1. Introduction
Effective waste management offers a variety of advantages, including the creation of a cleaner, greener environment, energy conservation, job opportunities, etc. Waste biomass has significant value-adding potential, but adopting these solutions in developing countries will require significant efforts [1].
Waste cooking oil (WCO) could be produced from domestic and industrial sources. This type of waste is generated by food premises, food industry, and households [2]. It is harmful to human health and environmentally unfriendly when it is improperly disposed in nature without undergoing any treatment. Oil and grease management is a significant challenge due to their unsustainable disposal issues, which can cause contamination and pollution of water and land resources [3]. However, due to careless and irresponsible practices and the absence of adequate legislation and its enforcement, majority of household WCO is inappropriately disposed. Inappropriate management of the WCO creates several socioeconomic and environmental problems. Open burning of used cooking oil and direct discharge into soil and water streams are the main inappropriate management methods for used cooking oil. According to the Environmental Protection Agency (EPA), WCO can cover and suffocate animals, plants, and their environment by causing oxygen depletion. WCO causes rancid odors, fouls shorelines, and clogs water treatment plants as well as household kitchen pipes [1, 2].
One of the solutions for recycling used cooking oil is its conversion into bioenergy, preventing competition from the use of edible oil. WCO has also recently become a leading product as a raw material for a variety of bio-based materials. On the other hand, WCO can also produce a wide range of added-value green chemicals such as lubricants, epoxies, soap, surfactants, polymers, plasticizers, etc [4, 5, 6].
The demand for energy has greatly increased, but the sources are limited, with most of the energy coming from fossil fuels [7]. The demand for energy is unpredictable due to the future development of technologies and demography. Environmental degradation is another problem associated with the burning of fossil fuels [8]. Thus, for the last decades, researchers had been conducting investigations looking for alternative fuels [9]. Previously, many researchers have suggested that biodiesel can become a promising fuel alternative to substitute petroleum-based diesel. Biodiesel is a fatty acid methyl ester (FAME) or a fatty acid ethyl ester (FAEE). It can be synthesized by transesterification of triglycerides with a monohydric alcohol in the presence of a catalyst in either an alkaline or an acidic environment [10].
Biodiesel is used due to its oxygenated, renewable, biodegradable, economically viable, environmentally friendly origin, high combustion efficiency, and reduced emissions [11]. According to researchers, biodiesel can reduce emissions of non-burnt hydrocarbons, carbon monoxide and particulates. In addition, this fuel contains no sulfur-containing elements, avoiding sulfur oxide emissions and sulfide production, and contains only 11% oxygen by weight [12]. It plays an important role in the reduction of environmental degradation, global warming, and climate change [13].
Compared to new oil, WCO has a high free fatty acid (FFA) content, which could retard the transesterification process and thus require more advanced transesterification conditions [14]. As this involves high material and energy intensity, mass transfer restrictions, high losses, waste generation, and water use, acid removal have generally the greatest economic impact during WCO pretreatment [15].
For example, when pretreatment by esterification is carried out by the addition of acids such as sulfuric acid, special precautions must be taken to avoid risks and corrosion. In addition, the reaction is carried out at high temperatures and generates a large amount of salt. Also, glycerolysis requires a high temperature of up to 200 °C, a long reaction time of 2 to 6 h, and has a slow reaction speed. In addition to the formation of process residues, adsorption pretreatment requires additional steps for the adsorbent preparation, such as high temperature calcinations [16, 17].
The transesterification reaction is the most used method to produce biodiesel from the WCO (Figure 1). It consists of reacting triacylglycerol from vegetable oils or animal fats with alcohol, usually methanol or ethanol, to produce methyl or ethyl esters known as biodiesel. It takes three moles of alcohol to react with a triglyceride (oil) to produce a fatty acid methyl or ethyl ester and glycerol as a by-product [18, 19, 20].
Transesterification reaction for biodiesel production from WCO.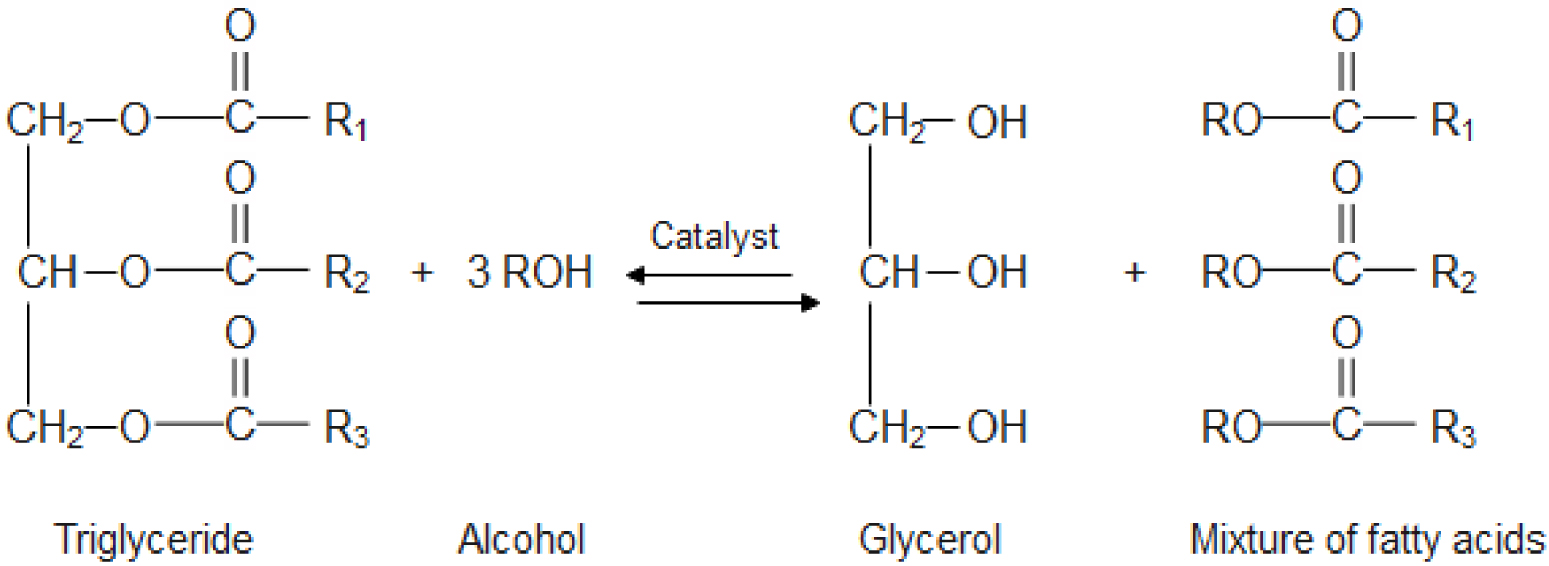
Some of the important parameters affecting the ester yield during the transesterification reaction are reaction time, alcohol to vegetable oil molar ratio, stirring rate, catalyst concentration, and reaction temperature [6, 9, 19, 21, 22].
