calculated to be 0.13 μM. To establish the binding mode of sensor 31, the model compound 32 has been designed and synthesized (Fig. 20b). The binding mode of sensor 31 toward Cu2+ (Fig. 21a) was established on the basis of absorption measurement of synthesized model compound 32 with various cations, 1H NMR titrations, and ESI-MS of the 31·Cu2+ complex (Fig. 21a). The formation of complex 31a is more favorable than that of 31b because of tridentate ligand formation (imine and two hydroxyl groups). The binding mode of sensor 31 with Cu2+ proposed previously was further supported by DFT calculation, and it was concluded that triazole nitrogen and two hydroxyl groups are involved in the formation of the 31·Cu2+ complex after deprotonation of the aromatic –OH group (Fig. 21b).
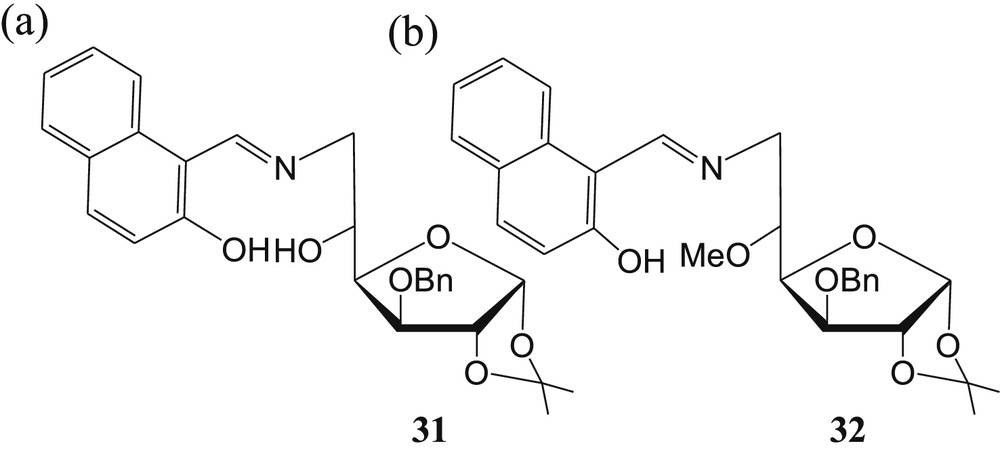
Carbohydrate-based imine-linked (a) sensor 31 and (b) model compound 32.
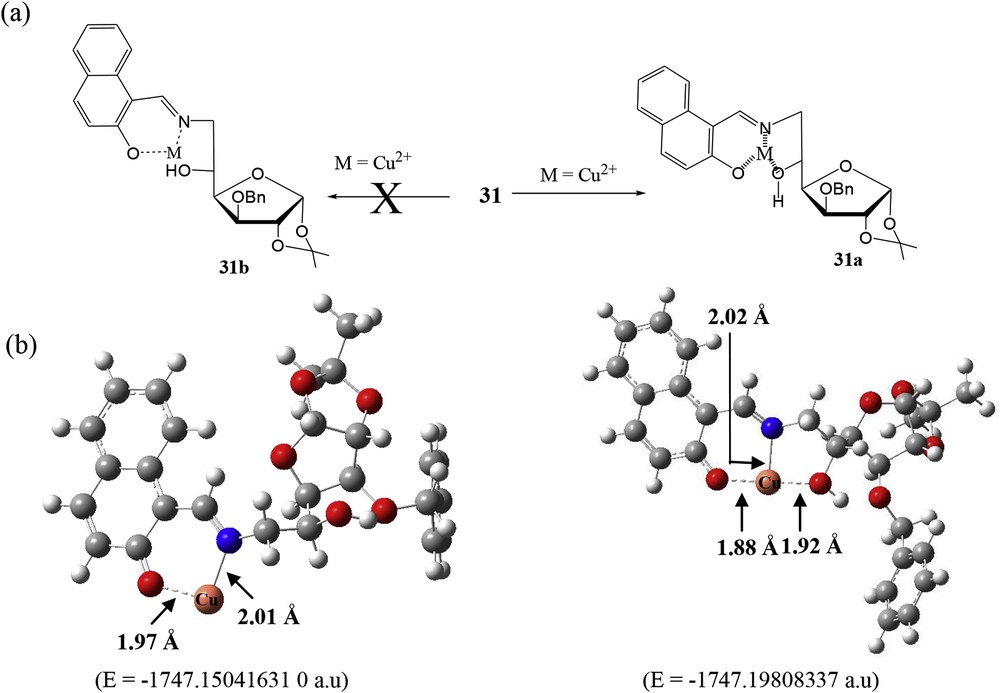
(a) Proposed binding mode of sensor 31 with Cu2+. (b) DFT-optimized structures of the proposed complexes. DFT, density functional theory.
