1. Introduction
Protein as a therapeutic active ingredient is a good alternative compared to small molecule drugs due to its specificity. Using proteins as therapeutic drugs is an alternative to treat some specific illness without interference on other natural biological processes and proteins induce less side effects when compared to synthetic drugs [1]. Thinking about the need of protein consumption, the interest for protein use as food supplement, vaccines, antibiotics and biopharmaceutical enzymes increased in the last two decades.
In the case of a protein function, two important parameters are followed: stability and efficiency. These factors are correlated and depend on protein conformation, normally the native state is required for high efficiency [2]. However, the native state can unfold into an inactive form under stress conditions such as temperature and pH changes, elevated pressures, use of solvents, shearing, surface contact, etc., and any modification in the structure of the protein can lead to loss in its efficiency [3]. These structural modifications can be part of protein process during filtration, purification, lyophilization, sterilization, storage, transport and drug application.
Temperature is one of the most important environmental parameters that directly impact the quality of the pharmaceutical and food products from the beginning of the formulation/processing until the administration/consumption [4]. For example, protein drugs need to be conserved under cold temperatures during storage and transportation in order to avoid denaturation and physical aggregation because the protein is thermosentitive [5]. Removal of microbial contamination of food product in its final container by heat (sterilization) is not recommended for protein-based products because it can lead to product degradation due to irreversible denaturation of enzymes [6].
In recent years, pasteurization has been considered to be adapted to protein industry in the manufacturing processes. Pasteurization represents a mild thermal process (compared to sterilization) which can be divided into 4 different methods as follow: LTLT (Low Temperature, Long Time—63 °C for minimum 30 min), HTST (High Temperature, Shorter Time—72 °C for minimum 15 s), flash pasteurization (85–90 °C for 1–4 s) and UHT (Ultra High Temperature—135 °C for minimum 1 second) [7]. The method chosen for pasteurization takes into account the composition and characteristics of the compound. As pasteurization uses temperature, it might lead to impact on the properties of the product. In order to avoid that, time and temperature have to be balanced to be enough for decontamination of bacteria while not affecting the product quality [8].
An alternative to eliminate microorganisms without using extreme conditions is to combine more than one method of purification and sterilization. For example, as presented by Subramanian [9], pasteurization could be used in upstream process in order to ease the downstream process done by ultrafiltration [9]. Ultrafiltration is a simple membrane process that can be used concomitantly for the concentration, purification, separation and microorganisms removal of protein solutions [10]. The mechanisms responsible for the separation are size-exclusion and electrostatic effects, the latter being dependent on membrane material surface [11]. The size-exclusion or sieving mechanism is dependent on the ratio between the membrane pore size and the actual size of the studied molecule.
In these conditions, it is interesting to observe the behavior of proteins that have been subjected to pasteurization (high temperatures) and ultrafiltration. In the present study, the hydrodynamic properties and the antibacterial properties are used to elucidate the changes occurring to lysozyme (model protein) due to temperature and filtration. Lysozyme is considered a model protein due to its structure and functions. Discovered in 1922 by Alexander Fleming, lysozyme is a basic enzyme with four disulphide bridges and a compact ellipsoid shape [12]. A key feature of lysozyme is the direct link between its structure and its antimicrobial activity. Due to the strong link between conformation and function, any modification of the lysozyme can temporally or completely change its antimicrobial activity [13].
In literature, works are in agreement and proove that the application of high temperature (higher than 74 °C) decreases the antibacterial activity of the lysozyme [14, 15, 16, 17, 18], but, there is no report about the influence on LSZ antibacterial activity caused by the combination of temperature and shear stress (ultrafiltration process).
2. Experimental section
2.1. Materials
2.1.1. Materials and solution preparation
The chemical reagents used in the present investigation were vitamin B12 (VB12) from Alfa Aesar and hen egg white lysozyme from Sigma-Aldrich. The solutions used were obtained by dissolving the desired amount of powder (LSZ—0.025 mM and VB12—9.22 × 10−3 mM) in deionized water. For the antibacterial assay, Micrococcus Lysodeikticus was purchased from Sigma-Aldrich and prepared with 0.01 M phosphate buffer solution.