The effect of alcohol type on the transesterification process revealed that methanol was the most suitable, with a faster reaction and good ester isolation [23]. The feedstock quality, mainly the FFA content and fatty acid composition, influences the properties of the biodiesel, The high amount of FFA in the oil results in a very low yield of biodiesel through the conventional base catalyst transesterification process [24].
Hence, the high FFA content of more than 2% tends to generate soap when it reacts with the catalyst for an alkaline transesterification reaction, so a pre-treatment process is necessary to reduce the FFA content present in waste cooking oils [3].
On the other hand, attractive and productive biodiesel sources are oil palm, beauty leaf tree, pongamia, Jatropha, coconut, sunflower, soy, rapeseed, jojoba, neem, moringa, cotton, rice bran, castor, and microalgae. The main raw materials are lipids of vegetable or animal origin [25].
During the frying process, the oil is heated in the range of 160 to 200 °C, and the transfer between the food and the oil reacts with moisture and oxygen repeatedly. It undergoes several degradation reactions, such as hydrolysis, oxidation, and polymerization, as well as many physicochemical changes [20]. The degradation of oil could be affected by the heat and mass transfer that occurred due to the nature of the fried food. Frying food in the presence of air and water triggers a series of interrelated reactions. It has been shown that the moisture and fat content, as well as the thermal conductivity of fried foods, are important factors causing the deterioration of oil [26].
Several types of pretreatment processes for waste cooking oil are available, such as steam injection, neutralization, vacuum evaporation, alcohol extraction, steam distillation, esterification reaction, and adsorption. Neutralization for deacidification has many limitations because glycerides can also be saponified by alkali, and separation of glycerides from the soap paste formed is then very difficult, resulting in a significant loss of glycerides. In addition, alcohol extraction for deacidification requires a large amount of solvent and several extraction stages due to the limited solubility of FFAs in alcohol. Steam distillation for deacidification requires high temperatures and consumes a large amount of energy with low efficiency. Studies have indicated that other pretreatment methods were also used to reduce FFA and water content in WCO, such as filtration and the use of a magnesium sulfate drying agent followed by vacuum filtration to remove any suspended matter and magnesium crystals [16]. Adsorption can be an alternative procedure to reduce or remove FFA from oils. Adsorbent treatment has the advantage of reducing oil loss and soap contamination [27]. There are several types of adsorbents, such as activated carbon, bleaching earth, montmorillonite, sepiolite, kaolinite, cristobalite, and bentonite [28]. Recently, there have been studies on the adsorption of FFA using anionic resins [20]. In addition, the mixture of activated carbon and silica gel can reduce the acid number by up to 53.9%. However, the most widely used method to remove FFA content from the oil is the esterification process with acid as a homogeneous catalyst, converting FFA to free fatty acid ester [29].
Several investigations have examined the treatment of waste oils in order to reduce their FFA content for their recovery in biodiesel. Few studies considering parametric studies and optimization of operating conditions for various processes of pretreatment, are presented.
Mueanmasa [30] studied the optimization of esterification of extracted oil from waste coffee grounds (WCGs) using response surface methodology (RSM), with an initial FFA of the WCGO of 16.5%. The results showed that the FFA percentage was 0.83 when the methanol to FFA ratio was 10, the amount of catalyst was 15 wt%, the reaction time was 115 min, and the reaction temperature was 65 °C. The FFA of WCGO was reduced by 94.97%.
Alptekin and Canakci [31] used as factors: sulfuric acid, hydrochloric acid, and sulfamic acid (amidosulphonic acid) as catalysts; methanol as the alcohol; and reaction temperature at 60 °C for the pretreatment of chicken fat. The used chicken fat had an acid value of 26.89 mg KOH/g oil, which corresponds to an FFA level of about 13.45%. The results showed that the FFA level of the chicken fat with 15% FFA may be reduced to below 1% when using 20% sulfuric acid and a methanol molar ratio of 40:1 during 60, 70, and 80 min at 60 °C.
Siow et al. [32] performed an optimization study of sulfuric acid-catalyzed esterification using response surface methodology (RSM) in order to reduce the high level of FFA content below to 1%. Hence, it could be suitably used for biodiesel production via alkali-catalyzed transesterification, when the acid value of mealworm oil was 21.57 mg KOH/g, which corresponded to a FFA level of about 10.84%.
Asri et al. [27] studied waste frying oil collected from a fast food restaurant serving California fried chicken (CFC) with a FFA % of 2.82. To reduce the FFA and water content of waste frying oil, various types of adsorbents were used, such as activated carbon, bleaching earth, and coconut coir (Cocos nucifera L.). Coconut coir was found to be the best one for reducing the FFA content of waste frying oil compared to the others (activated carbon, bleaching earth, or their mixture), and FFA % decreased to 0.31%.
Díaz and Brito [16] performed the FFA adsorption using an anion-exchange resin for reducing or removing FFA from oils. Oils with different acid values (AV) or FFA contents were used as raw materials; waste oil (AV = 2.13 mg KOH/g oil) from frying in a canteen and Jatropha curcas oil (AV = 6.62 mg KOH/g oil). After adsorption, the AV were 0.25 and 0.28, respectively. FFA adsorption is an easier method comparing to esterification since it can be carried out at a low temperature and does not require alcohol, reducing the costs of raw materials.
Miroud et al. [17] used two pretreatment methods: acid esterification (neutralization) and adsorption onto activated charcoal. The results showed that the two methods reduced the FFA to almost 50% of its initial value.
On the other hand, the transesterification process is used in biodiesel synthesis under the influence of many parameters, such as temperature, reaction time, catalyst concentration, and oil to alcohol molar ratio. Various researchers investigated this synthesis with and without pretreatment.
Siow et al. [32] applied esterification pretreatment in mealworm oil for reducing FFA% in order to increase biodiesel yield to 92%.