4 Carbohydrate-based non–triazole-/imine-linked sensors
Two N-pyrenylacetamide–appended SAC ethers 33 and 34 (Fig. 22) have been designed and synthesized by Xie et al. [72] for selective recognition of Cu2+ ions in MeOH.
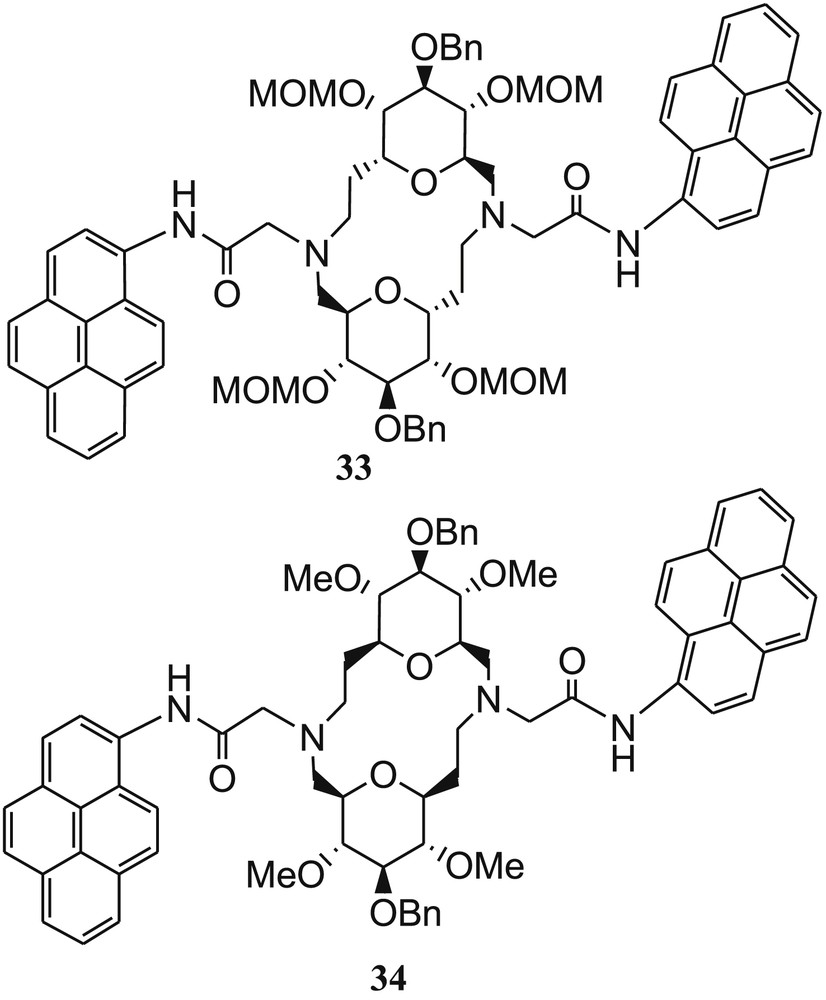
Bispyrenyl sugar-aza-crown ethers 33 and 34.
The complexation property of both the fluorescent molecular sensors with Cu2+ ions was established by absorption and fluorescence measurements. The fluorescence intensities of 33 and 34 were not affected by pH and other metal ions, such as Ca2+, Mg2+, Co2+, Mn2+, and Ni2+. The fluorescence intensity of both the sensors was remarkably induced in the presence of Cu2+ ions, which was explained by PET, from excited pyrene to the complexed Cu2+ cation [73]. The sensors showed to some extent fluorescence quenching effect with Pb2+, Zn2+ (for 33), and Cd2+ (for 34). Both the sensors exhibited 1:1 metal–ligand binding ratio with high binding constants as log K (Cu2+·34) = 6.7 ± 0.2 and log K (Cu2+·34) = 7.8 ± 0.2. The detection limits of both the sensors were found to be 40 nM. The probable binding mode was established from DFT calculations, which suggested that two carbonyl groups and two N of SAC ethers take part in coordination (Fig. 23).
