1. Introduction
Mineral resources play an essential role in the technologies associated with energy transition. These resources contain elements designated as critical by many countries. In particular, the growing deployment of electric vehicles requires considering the use of certain elements as a geopolitical issue. This is particularly the case for elements present in lithium-ion batteries (LiB) such as cobalt, lithium, nickel, manganese, or graphite, whose increasing demand has revealed interdependencies and vulnerabilities [1]. In response to the urgency of the situation, the European Union is seeking to reduce its dependence while initiating a more environmentally friendly cycle by proposing a revision of the old Battery Directive [2]. The newly proposed directive [3] sets very ambitious recycling rate targets which must increase, between 2027 and 2031, from 90 to 95% for Co and Ni, and from 50 to 80% for Li, but also establishes, for the first time, requirements for recycled content in new batteries. These recycled contents must reach, by 2036, 26% for Co, 15% for Ni and 12% for Li. Recycling is, therefore, a strategic aspect since, by 2027, it is estimated that around 50,000 tons of used batteries will need to be processed in Europe, allowing for the recovery of 25,000 to 30,000 tons of metals of interest. Currently, recycling rates struggle to increase (less than 1% of Li is actually recycled globally), as recycling faces many barriers that hinder its development. Among these, the consumption of energy and chemicals is a significant factor in the cost of operations. This is particularly the case for pyrometallurgical and hydrometallurgical methods which are used at the industrial level to recycle elements such as cobalt, lithium, nickel, copper or manganese [4, 5, 6]. Pyrometallurgy, although it remains a frequently used process for the extraction of high-value metals such as cobalt and nickel, suffers from several drawbacks such as high operating temperature, production of toxic gases, high energy costs and the limited number of materials recovered (Li and Al are lost in the slag). Although hydrometallurgy (dissolution in acidic media in the presence of a reducing agent) is the preferred process for LiB recycling [7, 8] due to high purity and recovery rate of metals, this process suffers from high consumption of quite aggressive media, unavoidably leading to the production of acidic effluents, and from the use of expensive extractants [9]. In order not to add acid, interesting studies have been carried out using subcritical water and an addition of chlorinated polyvinyl chloride (CPVC) or polyvinylidene fluoride (PVDF) [10, 11]. The acid formed during the degradation of CPVC at 290 °C or PVDF at 350 °C (HCl or HF) induces the leaching of the valuable metals. Furthermore, several researches have been conducted using organic acids, ionic liquids (ILs) and deep eutectic-solvents (DESs), with the promise of a green approach [11, 12, 13, 14]. However, despite a great deal of research in these areas, it has proved impossible to transpose the studies carried out on a laboratory scale to an industrial scale. Among the probable causes of this failure, the excessive viscosity of these solvents, their difficulty in being recycled, their limited chemical stability and their prohibitive prices are frequently mentioned [15].
The so-called polyol process has been used for the synthesis of finely divided metal particles from their oxides, hydroxides, or salts in polyalcohols [16, 17, 18]. The polyol (e.g., ethylene glycol, diethylene glycol) acts as both solvent and reducing agent. This method can also be performed in a solvothermal way, which is a treatment performed in a closed reactor, above the boiling temperature of the solvent. In this case, a reduction of metal oxides is also observed, leading to the obtaining of several morphologies of the nanoparticles related to the concentration of the precursor [19]. Although ethylene glycol is often used as a component in mixtures (associated to a hydrogen bond acceptor) in DESs [20] or to a mild organic acid in an eco-friendly leaching system [21, 22], it has never been used alone to extract metals for recycling purposes.
Ethylene glycol (EG) is a major industrial organic compound used in the production of polyester fibers and films, antifreeze, coolant, and numerous other applications. This solvent presents favorable features like low toxicity and noncorrosiveness, and it is environmentally benign and readily biodegradable [23].
In this study, a new original approach with a low environmental footprint was developed to recycle lithium-ion batteries cathodes [24]. This process is based on a solvothermal treatment of the cathode material in the presence of EG and carbonate ions and without any acid. Performed at low temperature (225 °C), the process converts the phase present in the cathode compound into several products easily separable. Ni and Co ions are reduced to the zero valent state, together in an alloy. Manganese cations and lithium cations combine with carbonate ions to form manganese carbonate and lithium carbonate. The resulting powder (which is a mixture of Ni–Co alloy, lithium carbonate and manganese carbonate) may be separated from the solvent by centrifugation, and the several solid compounds isolated, thanks to their solubilities, by a succession of washings and magnetic separation. In this work, the characterization of the recovered compounds was performed, their purities were determined, and the recovery rates by elements were calculated. As some of the Li+ cations remain in EG after treatment, our research aims at showing that this solvent can be reused several times with the same efficiency, and that this reuse allows to recover all Li+ cations coming from the previous steps. The combination of solvent and source of carbonate ions was first ascertained in the case of LCO in terms of recovery rate and purity of the recovered powders. The possibility of reusing the solvent in order to reduce the environmental impact of the process was then assessed. Subsequently, the process was investigated both on NMC 111 cathode powder and on a black mass (BM) containing NMC cathode active material.