Díaz and Brito [16] studied adsorption as a treatment for reducing or removing FFA from oils prior to the transesterification reaction for biodiesel production, and they obtained biodiesel yields of 95 and 96% for waste oil from frying in a canteen and Jatropha curcas oil, respectively.
Alptekin and Canakci [33] studied the acid pretreatment (sulfuric, hydrochloric, and sulfamic acid) of chicken fat and transesterification reaction for producing biodiesel. The pretreatment was achieved in two steps. The results showed that the FFA level of the chicken fat with 15% FFA can be reduced to less than 1% when using 20% sulfuric acid and a methanol molar ratio of 40:1 for 60, 70, and 80 min at 60 °C, with a high ester yield obtained after the transesterification reaction with KOH. The methyl ester yield was 87.4%.
These methods used additional materials and chemicals and were often time-consuming, which affected the pretreatment cost and its impact on the environment, hence the total cost of the biodiesel production process from the WCO. The disregard for green chemistry’s guiding principles was the cause of all these drawbacks.
The principle of green chemistry recommends minimizing or eliminating hazardous materials, producing degradable products, preventing pollution and detecting problems before major emissions or accidents occur, and consuming less energy [34].
Recently, several researchers have proposed process intensification techniques to improve mixing, heat and mass transfer, and product separation. Examples are co-solvent, ultrasonic, and microwave heating methods; membrane reactors; reactive distillation; static mixers; and in situ biodiesel production [24].
Microwave ovens use a mechanism that involves the generation of electromagnetic radiation, which is absorbed by water molecules, sugars, and fats in food, causing their vibration and therefore generating heat. This mechanism is based on the interaction between electromagnetic radiation and the polar molecules in the food, which converts electrical energy into high-frequency electromagnetic waves [35].
Considerable attention has been paid to microwave heating techniques in biodiesel production from a variety of renewable feedstocks in batch mode. The interaction of microwaves with the reactants (triglycerides and methanol) to accelerate the transesterification reaction is already well-established, with a 95% yield obtained in a short reaction time [28].
The term “sonochemistry” is frequently used to describe the application of ultrasound to chemical reactions and processes. Ultrasonication is the application of sound waves to produce energy; this frequency is above the range of human hearing, 20 kHz. Low-frequency ultrasound is typically used in process intensification since it helps to maximize the physical effects (such as acoustic microstreaming, microturbulence, shock waves, and shear forces) by improving the mass transfer [36]. Ultrasonic irradiation produces cavitation in the sample liquid. The effect of cavitation produces bubbles in consecutive cycles of compression and rarefaction. Then, the bubbles collapse creates spatially resolved regions of extreme excitation as well as concomitant shock waves that may lead to nucleation in regions of supersaturated solution [37].
However, to the best of the authors’ knowledge, MW and US have not been used as pretreatment without any chemical additives, to reduce the FFA content of WCO in order to be used for biodiesel production.
In this study, a physical method is proposed and optimized with respect to conditions that allow reducing FFA without chemicals or the generation of residues. The goal of this work is the application of an intensification process to reduce the FFA content of frying oil and to improve the transesterification reaction in order to produce biodiesel with a good yield and minimal impact on the environment.
2. Methodology
The processes involved in this experiment were intensification pretreatment by microwave and ultrasonic irradiation, indices determination, analysis and transesterification reaction for biodiesel production. All chemical reagents used were of analytical grade and were used without any purification or treatments. The chemicals involved were methanol and ethanol (purchased from Sigma Aldrich), phenolphthalein, sodium hydroxide and HCl (purchased from Biochem), including collected waste cooking oil (100% soybean).
2.1. WCO collection and characterization
A sample of WCO used for frying was collected from the university restaurant at Constantine 3. The recovered oil was filtered through Whatman filter paper in order to remove food residues prior to its use for the experiments.
The WCO was first characterized in terms of acid and saponification values with titration methods and physical properties such as density measured by a Mettler Toledo hydrometer, the refractive index measured by a refractometer, pH measured by a Hanna Instruments pH meter, and humidity measured by a Denver Instrument.
The used cooking oil sample was evaluated using the standard titration method to determine the characteristics before and after pretreatment.
2.1.1. Acid value (AV)
The acid value represents the percentage of FFA in the oil after conversion by an appropriate factor [32]. It is defined as the amount of potassium hydroxide (KOH) needed to neutralize the FFA in 1 g of fat. It is used to determine the content of fatty acids in the oil.
A volume of 10 ml of methanol alcohol and phenolphthalein were used as solvent and indicator, respectively and mixed with 1 g of oil sample. The solution was titrated with a 0.1 M KOH solution [19]. The amount of FFA present in the sample defines the acid value (AV) in mg KOH/g [38]. The acid value and the FFA percentage can be calculated using the equations below [3]:
| (1) |
MWKOH: Molecular weight of potassium hydroxide (g/mol).
VKOH: The volume of KOH needed to neutralize the FFA (ml).
CKOH: Normality of the KOH solution (0.1 M).
Wreal: Weight of the sample (g) [39].
Where FFA% [39] is calculated using:
| (2) |
2.1.2. Saponification value (SV)
The saponification value (SV) represents the mass (in mg) of KOH required to saponify 1 g of fat [32]. It describes the total amount of saponifiable oil units per unit weight of oil. A relatively high SV indicates the existence of a large amount of low molecular weight fatty acid chain. A higher SV of crude oil shows the existence of a high proportion of fatty acids that can guide soap creation throughout the transesterification process [38]. A volume of 10 ml of 0.5 M ethanolic KOH solution and a few drops of phenolphthalein were used as solvent and indicator, respectively. The mixture of oil and solvent was heated up to boiling for 30 min. After cooling, the mixture was titrated with a 0.5 M HCl solution until the pink color disappeared. The saponification value can be calculated using the following equations:
| (3) |
V′: Volume of acid required for blank test (with acid and base only) (mL).
V : Volume of acid required to neutralize sample (mL).
MWKOH: The molecular weight of KOH (g/mol).
CHCl: The concentration of hydrochloric solution (0.5 M).
Wreal: Weight of the sample (g).