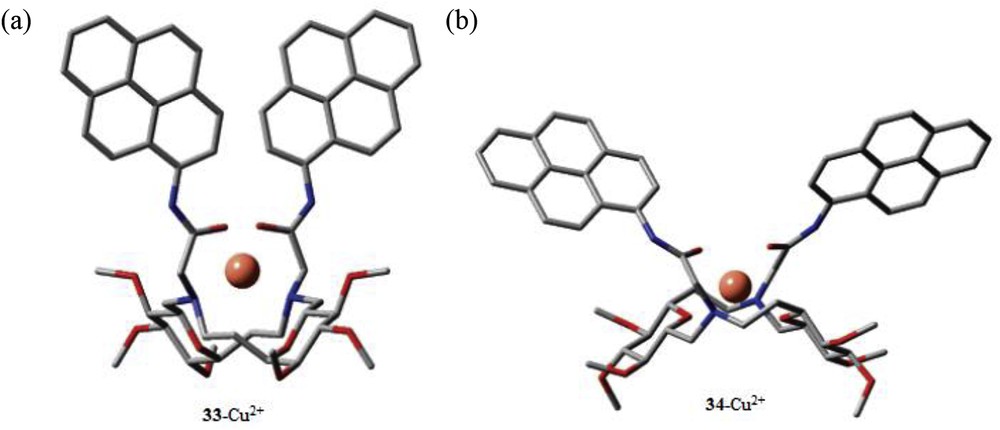
DFT-calculated energy-minimized structures of the 33·Cu2+ and 34·Cu2+ complexes. (To simplify the DFT calculation, methyl ethers were considered as hydroxyl-protecting groups.) DFT, density functional theory.
Hseih et al. [74] again reported another two pyrenylmethyl groups–containing SAC ether–based fluorescent sensor 35 (Fig. 24) for detection of Cu2+ and Hg2+ in MeOH. The fluorescence of 35 is measured in different solvents (dichloromethane (DCM), MeOH, tetrahydrofuran (THF), CH3CN, and dimethyl sulfoxide (DMSO)), and it was found that monomer and excimer ratio of 35 is not affected with the polarity of the solvents, whereas by increasing the polarity of solvents, only the monomer is predominant as compared with the excimer, in which the excimer emission is quenched completely because of strong solvation. This phenomenon indicated that two pyrenes of 35 do not exist parallel. In the presence of Cu2+ and Hg2+, the fluorescence enhancement efficiency of sensor 35 was ∼128% and 51% more than the free sensor. The fluorescence enhancement in the presence of Cu2+ and Hg2+ was explained by the PET process between the amino group of SAC ether and the pyrene fluorophore. The binding constant for the 35·Cu2+ complex was calculated as 7.4 × 101 M−1 from fluorescence titration data. The aforementioned fluorescence titration data were used to calculate the limit of detection of 35 for Cu2+ and found to be 1.3 × 10−4 M. The metal–ligand binding ratio of the 35·Cu2+ complex was established from the Job plot and supported by ESI-MS spectra of the complex. Hseih et al. [74] have established the binding mode of 35 toward Hg2+ ion by DFT calculation and concluded that two ribosyl ring oxygen atoms and two linker nitrogen atoms form coordination with Hg2+ in MeOH.
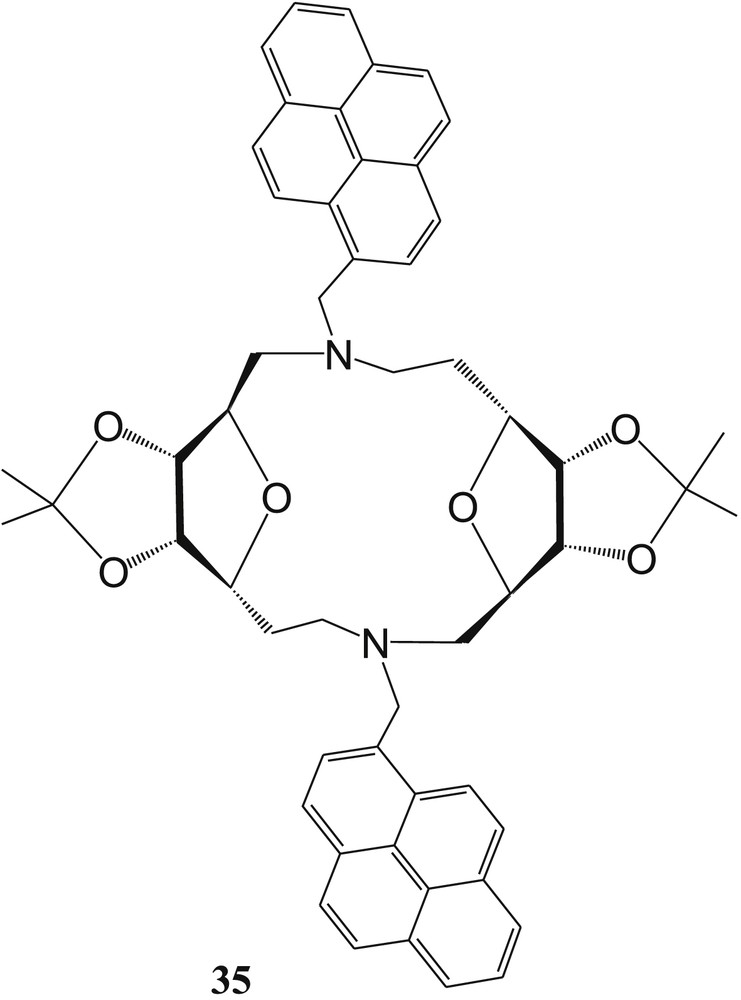
Two pyrenylmethyl groups containing sugar-aza-crown (SAC) ether–based fluorescent sensor 35.