2.1.2. Preparation of thermally denatured lysozyme
Thermally denatured LSZ was prepared by heating the solution on a heating plate at 60, 70, 80 and 90 °C in a glass vial for 1 h after the denaturation temperature was reached. The solution was then cooled down until it reached room temperature and filtered with a 0.2 μm syringe filter.
2.2. Experimental protocol
The filtration procedure and the laboratory pilot-plant were described in previous studies [19, 20]. Filtrations were performed with three tubular mono channel ultrafiltration titania membranes with a commercial cut-off of 1 kDa (M1, M2 and M3). The active layer of the membrane is globally negatively charged for pH higher than 4.1. In this work, all experiments were done with deionized water (pH around 6.0, ionic conductivity <1 μS∕cm). Temperature was maintained constant at 25 °C using a cooling system composed of a refrigeration system and heat exchanger. The applied flow rate was 700 L/h (feed velocity about 5 m/s, turbulent flow Re > 39,000) and the pressure varied from 4 to 12 bar. At each pressure, samples from retentate (solution in the feed tank) and permeate (solution that passed through the pores) were analyzed. VB12 was used as a neutral molecule to assess the steric hindrance assuming that the separation is only due to steric effect. The membrane hydraulic properties (water permeability) are calculated from pure water filtration tests.
2.3. Characterization techniques
2.3.1. UV-VIS spectroscopy
This technique was used to quantify the lysozyme after solution preparations and to determine the rejection rate of ultrafiltration membrane. Using the Lambert–Beer law and a calibration curve, LSZ quantification was performed measuring the absorbance in triplicate of protein solutions at 280 nm (Lambda 750, Perkin Elmer Instrument). On the basis of the obtained concentration, solution was diluted for enzymatic tests.
The rejection rate (R) was calculated based upon Equation (1) taking into account the absorbance of the permeate (Aperm) and the retentate (Aret). The absorbance was measured at 360 nm for VB12 and 280 nm for LSZ.
| (1) |
2.3.2. Size-Exclusion High Performance Liquid Chromatography analysis
For this analysis, 100 μL of LSZ were injected into the SEC-HPLC system (Agilent 1100 Series chain equipped with a UV detector and a quaternary pump) with a chromatographic 9.4 × 250 mm Zorbax Bio Series GF-250 column (range 400,000–4000 g⋅mol−1). The mobile phase was composed of phosphate buffer saline PBS (1 tablet in 200 mL deionized water), sodium dodecyl sulfate SDS (0.1 wt%) and sodium azide NaN3 (0.005 wt%). All reagents were purchased from Sigma-Aldrich. The measurements were made at a wavelength of 280 nm, constant temperature of 25 °C and a flow rate of 1.0 mL⋅min−1.
2.3.3. Antibacterial activity
The antibacterial activity of LSZ was determined by monitoring the decrease in turbidity of a Micrococcus Lysodeikticus suspension at 450 nm for 10 min with 15 s intervals. In a 96-well microplate, 20 μL of LSZ were put in contact with 200 μL of bacteria culture (0.3 mg⋅mL−1). The solutions were shaken for 30 s before measurements and incubated at 30 °C. The absorbance measurements were repeated 9 times at a wavelength of 450 nm. The reaction rate was estimated by the slope of 1∕A450 nm versus time graph (second order reaction between Micrococcus Lysodeikticus and LSZ). The activity of LSZ was determined by Equation (2).
| (2) |
An activity index was calculated using Equation (3) that normalizes the antibacterial activity of the sample with the one of the reference (untreated LSZ).
| (3) |
2.3.3.1. Statistical tests
Two-samples t-test were performed using Origin Pro 2021 with a 95% level of confidence and statistical significance at p < 0.05 to compare the native lysozyme with the filtered one.
3. Results and discussion
3.1. Study of membrane influence in native lysozyme antibacterial activity
In a previous study [21], the relationship between membrane hydraulic properties and LSZ antibacterial activity was evaluated using different membranes with the same commercial cut-off of 1 kDa. However, it was showed that size distribution of pores and real cut-off could be different and membranes with small and bigger pores were compared. Results indicated that the loss of activity was observed for the membrane with smaller pores due to the shear forces applied in the molecule during the filtration. When LSZ passed through small pores, three-dimensional structure is modified with consequently a loss of antibacterial action.