2.1.3. Determination of FFA recovery (%)
The efficiency of FFA recovery (%) was determined as an increase in FFA content in the WCO sample. The equation can be used to calculate the FFA recovery (%):
| (4) |
2.1.4. Environmental factor (E-Factor)
The first principle of green chemistry, known as the prevention principle, can be quantified by the E-Factor (or environmental factor of Sheldon) [34]. The E-factor is a measure of the actual waste generated during a process, which is defined as everything other than the desired product. It includes reagents, solvent losses, process aids, fuel, and the chemical yield (energy input). Since it includes all processes and water, it can frequently result in exceptionally high E-factors and makes meaningful process comparisons challenging. However, water is typically removed from the E-factor [40]. In simple terms, it is the total mass of raw materials minus the total mass of the product, all divided by the total mass of the product, defined as early as 1992 as follows [34]:
| (5) |
2.1.5. Energy consumption
In order to compare the energy consumption of each pretreatment method, the energy corresponding to each method was calculated as follows [40, 41]:
| (6) |
2.2. Sample analysis
2.2.1. FTIR
Infrared spectroscopy is a rapid method for characterizing the functional groups and major components of different samples. Fourier transform infrared spectroscopy (FTIR) is used to predict the structural and chemical binding (targeting functional groups) between different molecules in the samples. The principle of FTIR spectroscopy is to send IR radiation onto a sample to be tested. Certain wavelengths are then absorbed by the chemical bonds of the molecules in the sample. We then generate an FTIR spectrum, which permits determining these chemical bonds by detecting the transmission according to their wavelengths. The FTIR spectra were recorded using the infrared spectrophotometer JASCO, in the range of 0–4500 cm−1 at a resolution of [42].
2.3. Intensification pretreatment methods
2.3.1. Microwave pretreatment
The microwave used in this work is of the Condor type with a frequency of 2450 MHz, a maximum power of 800 W, and a range of 10 to 100%. A mass of 50 g of frying oil (100% soybean) at an initial temperature of 18 °C was put in a closed Erlenmeyer in the microwave for a pretreatment time of 20 s to 2 min, without agitation or adding chemicals. After the set time, sample temperature, acid value and saponification value were measured.
2.3.2. Ultrasonic pretreatment
In this method, the laboratory ultrasonic bath has a 24 MHz frequency and varies in time and temperature. A 50 g sample of frying oil (100% soybean) with a temperature of 18 °C was placed in a closed Erlenmeyer in an ultrasonic bath for temperatures ranging from 20 to 40 °C and a pretreatment time of 5 to 40 min with no agitation or chemical addition. After the residence time, the sample temperature, acid number and saponification number were measured for each sample.
2.4. Production of Biodiesel from pretreated WCO
In this study, biodiesel was made from pretreated WCO via a transesterification reaction, with 50 g of pretreated oil used at the optimal conditions obtained by the conventional method mentioned in a previous study [6]. In brief, the conditions of the reaction were; KOH catalyst concentration of 2 wt%, a methanol to oil molar ratio of about 4.73 and a minimum temperature of 45 °C. The mixture was carried out in a reflux assembly for 30 min. The reacting mixture was stirred during the heating to ensure a good mixture. After the reaction, the content was transferred to a separating funnel for separation and allowed to settle in two phases. From the separating funnel, the glycerin layer was removed, fatty methyl ester was collected, and then FAME (fatty acid methyl ester) was washed with distillate water to remove excess residual FFA. Finally, the sample was dried to remove excess methanol and catalyst from the reaction mixture. The yield was calculated using Equation (7) [28] and the transesterification reaction stages are shown in Figure 2.
| (7) |
3. Results and discussion
3.1. Oil characterization
Table 1 shows the measured characteristic parameters of WCO after drying as well as the range of Codex norms for soybean oil.
Characterization of WCO and dried WCO and the CODEX norms
| Parameters | WCO | Dried WCO | Codex norms for Soybean oil |
|---|---|---|---|
| AV (mg KOH/g) | 4.48 | 3.268 | 0.6 max |
| FAA% | 2.24 | 1.634 | - |
| SV (mg KOH/g) | 188.5 | 186.618 | 189–195 |
| RI at 20 °C | 1.473 | 1.47 | 1.466–1.470 (40 °C) |
| pH | 5–6 | 5–6 | - |
| Density (g/cm3) at 20 °C | 0.920 | 0.920 | 0.919–0.925 |
| Humidity (%) | 0.02 | - | - |
The waste and dried cooking oil densities were similar at 0.92 g/cm3 and were within the Codex norms range. Due to the heating process during cooking and the contact with food as well as cooling to which the oil was subjected, the FFA content generally increased with the used quantity of WCO [43]. The acid value of the WCO was 4.48 mg KOH/g oil, over seven times the maximum established limit of 0.6 mg KOH/g so that the oil could be consumed. On the other hand, it was necessary to decrease the FFA to less than 2% to be considered an acceptable feedstock for biodiesel production with important yields, in which, if the percentage of FFA is more than 2%, the WCO needs to undergo a pretreatment process for a reduced acidity value [3].
As the saponification value for all the analyzed oils was lower than the ones reported in the Codex range, The literature mentioned that the higher the saponification value, the higher the number of carboxyl groups present in the oil or fat [32].
Note that the drying of the oil reduces the acidity value from 4.48 to 3.27 and saponification value from 188.5 to 186.6 before the application of the pre-treatment, i.e. a reduction of 27% of FFA and 0.99% for saponifiable compounds in the WCO.
3.2. Pretreatment of waste oil by microwave
The experiments are investigated in a Condor type laboratory microwave and the various pre-treatment steps are as follows:
3.2.1. Effect of power and temperature
In this part the power and time of the microwave were varied and the indices for each case were measured. The results are presented in Table 2.
Final temperature, acid and saponification values at different powers and residence time in the microwave oven
| P (W) | 100 | 240 | 400 | 800 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t (s) | Tfin | AV | SV | Tfin | AV | SV | Tfin | AV | SV | Tfin | AV | SV |
| 20 | 22 | 2.239 | 184.71 | 24 | 1.878 | 171.60 | 31 | 2.71 | 187.8 | 36 | 1.93 | 179.70 |
| 30 | 22 | 1.679 | 182.09 | 27 | 1.568 | 169.60 | 31 | 2.20 | 185.84 | 40 | 1.36 | 176.82 |
| 40 | 22 | 1.387 | 179 | 32 | 1.342 | 172.12 | 32 | 1.65 | 185.12 | 51 | 1.06 | 176.47 |
| 50 | 22 | 1.056 | 178.6 | 32 | 1.394 | 171.96 | 36 | 1.39 | 173.17 | 63 | 1.11 | 171.8 |
| 60 | 24 | 1.853 | 179.85 | 34 | 1.116 | 169.66 | 39 | 1.96 | 159.54 | 68 | 1.68 | 162.57 |
| 120 | 30 | 1.903 | 198.81 | 44 | 1.669 | 146.92 | 50 | 1.93 | 206.50 | 90 | 1.95 | 167.43 |
According to the obtained results, the acid value decreased with the microwave pretreatment time and then increased indicating an optimum for each power (Figure 3). The best values were obtained for times of 50 s at 100 W and 40 s at 800 W while the sample temperature rose from 22 to 51 °C from the first to the second conditions. Emma Chiavaro et al. [44], showed that for high-oleic sunflower oil with an initial FFA percentage of 0.17, decreasing to 0.14% at 3 min. On the other hand, the FFA % of other oils increased in proportion to the microwave heated duration. Hence, the effect of heated microwave treatment depends not only on their specifications (power and time) but also on the type of oil and their chemical composition.