2. Materials and methods
2.1. Materials
The cathode powders (LiCoO2: LCO or LiCo0.33Ni0.33Mn0.33O2: NMC 111) were either obtained from MSE Supplies or synthesized through the ceramic route. X-ray diffraction (XRD) characterizations and chemical analyses of these powders are presented in Supporting Information Figures S1 and S2. The black mass (BM) was obtained from mobile phone batteries (Xiaomi model BN44), by discharging, opening in a glovebox, shredding in a cutting mill (Retsch SM 300), and sieving in a vibratory sieve shaker (Fritsch Analysette 3 Spartan) using a 100 μm grid size. XRD characterization of the sieved BM and inductively coupled plasma (ICP) analysis are shown in Supporting Information Figure S3. As graphite peaks are observed (ICDD file No. 65-6212), one can conclude that sieving was not efficient to remove graphite present in the BM. In fact, other separation techniques exist or are currently being developed to extract graphite from black mass before any processing, such as froth flotation [25, 26]. Furthermore, ICP and Energy-dispersive X-ray spectroscopy (EDX) analysis (Supporting Information Figure S4) indicate that the cathode active material is a NMC-type.
The process is performed in a Teflon-lined bomb provided by Parr Instruments (4744 model, 45 mL) or by Top Industrie (2148 5000, 500 mL or 2148 4000, 1 L), depending on the amount of solvent used. The solvent used is ethylene glycol (Merck 99%) in the presence of carbonate ions (e.g. potassium carbonate Alfa Aesar 99.0% min), with an amount corresponding to 1.2× the stoichiometric amount of carbonate ions necessary to form Li2CO3 from Li+ cations contained in the cathode powder. A pressure calculation based on the saturation vapor pressure of ethylene glycol and the air pressure at 225 °C, indicates that in the 45 mL reactor using 30 mL of ethylene glycol, the pressure is expected to be 3.9 bars (i.e. an overpressure of 2.9 bars). Table 1 summarises the operating conditions for the treatments performed in this study.
Conditions applied for the treatments of LCO and NMC cathode powders and for BM
| Sample name | Mass of powder treated (g) | EG volume (mL) |
|---|---|---|
| LCO1 | 1.5 | v-EG 30 |
| LCO2 | 15 | v-EG 300 |
| LCO3 | 30 | v-EG 600 |
| LCO4 | 15 | r-EG 300 |
| NMC | 1.5 | v-EG 30 |
| BM Xiaomi | 2 | v-EG 30 |
v-EG: virgin ethylene glycol; r-EG: reused ethylene glycol.
2.2. Experimental procedure
After introducing the cathode powder or the black mass, the reactor was filled with ethylene glycol in which potassium carbonate was previously dissolved. The reactor was then closed and heated to 225 °C for 10 h in an oven. This choice of experimental conditions comes from the conditions usually applied during polyol reduction treatments of metal oxides under solvothermal conditions (temperature higher than the boiling point of the solvent). After cooling, the reaction mixture comprises of both powder and liquid, and several steps of centrifugation, rinsing and magnetic separation (if needed) were carried out. These steps are illustrated in Figures 1a and b for the LCO and NMC cathode powder treatments, respectively. For LCO (Figure 1a), the solvent was first separated from the powder by centrifugation and stored for possible reuse. Then, the extracted powder was rinsed with distilled cold water while undergoing ultrasound dispersion, and separated again from water by a second step of centrifugation (P2). The rinse water was evaporated in an oven to recover a white powder (P1).