Based on these results, in the present investigation, other membranes were studied according to their permeability and selectivity determined by water and VB12 filtration, respectively. The values are presented in Table 1.
Membrane performances determined by VB12 filtration and pure water filtration
| Membrane | Rejection rate (%) | Permeation flux (10−14 m3⋅m−2⋅s−1) |
|---|---|---|
| M1 | 54 | 5.5 |
| M2 | 56 | 2.9 |
| M3 | 59 | 5.4 |
Regarding the selectivity, membranes M1, M2 and M3 presented similar values around 50% and it could be affirmed that they have smaller pores. To understand which parameter between rejection rate and permeation flux interferes more in the antibacterial activity, an enzymatic test was conducted for retentate and permeate solutions. As the retentate solutions are the ones that did not pass through the pores, in the previous work [21] no activity loss was observed and consequently all the retentate results are summarized in Figure 1 (transmembrane pressure = 0 bar).
Evolution of antibacterial activity index (geometric mean between measures of M1, M2 and M3) for permeate solutions of LSZ filtered under different applied pressures.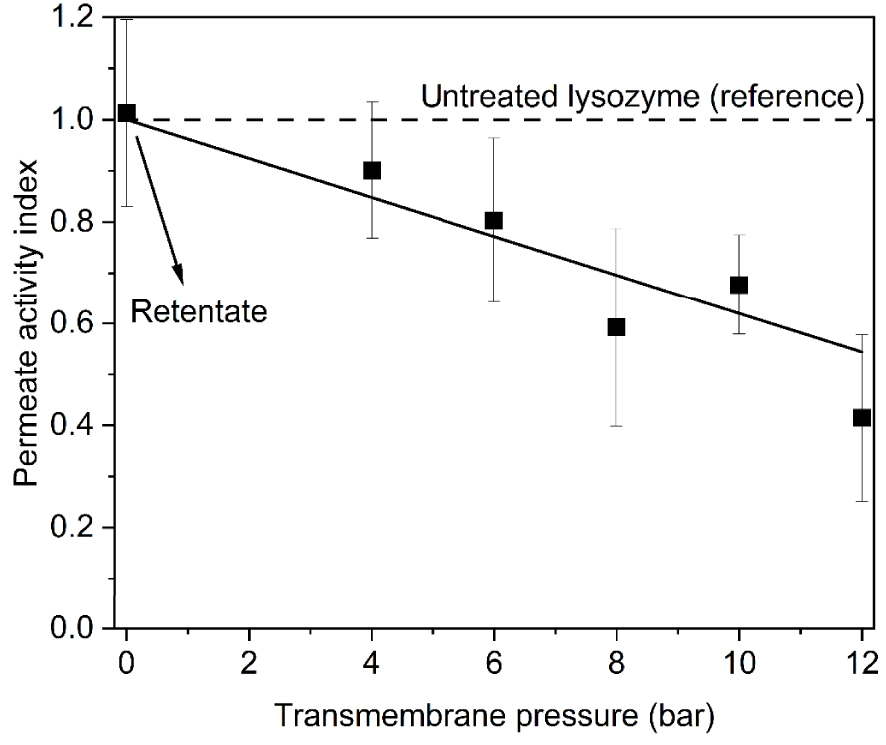
For the permeate solutions, the three membranes studied presented similar results of antibacterial activity decrease and Figure 1 shows the average between 27 measures (9 for each membrane).
The activity loss increased with applied pressure for the membranes with similar values of rejection rate. The maximum of activity loss was approximately 60% for 12 bar. In addition, a linear relationship between activity index and pressure was obtained in the studied range of applied pressure (IA u = −0.038𝛥P). The similarity between permeate results in these three membranes indicates that the parameter of the membrane that plays a significant role in antibacterial action is the pore size and so, the steric hindrance.