Effect of microwave power for the acidity value of WCO.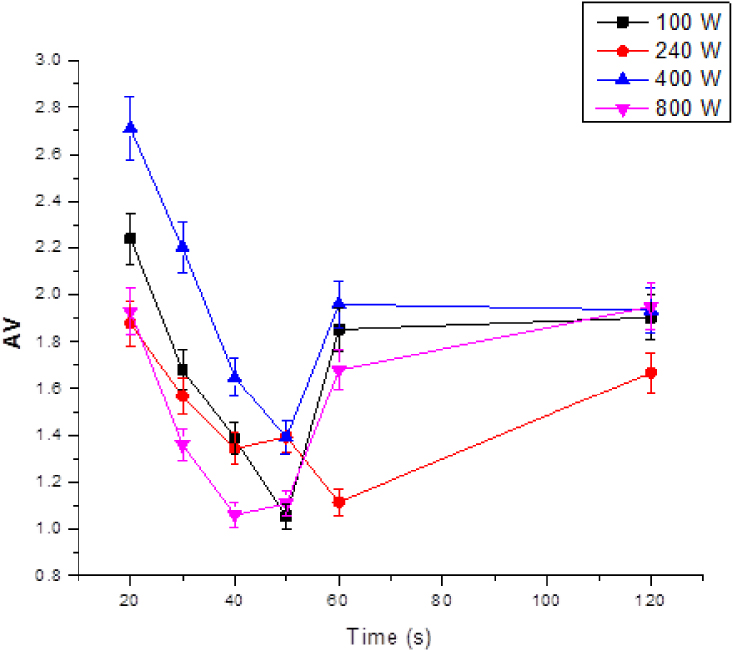
Borges et al. [45] demonstrated that soybean oil was not regularly affected by the increasing exposition time, even at 15 min. The 0.75% FFA of the treated oil decreased to 0.70% at 1 min, then, increased to 0.72 and 0.73% at 3 and 5 min, respectively, then decreased back to 0.72% at 10 min and finally increased to 0.75% at 15 min. For soybean oil, no correlation was observed between FFA % values and the exposure heating time.
The saponification index also decreased and remained within the normal values of the Codex Alimentarius. Saponification value represents all the saponifiable compounds present in the oil, so the reduction of FFA by reducing the acidity value leads to a reduction in the saponification value.
The results show that for each power there was an optimal residence time for maximum AV reduction.
For microwave pretreatment at a power of 800 W for a duration of 40 s, the acidity value went from a value of 4.48 to values of 1.06 (without drying) and 1.12 (with drying) corresponding to 76.34 and 75% reductions of the free fatty acid content, respectively.
3.3. Ultrasonic pretreatment of WCO
The pre-treatment process in this case was carried out in an ultrasonic bath containing water. The results are shown in Table 3.
Final temperature, acid and saponification values at different initial temperature and residence time in the ultrasonic bath
| T (°C) | 20 | 30 | 40 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| t (min) | Tfin (°C) | AV | SV | Tfin | AV | SV | Tfin | AV | SV |
| 5 | 20 | 2.756 | 176.57 | 31 | 2.185 | 182.98 | 43 | 2.175 | 192.14 |
| 10 | 21 | 2.767 | 176.41 | 36 | 1.868 | 175.97 | 43 | 1.884 | 185.88 |
| 20 | 21 | 2.713 | 182.1 | 39 | 1.339 | 175.81 | 44 | 1.104 | 185.27 |
| 30 | 21 | 2.468 | 172.51 | 39 | 1.399 | 139.79 | 46 | 1.014 | 185.36 |
| 40 | 40 | 1.619 | 204.27 | 45 | 1.605 | 212.23 | 50 | 1.354 | 204.97 |
| 50 | 41 | 1.542 | 192.02 | 48 | 1.625 | 191.7 | 58 | 1.392 | 205.99 |
From Figure 4, it can be seen that the acid value decreased with the time of pretreatment with the ultrasonic bath at different temperatures of 20, 30 and 40 °C. It was observed that at 20 °C the acidity value decreased down to 1.542 mg KOH/g during 50 min. On the other hand, for the other temperatures of 30 and 40 °C there was another increase, indicating the presence of an optimum at 20 min with an acidity value of 1.399 mg KOH/g and at 30 min with an acidity value of 1.014 mg KOH/g respectively. Fluctuations in the values of the saponification index for the different temperature levels could be noted.