Summary diagram of the different stages of the solvothermal process for LCO cathode powder (a) and for NMC cathode powder and Black Mass (b).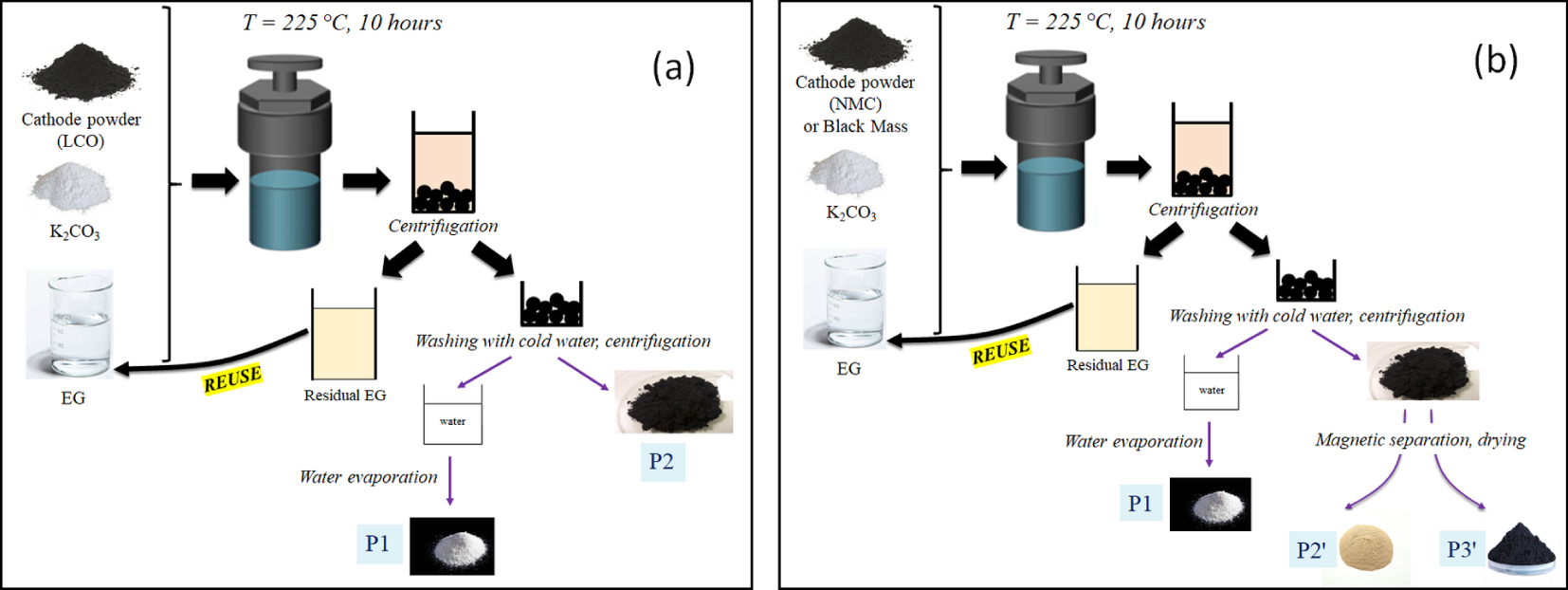
For NMC (Figure 1b), a subsequent step was added, in which the rinsed black powder was magnetically separated to isolate magnetic and non-magnetic fractions, each of which was dried. Magnetic separation was performed by mixing, under ultrasonic dispersion, the powder to be separated with water or ethanol in a beaker, and by approaching a Nd2Fe14B magnet close to the side of the beaker, which attracts magnetic part of the powder (this operation was repeated twice). The fraction that was not attracted to the magnet was a beige powder (P2′), while the fraction that was attracted to the magnet was a black powder (P3′).
2.3. Characterization methods
The crystallinity and purity of the obtained materials were characterized using an Empyrean diffractometer (PAnalytical) or a D8 diffractometer (Bruker AXS), using Co Kα radiations. The intensity was measured with a 2𝜃 step of 0.01°. Phase identification was done using DIFFRAC.EVA (Bruker AXS). Powders were observed by Scanning Electron Microscopy (SEM) equipped with an Energy-dispersive X-ray spectroscopy (EDX) detector (Zeiss 1530). Infrared spectroscopy analyses were performed with a Shimadzu IR affinity-1 spectrometer. ATR spectra of liquid samples were obtained using a GladiATR instrument equipped with a monolithic diamond by accumulating 32 scans in the spectral range from [4000–400] cm−1 with a resolution of 4 cm−1. Elemental quantification was performed by Crealins (Lyon, France) using inductively coupled plasma-atomic emission spectrometry (ICP-AES) iCAP 6500 Duo (Thermo Scientific) after acidic dissolution of the samples in the presence of sulfuric acid/nitric acid mixtures in an open environment at 220–240 °C. The relative error on the mass % of the elements is 5%.