Diwan et al. [75] have reported nonfluorescent water-compatible fluorescein-sugar conjugate 36 (FG) for selective detection of Cu2+ ions (Fig. 25). The sensor 36 exhibited strong fluorescence and remarkable color change after spirolactam ring opening upon binding with Cu2+ ions in 70% aqueous HEPES buffer solution (H2O:CH3CN = 70:30, v/v, pH 7.4). In the presence of Cu2+ ions, the absorbance of 36 was significantly increased in the visible region and concomitantly, color change was noticed from colorless to blue. These results suggested the spirolactam ring opening of FG in the presence of Cu2+. The UV-vis spectrum of FG was also influenced in the presence of higher amount of Hg2+ ion. The fluorescence intensity of 36 was significantly increased in the presence of Cu2+, but fluorescence was not affected by the addition of Hg2+ and other metal ions under the same experimental conditions. But the emission spectral pattern of FG was perturbed in the presence of Hg2+ at higher concentration of FG. In the presence of Cu2+ ions, the sensor showed strong blue-green fluorescence. The 1:1 metal–ligand (FG-Cu2+) binding ratio was established from the Job plot and further confirmed by ESI-MS study of the 36·Cu2+ complex. The association constants (Ka) for FG with Cu2+ were calculated from UV-vis and fluorescence titration data and found to be (1.02±0.08) × 105 M−1 and (2.51±0.23) × 106 M−1, respectively. The detection limits for Cu2+ were found to be 1.84 × 10−7 M and 6.32 × 10−9 M from absorbance and fluorescence spectroscopic methods, respectively [76,77]. Furthermore, the coordination mechanism was established from 1H NMR titration experiment (Fig. 25).
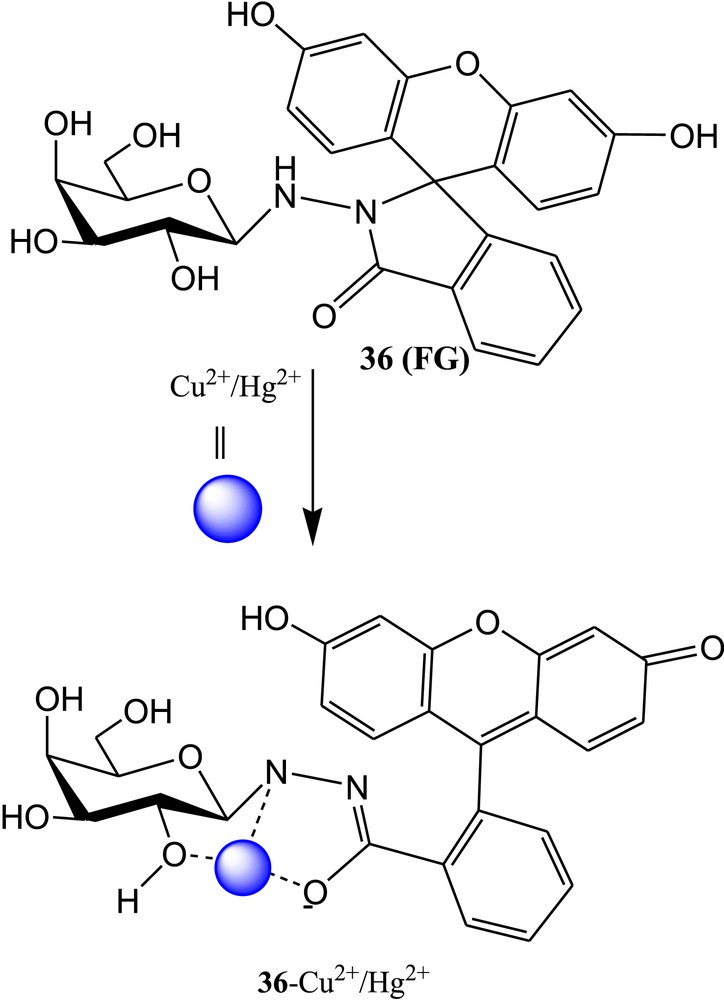
Chemical structure of fluorescein-sugar conjugate 36 and proposed binding mode of 36 with Cu2+/Hg2+.