Effect of ultrasonic on the acidity value of WCO.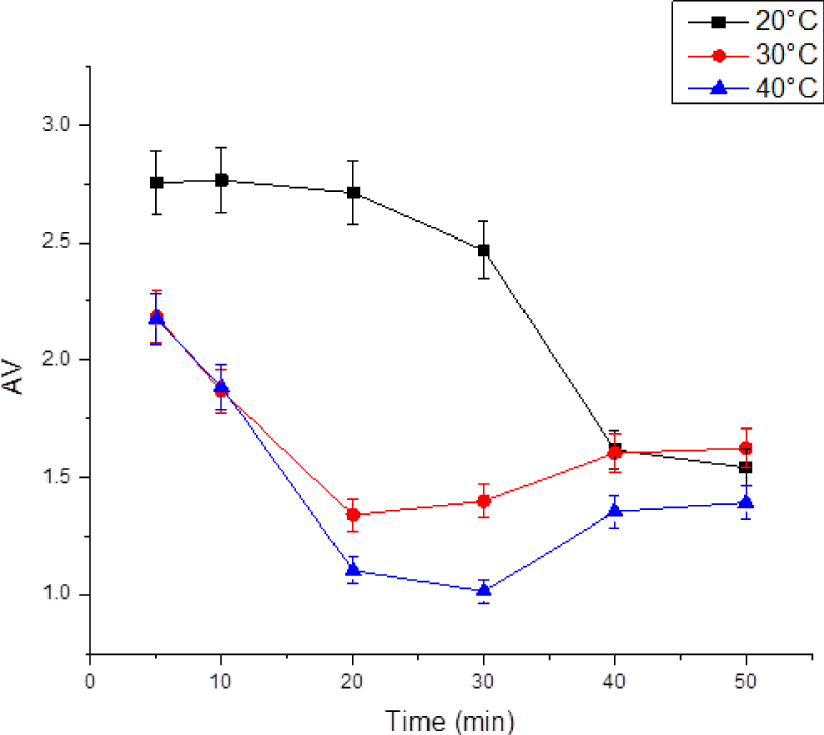
In comparison with previous pretreatment work using conventional methods (esterification and adsorption), the pretreatment of the filtered WCO by activated coal adsorption was carried out by adding 10% by mass of activated carbon and stirring at 300 rpm for 30 min at a temperature of 55 °C. The acid number was reduced by 17%. The pretreatment by esterification was carried out by adding more than 50% by volume of methanol and 1% sulfuric acid at 55 °C for about an hour. The content of FFA was reduced by 26%. The optimum of our study for microwave pre-treatment was achieved with a power of 800 W/40 s. The acidity value was reduced by 76.34%. On the other hand, the ultrasonic pre-treatment was applied with a power of 120 W for 30 min and the acidity value was reduced by 74.55%.
3.4. Fourier transform infrared spectroscopy (FTIR) analysis of samples
The fresh soybean oil, the WCO and pretreated cooking oil with microwave and ultrasonic bath were analyzed. Figure 5 shows the spectra of four oils, the various functional groups relating to the peaks in the spectrum (cm-1) at corresponding wave numbers are identified and tabulated in Table 4.
Fourier transform infrared spectroscopy (FTIR) analysis of samples.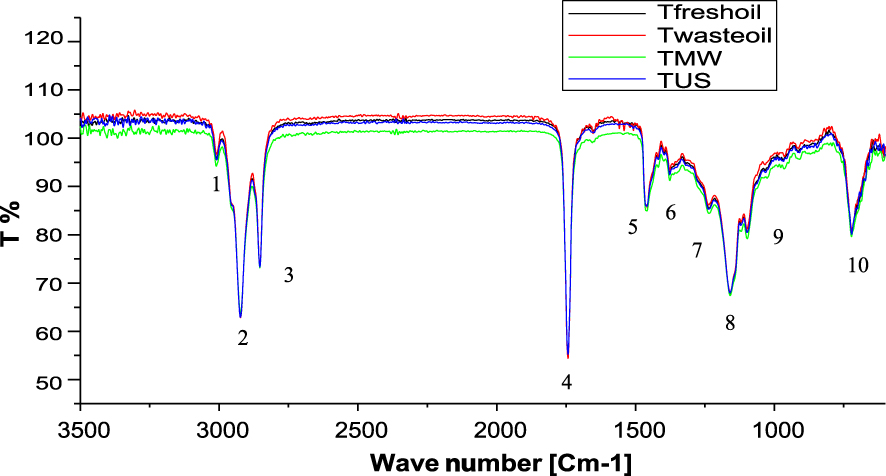
Descriptions of wave number observed from FTIR analysis between current and literature values [42, 46, 47]
| Wave number (cm-1) (current study) | ID | Ref. wave number (cm-1) | Description |
|---|---|---|---|
| 3003.6–3010.5 | 1 | 2989–3029 | C–H Stretching vibration of cis-double bond (=CH) |
| 2919.7–2926.5 | 2 | 2850–2970 | C–H Stretching (asym)–C–H (CH2) |
| 2850.3–2853.2 | 3 | C–H Stretching (sym)–C–H (CH2) | |
| 1743.3–1746.2 | 4 | 1690–1760 | C=O Carbonyl group Stretching |
| 1455–1463 | 5 | 1340–1470 | C–H Alkane Bending (scissoring)–C–H (CH2) |
| 1373.1–1375.9 | 6 | C–H Alkane Bending (sym)–C–H (CH3) | |
| 1232–1240 | 7 | 1050–1300 | C–O Stretching |
| 1154– 1163.8 | 8 | ||
| 1086.7–1100 | 9 | ||
| 719.3–723.2 | 10 | 690–900 | C–H Aromatic Rocking –(CH2)n– |
Significant information on the oxidative status of oils can be obtained by studying the intensity and absorbance values of many bands of the infrared spectrum [48]. An increase or decrease in some of the wave number regions was observed in this study for the comparison between the fresh soybean oil, waste soybean oil and treated oils with intensified methods. Table 4 shows the most significant bands: band near 3009 cm-1 due to the cis double-bond stretching vibration; bands near 2926 cm-1 due to the asymmetric and symmetric stretching vibration of the aliphatic CH2 functional group; band at 1743 to 1746 cm-1 due to the ester carbonyl functional group of the triglycerides; band near 1455 cm-1 due to the bending vibrations of CH2 aliphatic groups; band at 1373 to 1376 cm-1 due to bending vibrations of CH3 groups; band at 1050–1300 cm-1 associated with the stretching vibration of the C–O ester groups. Finally, the band near 720 cm-1 due to the C–H Aromatic Rocking –(CH2)n–.
The FTIR spectra of the different oil samples show that there is a remarkable decrease in the band 3010 cm-1 for fresh oil to 3004 cm-1 for the used oil. Thus, the value of this frequency in the non-oxidised soybean oil samples is shown in [49] with an absorbance of 3007 cm-1. The intensity of this band is increased to around 3005 cm-1 for oils pretreated by microwave and ultrasound. Then, a small increase in the band at 1745 cm-1 to 1746 cm-1 for fresh soybean oil and waste oil respectively corresponds to the carbonylic compounds (Table 4) generated from the hydroperoxide decompositions during the heating [48]; the intensity of it tended to increase with oxidative treatment and formation of secondary oxidation products [50]. This band is decreased to 1741 cm-1 for oil treated by microwave and ultrasonic bath.
It should be noted that these pretreatment methods are significant for oil used for frying with a low acidity assay. On the other hand, as the previous work mentioned, there is a change through the heating of oil for 10 min in a microwave.