3. Results and discussion
3.1. Application of the process to LCO material
3.1.1. Use of virgin ethylene glycol
For this first experiment (LCO1), XRD analysis and Scanning Electron Microscopy results of P1 and P2 are presented in Figure 2. P1 powder (Figure 2a) consists of grains of few tens of micrometers and contains only one phase of lithium carbonate (monoclinic, ICDD file No. 22-1141). P2 powder (Figure 2b) contains particles of a few hundreds of nanometers which are formed through the aggregation of smaller particles. Phase analysis shows that two phases are present: a face-centered cubic (fcc) phase and a hexagonal close-packed (hcp) corresponding to metallic cobalt (ICDD files No. 15-0806 and 89-4308 respectively). This morphology and the coexistence of both phases is classically observed in the case of synthesis of cobalt nanoparticles by polyol way from cobalt salts [17, 27].
XRD patterns and SEM micrographs of P1 (a) and P2 (b) obtained after treating LCO compound.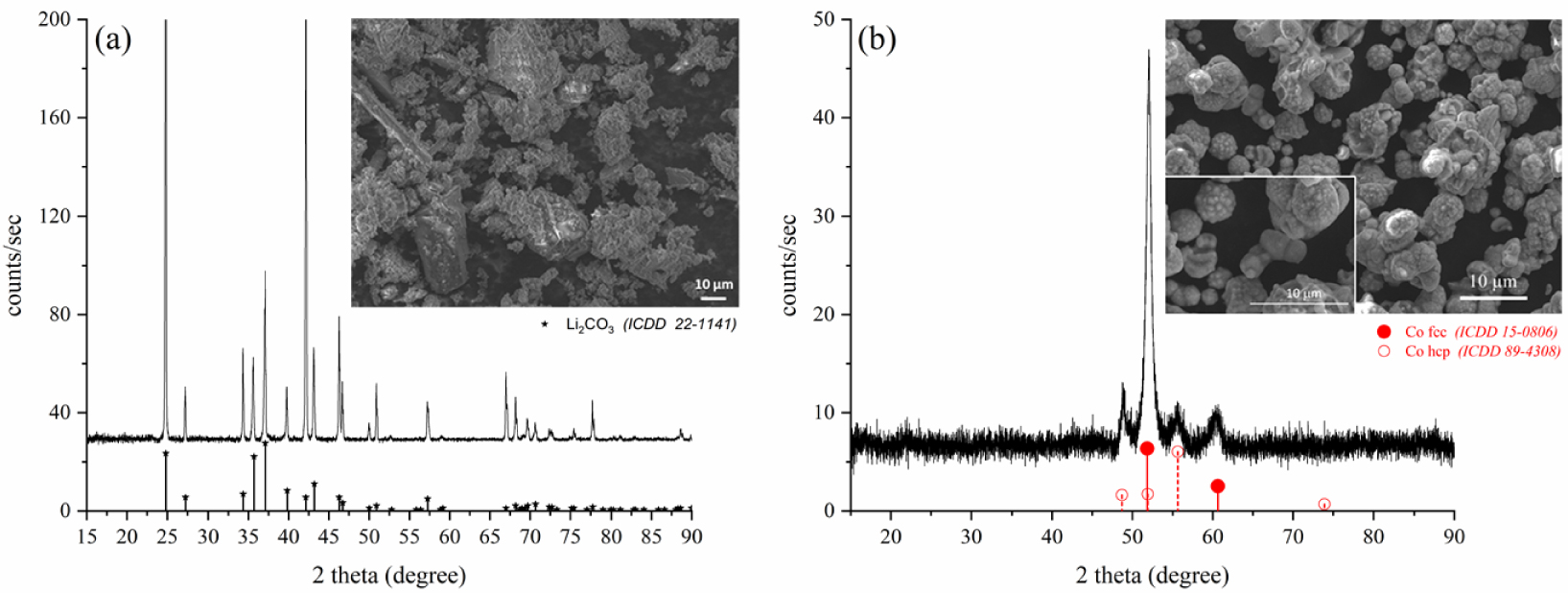
Chemical analysis of the residual EG, after separation from the solid by centrifugation, shows that it contains dissolved lithium, with an amount corresponding to approximately 30 wt% of the initial lithium present in the LCO compound.