Finally, the characteristics of reported Cu2+-sensitive colorimetric and fluorometric carbohydrate-based sensors are demonstrated in Table 1.
Characteristics of Cu2+-selective reported sensors till date.
| Sensor | Method | Solvent system | LOD | Binding constant | Ref |
| 1 | Fluorescence | MeOH | 1.39 μM | 4 × 105 M−1 | [37] |
| 2 | Fluorescence | MeOH | 1.39 μM | 2.5 × 104 M−1 | [41] |
| 3 | Fluorescence | MeOH | …… | 4.82 × 105 M−1 | [42] |
| 4 | Fluorescence | H2O | 0.89 μM | 2.15 × 105 M−1 | [43] |
| 6 | UV-vis and fluorescence | CH3CN | ….. | log K = 5.64 | [44] |
| 7 | UV-vis | CH3CN | 0.75 μM | ……. | [49] |
| 8 | …… | …….. | |||
| 9 | Fluorescence | CH3CN | 6.99 μM | 3.34 × 103 M−1 | [51] |
| 10 | 7.30 μM | 5.93 × 103 M−1 | |||
| 11 | UV-vis | CH3CN/phosphate buffer (1/4, v/v, pH 7.4) | 3.50 μM | 4.2 × 105 M−1 | [54] |
| 13 | UV-vis | HEPES buffer | ………… | 50,500 ± 1000 M−1 | [59] |
| Fluorescence | 50,500 ± 700 M−1 | ||||
| 17 | Fluorescence | CH3CN/H2O (1/4, v/v) | 0.188 μM | …… | [60] |
| 20 | UV-vis and Fluorescence | CH3CN/H2O (2/3, v/v) | 15.7 μM | ……… | [62] |
| 21 | |||||
| 22 | |||||
| 25 | Fluorescence | CH3CN | 0.15 μM | ……. | [68] |
| 29 | UV-vis and Fluorescence | EtOH | ….. | …… | [69] |
| 30 | UV-vis and Fluorescence | Aqueous HEPES buffer with DMSO | 0.11 ± 0.016 μM. | ……. | [70] |
| 31 | UV-vis | CH3CN/H2O (1/4, v/v) | 0.13 μM | 9.7 × 105 M−1 | [71] |
| 33 | Fluorescence | MeOH | 0.04 μM | log K = 6.7 ± 0.2 | [72] |
| 34 | log K = 7.8 ± 0.2 | ||||
| 35 | Fluorescence | MeOH | 130 μM | 7.4 × 101 M−1 | [74] |
| 36 | UV-vis | 70% aqueous HEPES buffer (H2O:CH3CN = 7:3, v/v, pH = 7.4) | 0.184 μM | (1.02 ± 0.08) × 105 M−1 | [75] |
| Fluorescence | |||||
| 0.0063 μM | (2.51 ± 0.23) × 106 M−1 |
5 Conclusion
In this minireview, we covered almost all the reported carbohydrate-based colorimetric and fluorometric sensors for recognition of Cu2+ ions. Our study revealed that limited numbers of carbohydrate-based Cu2+ sensors have been developed till date, although carbohydrate moiety has several advantages such as low cost, biocompatibility, presence of hydroxyl groups and oxygen atoms, and ring-flipping tendency. Some of the reported carbohydrate-based sensors act as potential sensors in terms of solubility, wavelength, nontoxicity, selectivity, and sensitivity. It is also noticed that some carbohydrate-based sensors for Cu2+ show poor water solubility because of inappropriate design. In this field, more research is required to design and develop effective sensors for detection of Cu2+ ions for real applications. The binding mechanism of sensors with Cu2+ ions was described successfully along with their DFT calculations which suggested that the solvent has a major role for specific selection of ions by the carbohydrate-based sensor. It is worth to mention that the structure of glucose in 17 and 23 reported in original articles [60,62] might be not correct. Finally, we can state that this review article will give the important way out for design and development of carbohydrate-based simple sensors for specific detection of metal ions in water medium and real-world applications.
Acknowledgements
This work was supported by CSIR (Project No.: 02(0277)/16/EMR-II). The authors would like to thank the Technical Education Quality Improvement Programme of Government of India (TEQIP) for giving FTIR facility at NIT AP. A.K.A. thanks the present group members.