Taking this into account, membranes with VB12 selectivity bigger than 54% were used to evaluate the ultrafiltration of LSZ thermally treated to understand if the shear stress in the pores could change the activity of a LSZ that had already undergone a denaturation treatment (heating). Consequently, all the following experiments were conducted with one of the membranes.
3.2. Study of antibacterial activity of thermally modified LSZ and hydrodynamic properties
Antibacterial activity indexes were obtained for LSZ treated at 60, 70, 80 and 90 °C and the results are presented in Figure 2 compared to antibacterial activity of native LSZ at room temperature (reference). Considering the box plot average, until 70 °C no relevant change was observed compared with untreated LSZ (reference). For the treatment at 80 and 90 °C the antibacterial activity loss was around 20 and 40% respectively. The increase in activity loss with temperature is in agreement with the literature [15, 14, 18, 17]. Furthermore Xing et al. [16] proved using Raman spectroscopy and antibacterial activity assay that thermal denaturation is a three-state mechanism that starts from 74 °C, which is consistent with the absence of activity loss for LSZ treated at 70 °C (LSZ 70).
Antibacterial activity for lysozyme treated at different temperatures.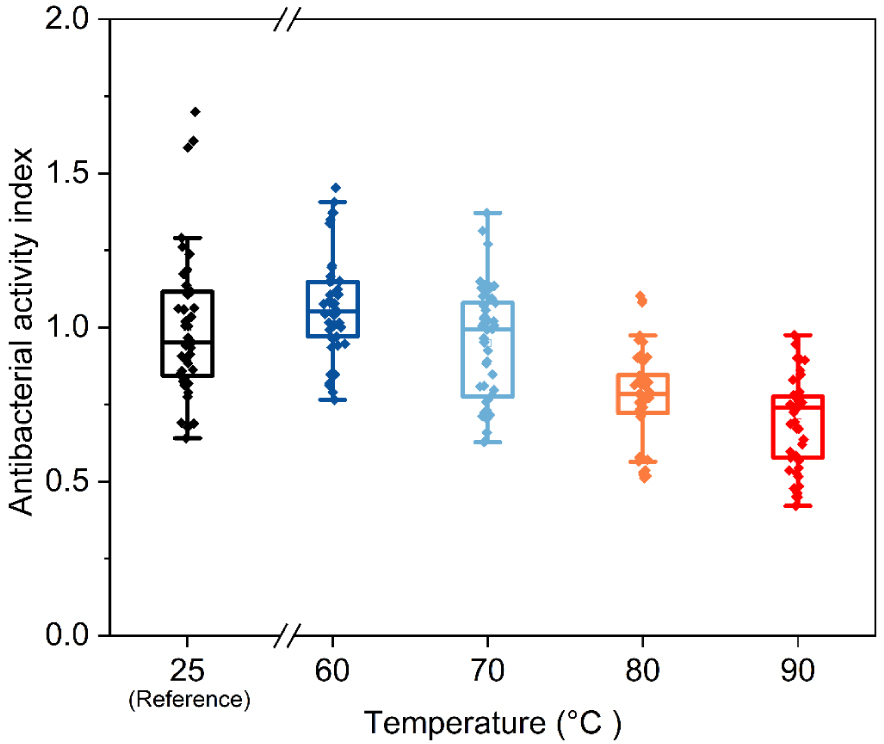
The mechanism of thermal denaturation states that during thermal treatment, LSZ tertiary structure changes prior to the secondary one. In the first stage (around 74 °C) the intermolecular interactions between the side groups are weakened forming an intermediate tertiary structure called molten state. It is estimated that the latter state persists in a 2 °C interval being followed by the secondary structure changes. Taking it into account, in the current work, temperatures of 70 and 90 °C were chosen to be compared in terms of biological activity before and after ultrafiltration.
Some works correlate the biological activity decrease with aggregate formation: the temperature increase induces soluble aggregate formation and when the protein is aggregated, the active site is less available to perform as an enzymatic antibacterial agent [14, 15]. To evaluate protein aggregation and/or changes in conformation, SEC-HPLC was performed and the chromatograms of LSZ thermally modified are shown in Figure 3.