At this stage, the mechanism involved in the process can be explained as follows: LiCoO2 phase is destroyed by the joint action of EG which reduces Co2+ cations to Co0, and carbonate ions which extract lithium cations to form lithium carbonate, according to (1)
| (1) |
The reduction is performed without any dissolution of cobalt cations, as cobalt was not detected in the residual EG. Lithium carbonate is partially soluble in EG, its solubility has been evaluated, under our solvothermal experimental conditions, to be approximately 3 g/L. After separation of the residual EG by centrifugation, the solid contains Co(s) and Li2CO3(s). Li2CO3 is soluble in water, especially cold water (13.1 g/L at 20 °C and 15.2 g/L at 0 °C [24]), so it dissolves in water during the rinsing, while Co remains as a powder (P2). Lithium carbonate can be recovered by evaporating water, this is P1.
Recovery rates for lithium and cobalt are calculated according to Equations (2) and (3) where mLCO treated is the mass of LCO treated (1.5, 15 or 30 g), mP1 and mP2 are the recovered mass of P1 and P2 powders, %mass(Li) is the Li content in P1 and %mass(Co) is the Co content in P2, these two values being determined by ICP analysis. The values 7.4% and 62.1% are the content of respectively Li and Co in the initial cathode powder obtained by ICP analysis (Supporting Information Figure S1).
| (2) |
| (3) |
Recovery rates of Li, Co, Ni and Mn and purity of P1, P2, P2′ and P3′ powders, calculated from ICP-AES analyses of the different powders extracted
| Sample | LCO cathode | |||||||
| Recovery rate (%) | Purity (%) | |||||||
| Lia | Co | Li2CO3 | Co metal | |||||
| LCO1 | 40.0 | 84.1 | 90.5 | 96.7 | ||||
| LCO2 | 69.7 | 99.1 | 95.2 | 99.9 | ||||
| LCO3 | 55.9 | 98.1 | 97.1 | 99.9 | ||||
| LCO4 | 101.3b | 99.3 | 95.4 | 99.9 | ||||
| Sample | NMC 111 cathode | |||||||
| Recovery rate (%) | Purity (%) | |||||||
| Lia | Co | Ni | Mn | Li2CO3 | NixCo1−x alloy | MnCO3 | ||
| NMC | 24 | 79 | 80.5 | 72 | 94.4 | 91 | 95.2 | |
aConsidering only P1 powder, without lithium dissolved in EG, bup to 100% due to the presence of Li ions in EG coming from the previous treatment.
As this first experiment was performed on a small amount of powder (1.5 g), unavoidable materials losses during the several steps (centrifugation, drying) have a strong influence on the recovery rates. The impact of these losses on the recovery rate is minimized by increasing the quantity of powder treated. The recycling process was performed on higher amounts of powder (15 g and 30 g, samples LCO2 and LCO3), with the same steps as described previously. As for the first experiment, a powder of Co was isolated, and a powder of Li2CO3 was recovered after evaporating the rinsing water. As it can be seen in Table 2, values of recovery rates and purity are greatly improved. The recovery rate of lithium in Li2CO3 form is between 55.9 and 69.7% with a purity between 95.2 and 97.1%. The recovery rate of cobalt is between 98.1 and 99.1%, i.e. identical for LCO2 and LCO3 taking into account measurement uncertainties, and with a purity of 99.9%.
3.1.2. Ethylene glycol reuse
In order to save EG and to recover more lithium, the solvent was reused (up to 4 times, but the result of only 1 time is shown here, sample LCO4). The phases obtained are the same that in Section 3.1.1, and the values of recovery rates and purities are indicated in Table 2. In this case, the recovery rate of lithium (calculated by comparing with the lithium amount present in 15 g of LCO treated) is higher than 100% because Li+ cations present in the solvent (and coming from a previous treatment of LCO) have precipitated as carbonate during the second treatment as saturation was reached more easily for this salt. This experiment also shows that EG is not degraded during the process, and that it remains efficient as a reducer for possible reuse.