3.5. Comparison of conventional biodiesel with pretreated cooking oil
The key response in terms of biodiesel economics is the yield of biodiesel production. Figure 6 shows the yield obtained for the transesterification reaction without pretreatment is 94%. We notice an increase in yield for the microwave pretreatment to 96% and it is increased to 98% for the frying oil pretreatment with ultrasound, which is a better result compared to some previous work that applied methods using additional components and generated waste (Díaz and Brito [16] studied the adsorption pretreatment and they obtained biodiesel yield with 95 and 96% for WCO in a canteen and Jatropha curcas oil respectively).
Biodiesel yield for different process: (a) no pretreatment; (b) MW pretreatment; (c) US pretreatment.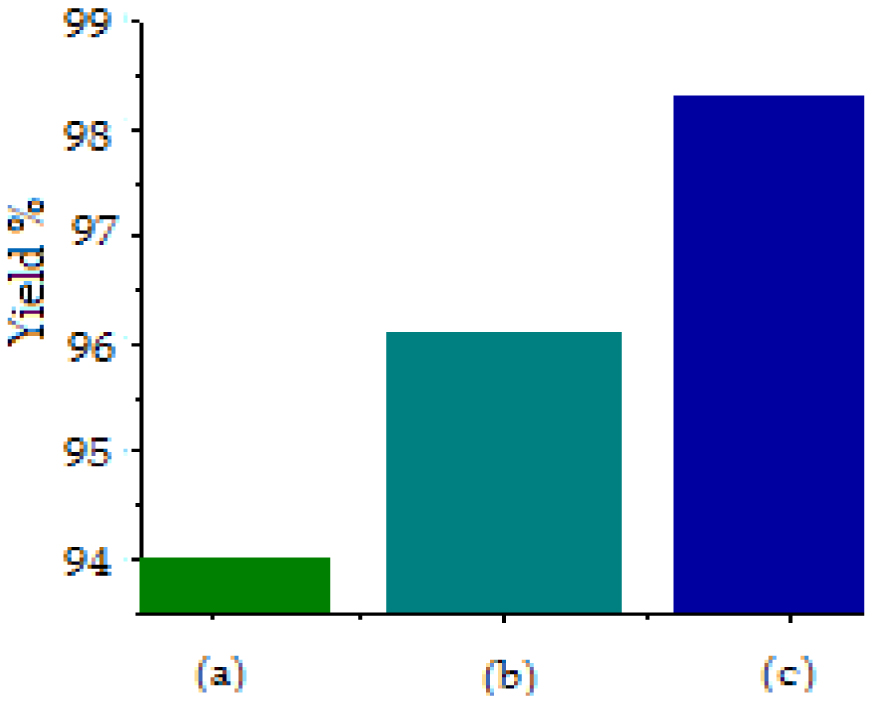
3.6. Quality of produced biodiesel
The biodiesel produced by using pretreated oil was tested for various physicochemical properties and compared with ASTM standards as shown in Table 5.
A comparison of the physicochemical properties of the biodiesel produced to international standards
| Properties | Conventional biodiesel | Microwave pret biodiesel | Ultrasonic pret biodiesel | ASTM norms of biodiesel [51] |
|---|---|---|---|---|
| Density at 15 °C (g/cm3) | 0.8884 | 0.8865 | 0.8856 | 0.875–0.900 |
| Kinematic viscosity (mm2/s) | 6.89 | 3.98 | 4.28 | 1.9–6.0 |
| Cloud point °C | −1 | −1 | −1 | −3–−12 |
| Flash point °C | >130 | >130 | 166 | 100–170 |
| Pour point °C | −4 | −3 | −3 | −15–−10 |
| Cetane number | 51 | 53.95 | 56 | 48–65 |
| Refractive index | 1.45679 | 1.45626 | 1.45348 | - |
- It can be seen from these results that the majority of the properties of the biodiesel produced from the oil pre-treated with microwave and ultrasonic methods are within the ASTM standard, such as density, viscosity, flash point, and cetane number.
- The cloud point and the pour point are not within the standards. Such value is due to the presence of small droplets of wash water influencing these two points.
- The refractive index also changes according to the pre-treatments, and the lowest refractive index is obtained for biodiesel produced from oil pre-treated with ultrasonic methods.
3.7. Green chemistry
Green chemistry is the conception of chemical products and processes that use and generate less hazardous substances, reduce pollution and waste, encourage the use of renewable feedstock, lead to safer designs, optimize materials and energy usage, and minimize costs [52]. The importance of generating as little waste as possible has emphasized the application of sustainable development and green chemistry principles [53]. In this study, the environmental factor was calculated since there was no reaction in the pretreatment process. The calculated environmental factor for microwave and ultrasonic bath methods was compared to the conventional pretreatment method used in the previous work (esterification and adsorption pretreatment) [17], and the results are shown in Figure 7.
E-Factor of pretreatment of WCO for conventional and non conventional methods: (a) US; (b) MW; (c) Adsorption; (d) Esterification.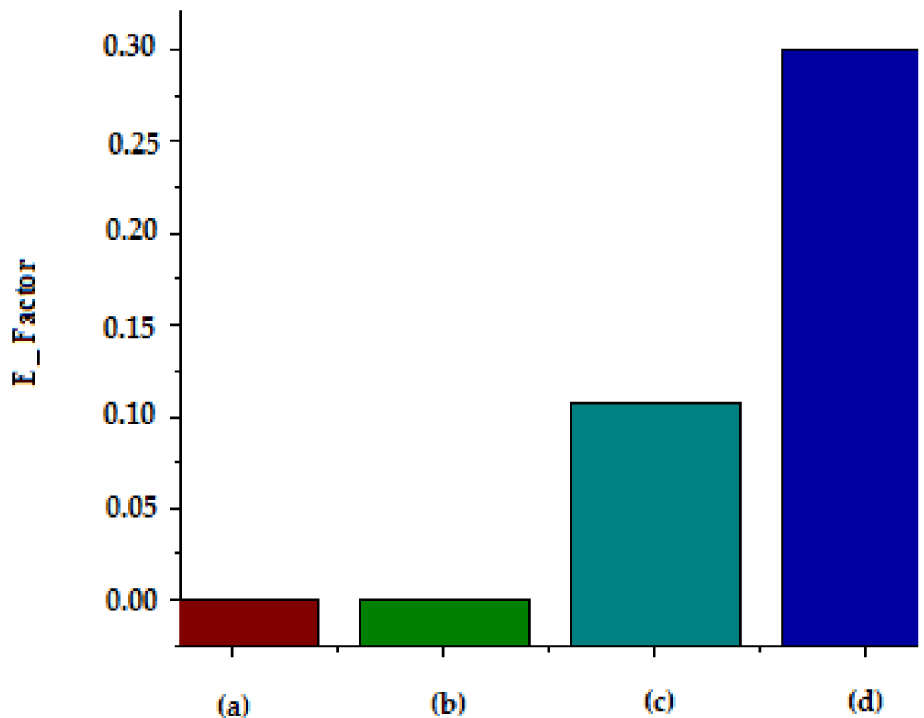
In addition, the time and the consumed energy are important factors for an economic consideration of the process and were also calculated in order to compare the present pretreatment with a previous adsorption and esterification [17] that used additional material to perform AV reduction [13].