SEC-HPLC chromatograms of lysozyme solutions thermally treated at 60, 70, 80 and 90 °C.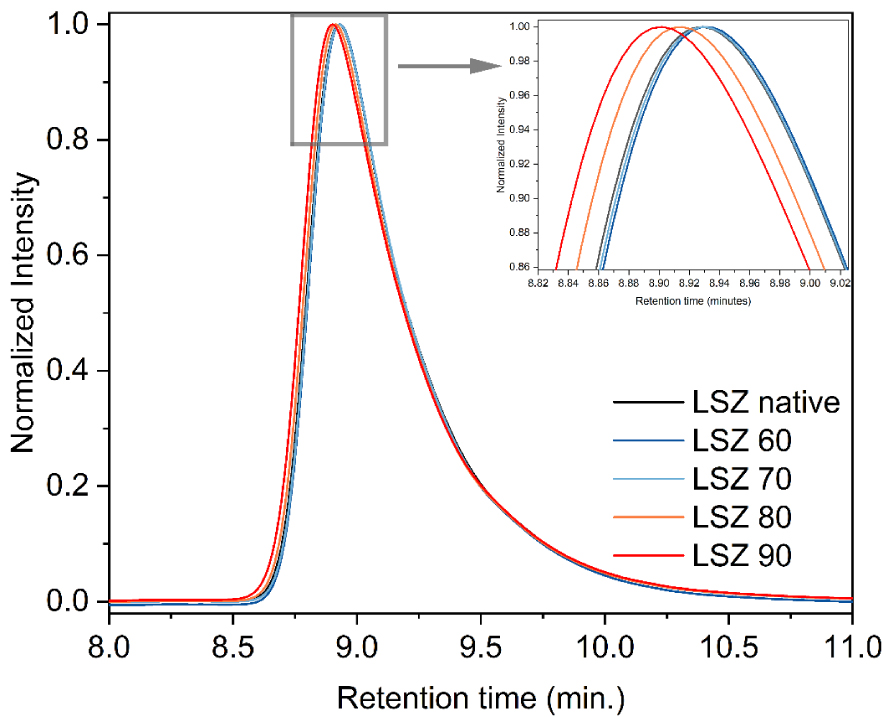
Superimposed chromatograms on Figure 3 show a monomeric peak without presence of oligomers or aggregates. Absence of soluble aggregates can be explained by the low concentration used in the present study (0.025 mM). Therefore, aggregation could not be an explanation for the activity loss in this case. A slight retention time shift is observed comparing the higher temperatures with the reference LSZ, LSZ 60 and LSZ 70 that have similar peaks. This shift for 90 and 80 °C indicates a modification of the hydrodynamic volume of LSZ with increasing temperature. This observation is in agreement with the literature that situates the lysozyme unfolding at temperatures higher than 70 °C [16, 18, 22]. Thus, antibacterial activity loss can be directly correlated with LSZ tertiary structure changes that probably modify the environment around the active site and as a consequence, decrease the antibacterial activity.
3.3. Ultrafiltration of LSZ thermally treated
Ultrafiltration was conducted on LSZ pre-treated at 70 and 90 °C varying the applied pressure from 4 to 12 bar to assess whether filtration can enhance the loss in biological activity of lysozyme. Both permeate and retentate were evaluated according to their hydrodynamic volume by SEC-HPLC and antibacterial activity.
The superposed chromatograms of the permeate and retentate for LSZ 70 are presented in Figure 4. Reference in this case is the LSZ treated at 70 °C before the filtration. Both permeate and retentate of LSZ 70 are characterized by one main asymmetric peak with tailing, corresponding to the non-fragmented lysozyme monomer. A second peak appears for the permeate at around 12 min, which can be associated with the presence of impurities. No soluble aggregates can be observed due to filtration (permeate sample) or temperature treatment as mentioned previously. After filtration however, there is an apparent slight shift in the retention time to higher values (around 0.07 min), which suggests that the molecule has a smaller hydrodynamic volume. This slight shift suggests a change in the hydrodynamic volume different from thermal denaturation at 80 and 90 °C. Smaller retention times were observed for these two cases, meaning that there was an increase in LSZ hydrodynamic volume.
Normalized response of the permeate, retentate and reference (untreated LSZ sample) for LSZ 70.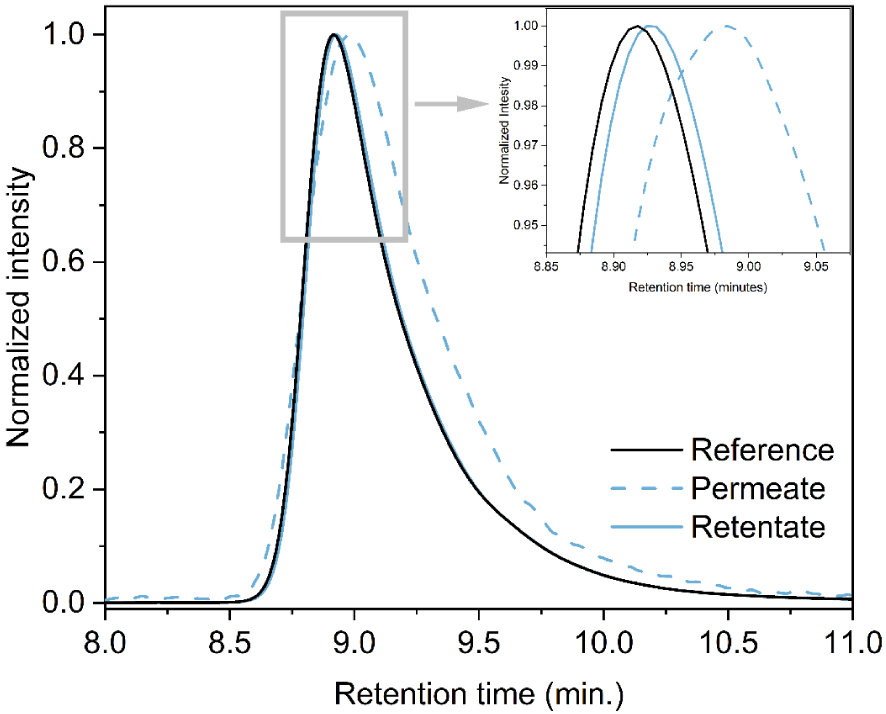
The difference observed in SEC-HPLC chromatograms for ultrafiltration (Figure 4) and thermal (Figure 3) treatments indicates two different denaturation mechanisms, where the LSZ treated at 70 °C had its structure changed after ultrafiltration and not due to heating.
In the current experimental conditions, protein adsorption in membrane pores was studied by an indirect method: the membrane properties (hydraulic permeability and selectivity) and the results are presented in Table 2.
Membrane properties before and after filtration of LSZ 70 and LSZ 90
| Robs (%) | Hydraulic permeability (10−14 m3⋅m−2) | |
|---|---|---|
| Filtration of LSZ 70 | ||
| Initial properties (VB12 filtration) | 57 | 5.5 |
| LSZ 70 | 98 | 4.8 |
| After filtration (VB12 filtration) | 72 | 4.7 |
| Filtration of LSZ 90 | ||
| Initial properties (VB12 filtration) | 47 | 5.0 |
| LSZ 90 | 95 | 2.9 |
| After filtration (VB12 filtration) | 75 | 3.1 |
The permeability loss after filtration of LSZ 70 is lower (14%) than for LSZ 90 (38%). Membrane selectivity increases by 15% for LSZ 70 versus 28% for LSZ 90. Hence, it seems that LSZ is adsorbed onto the membrane in a greater amount if it is pretreated at 90 °C, rather than 70 °C. When high temperatures are applied (superior to 75 °C), lysozyme is unfolded, exposing the hydrophobic groups to the aqueous environment thus facilitating adsorption onto surfaces [23]. In addition, as observed by HPLC (Figure 3), LSZ 90 was supposed to have a bigger hydraulic volume when compared to native and LSZ 70, which can explain this different behavior in membrane properties.