As shown in Figures 8 and 9, increased times of pretreatment led to an increase in energy consumption for each method. Conventional heating on a laboratory hot plate for esterification pretreatment required about 1800 kJ of energy for 3600 s whereas the adsorption pretreatment required about 900 kJ. While microwave and ultrasonic processes required 32 and 216 kJ of energy with residence time of 40 s and 1800 s respectively. These results showed that non-conventional heating and mixing (process intensification) techniques had the potential to reduce the process energy requirements significantly.
Time of pretreatment of WCO for conventional and intensified methods: (a) US; (b) MW; (c) Adsorption; (d) Esterification.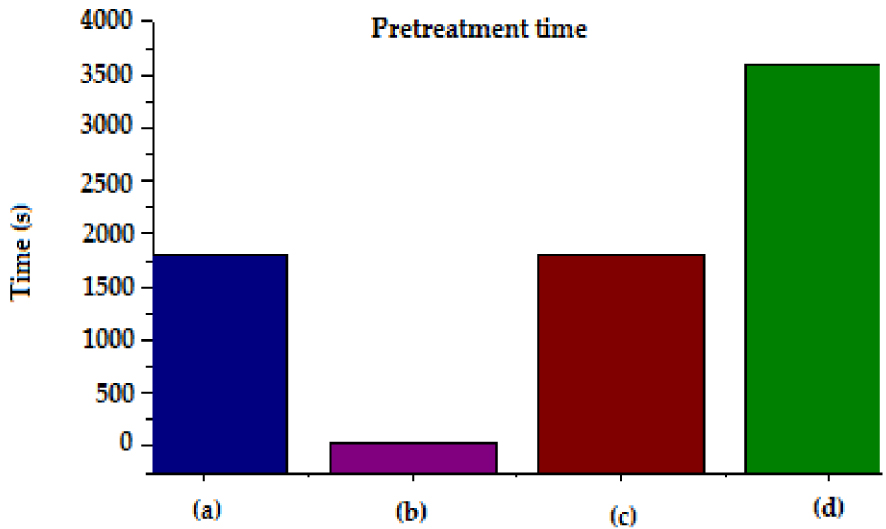
Consumed energies of pretreatment of WCO for conventional and non conventional methods: (a) US; (b) MW; (c) Adsorption; (d) Esterification.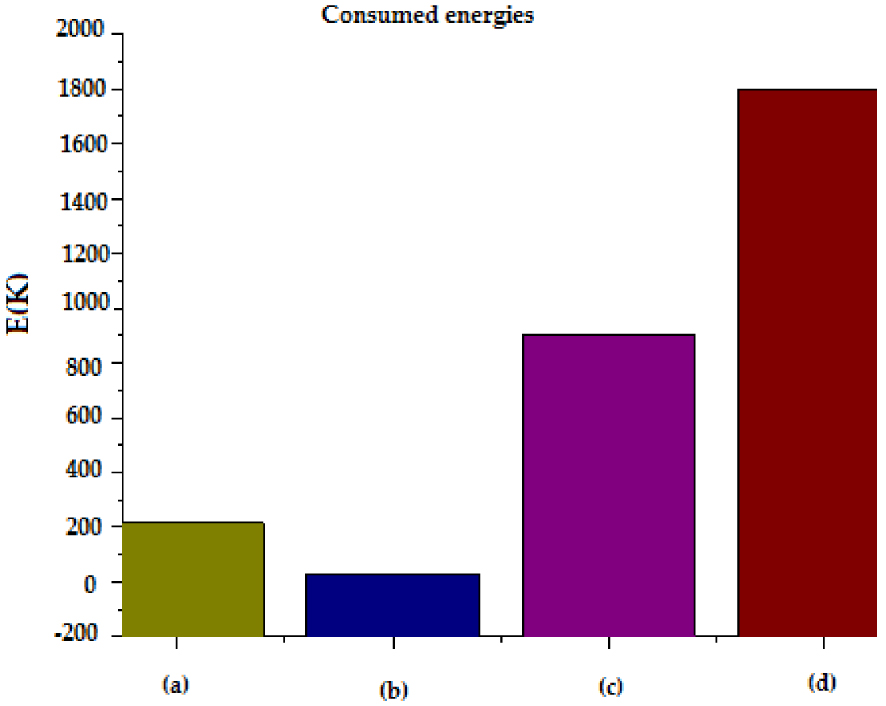
Another important gain with microwave process was that the treatment was carried out in few seconds compared to other two methods of biodiesel conversion.
Figure 9 shows that intensified pretreatment were more respectful for the environment compared with conventional pretreatment. Indeed, both adsorption and esterification consumed materials and generated waste. The adsorbent could be reused several times but its efficiency decreased with the number of uses.
For the esterification pre-treatment, it generated waste: the excess alcohol, the catalyst and the free fatty acid phase recovered after decantation. The MW and US pretreatments were respectful of green chemistry principles as they did not generate any waste.
4. Conclusion
A study on the effect of microwave irradiation and ultrasound for the pretreatment of frying oil recovered from the university campus restaurants (100% soybeans) was carried out? Results showed that the two processes, when applied under optimal conditions, allowed a reduction of FFA in reasonable time and without the use of products or generation of waste. These processes could be used in the chemical industries to refine crude oils in order to reduce their FFA content, as well as a pretreatment process in the case of using oils rich in free fatty acids in transesterification for the production of biodiesel or any other product with added value derived from used and recycled oils with the goal of valorizing. The efficiency of the two processes to effectively remove the FFA without deteriorating the quality of the oils with the objective of their recycling, in particular in biodiesel is confirmed.
For future work, a pretreatment synergy will be proposed to decrease the FFA in frying oils to a value within the range of the norm.
Conflicts of interest
Authors have no conflict of interest to declare.




 CC-BY 4.0
CC-BY 4.0