3.4. Study of antibacterial activity of thermally modified LSZ
Two parameters of ultrafiltration can be correlated with the antibacterial activity; the time-effect of the turbulent flow in the feed solution that is stirred during the test period (velocity of 700 L/h—Re ≈ 39,720) and the effect of shear stress in the pores (permeate flow for different transmembrane pressure). In this sense, retentate samples of LSZ 70 and LSZ 90 were studied and compared with LSZ native. All of the retentate samples presented a constant behavior during the test period, which means that stirring (turbulent flow) and surface contact with stainless steel material in tubing and feed tank did not induce any modification in lysozyme antibacterial activity.
Figure 5 shows permeate behavior according to the applied pressure for LSZ 70. The antibacterial activity of filtrated LSZ 70 decreases linearly with the increase of pressure (IA u = −0.035𝛥P) as observed before for filtration at room temperature. The slopes of the linear curves obtained at 25 and 70 °C are close (0.035 versus 0.038) indicating a probably identical denaturation mechanism. The difference of activity is statistically significant for 8, 10 and 12 bar compared to the reference. The loss for 8 bar is 23%, 37% for 10 bar and 42% for 12 bar. The decrease in activity can be correlated with the slight change in the LSZ hydrodynamic volume (Figure 4) to smaller size. In this case, the modifications could be in the amino acid environment (cleavages of bonds) [24, 25]. The results show that there is indeed a change in the structure of the lysozyme (structure is directly linked with the antibacterial activity) due to filtration and not due to the temperature treatment before filtration.
LSZ 70 antibacterial activity for permeate solutions as a function of applied pressure, § represents the significant difference for p < 0.05 with respect to LSZ reference (black line).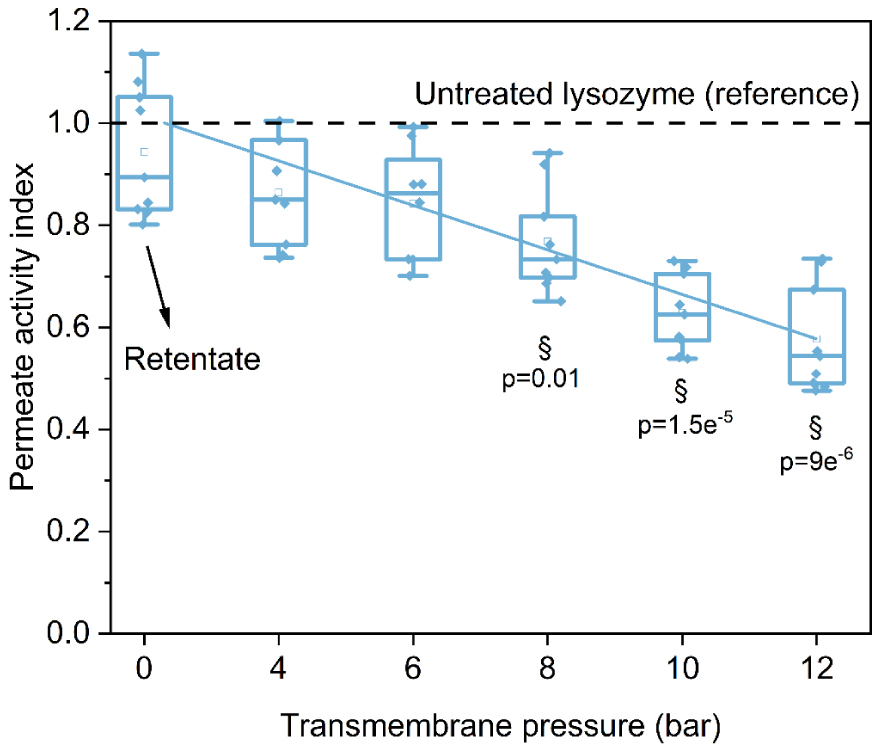
LSZ 90 shows first a decrease of antibacterial activity due to the temperature treatment (retentate sample Figure 6). Following the thermal treatment, lysozyme loses approximately 40% of its activity. Filtration of LSZ 90 does not further affect the antibacterial activity of the solution (Figure 6, permeate samples). The decrease in antibacterial activity is around 40% (same value as the retentate) regardless of the applied pressure. In the current case, the inactivation of the Micrococcus Lysodeikticus obtained with LSZ 90 is comparable to the one obtained with LSZ 70 filtered at around 10 bar (around 37%). However, there are differences in the hydrodynamic properties which confirm that different stress factors imprint different denaturation routes [26]. According to Ibrahim et al. [27] surpassing the denaturation temperature (72 °C) cleaves the disulfide bonds (characteristic of lysozyme’s structure) and exposes the tryptophan residues to the polar environment, which induces substantial conformational changes.
LSZ 90 antibacterial activity for permeate solutions as a function of applied pressure, LSZ reference (black line).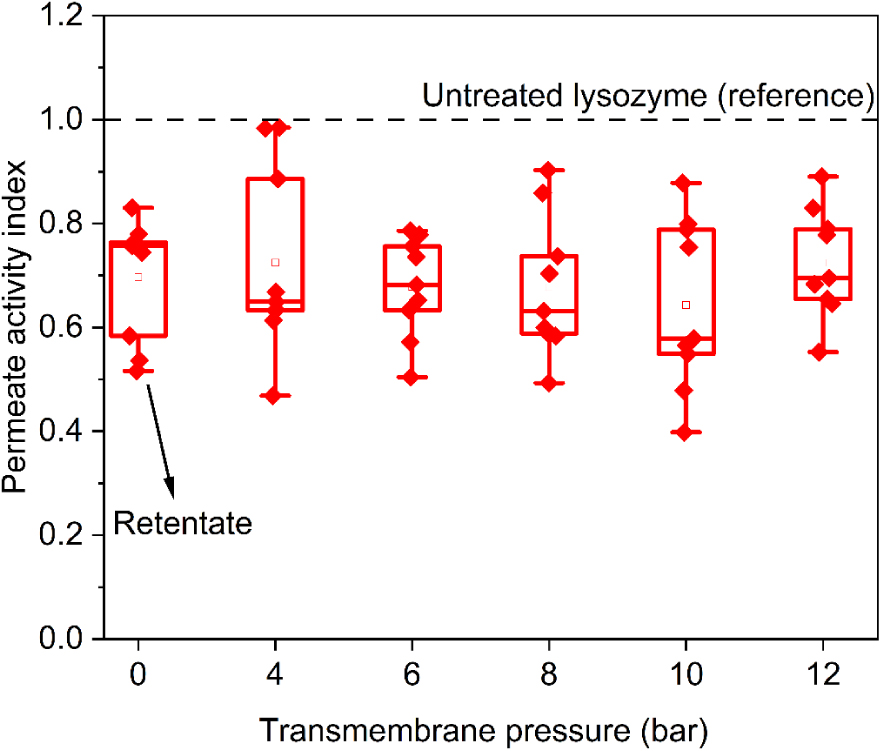
Overall, the changes in activity and in hydrodynamic properties suggest that filtrated lysozyme is further denatured by the filtration process, probably due to a combination of pore size, shear stress and protein-membrane interactions [28]. Nevertheless, the denaturation by filtration is produced to a different extent depending on the temperature used for the pre-treatment and the operational conditions.
4. Conclusion
The study focused on the hydrodynamic properties and on the antibacterial activity against Micrococcus Lysodeikticus of lysozyme denatured by heat and by a combination of heat and ultrafiltration operation. It was showed that antibacterial activity of heat-treated LSZ decreases in function of hydrodynamic volume modification as reported by SEC-HPLC. Depending on the temperature used for the pretreatment of LSZ, filtration imposes different degrees of change. By using a temperature of 70 °C, after filtration, the solution suffers a decrease in antibacterial activity similar to non-heated LSZ. The loss in antibacterial activity can be linearly correlated with an increase in pressure (in the range studied). On the other hand, by completely denaturing lysozyme (thermally treated at 90 °C), the antibacterial activity remains unchanged after filtration (same activity in the retentate and permeate) regardless of the applied pressure. Thus, the present study highlights the behavior of a model protein (LSZ) denatured by temperature or a combination of temperature and shear stress by using ultrafiltration.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0