In order to confirm the integrity of the ethylene glycol, infrared spectrometry was carried out on the solvent after extraction of the solid phase by centrifugation. Figure 3 shows the infrared spectra of virgin ethylene glycol, of the solvent after extraction of samples LCO1 and LCO4, and of a solvent after three successive reuses. The spectrum of the ethylene glycol presents absorption bands which correspond perfectly to what is expected [28]. After treatment, the solvent spectra still show all the absorption bands characteristic of ethylene glycol. Additional absorption bands appear at 1595, 1355 and 1125 cm−1, whose intensities increase with the number of times the solvent is reused. The two bands located at 1595, 1355 cm−1 could be attributed to asymmetric and symmetric elongation vibrations of the COO− group in a glycolate (CH2OHCOO−), or glyoxylate (CHOCOO−), both of which are oxidation products of ethylene glycol [29, 30, 31]. Doubt remains as to the attribution of the weak absorption band at 1125 cm−1, whose wavenumber is slightly higher than already observed for these two compounds [29]. These results confirm that the reduction of Co(III) to cobalt metal during treatment does indeed involve a redox mechanism with ethylene glycol. The exact nature of the ethylene glycol oxidation product(s) remains to be confirmed. In addition, the solvent being in very large excess compared with the cathode compound (EG/LCO molar ratio = 36/1 in the case of LCO1, LCO2 and LCO3), it can be reused several times before being significantly oxidized.
Infrared spectra of virgin ethylene glycol, EG after treatment and extraction of samples LCO1 and LCO4, and of EG after three successive reuses in the process.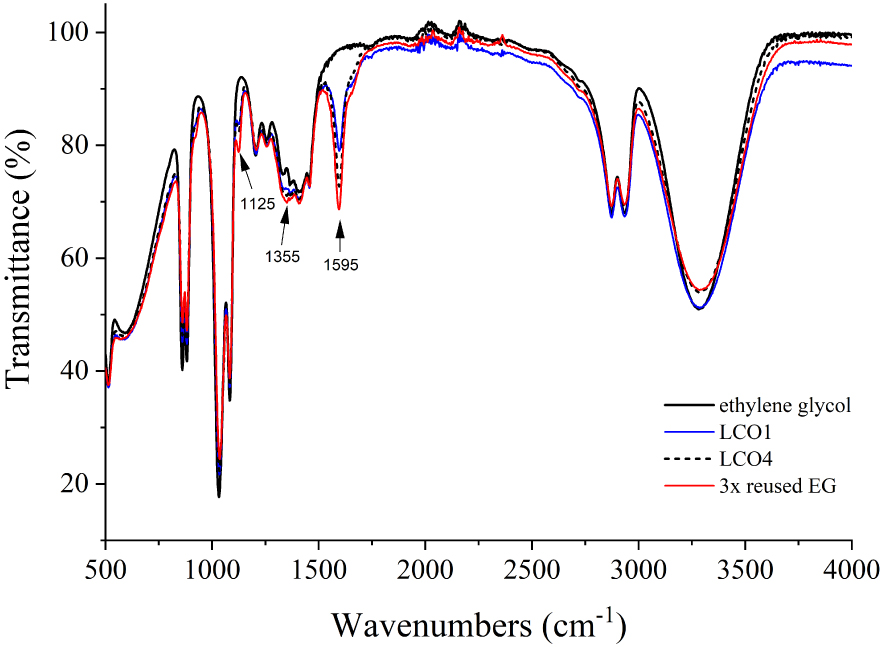
3.2. Application of the process to NMC material
The process applied to NMC 111 powder, and following the scheme of Figure 1b, leads to the obtaining of 3 fractions of powder named P1, P2′ and P3′. XRD analysis and Scanning Electron Microscopy results of these 3 powders are presented in Figure 4. As in the treatment of LCO, P1 (Figure 4a) only contains Li2CO3, with the same morphology that was previously observed (grains of few tens of micrometers). P2′ powder (Figure 4c) consists in octahedral-shaped grains of around 10 micrometers and contains MnCO3 as a single phase (rhombohedral, ICDD file No. 44-1472). P3′ powder (Figure 4b) mainly contains spherical particles with heterogeneous sizes between few hundreds of nanometers and several micrometers, which are formed through the aggregation of smaller grains. Some octahedral grains are also observed. Concerning P3′ powder, the XRD structural characterization shows the presence of diffraction peaks whose positions are between those of cobalt (ICDD file No. 15-0806) and nickel (ICDD files No. 87-0712), indicating the presence of a single-phase Ni–Co alloy. No hcp structure was identified, as usually observed while forming Ni–Co alloys by polyol way [17]. Peaks of MnCO3 with a low intensity were also observed in P3′ due to an incomplete magnetic separation, which explains the observation of octahedral-shaped grains in the SEM picture (Figure 4b).
XRD patterns and SEM micrographs of P1 (a), P3′ (b) and P2′ (c) after treating NMC 111 compound. A small amount of MnCO3 is present in P3′ due to an incomplete magnetic separation.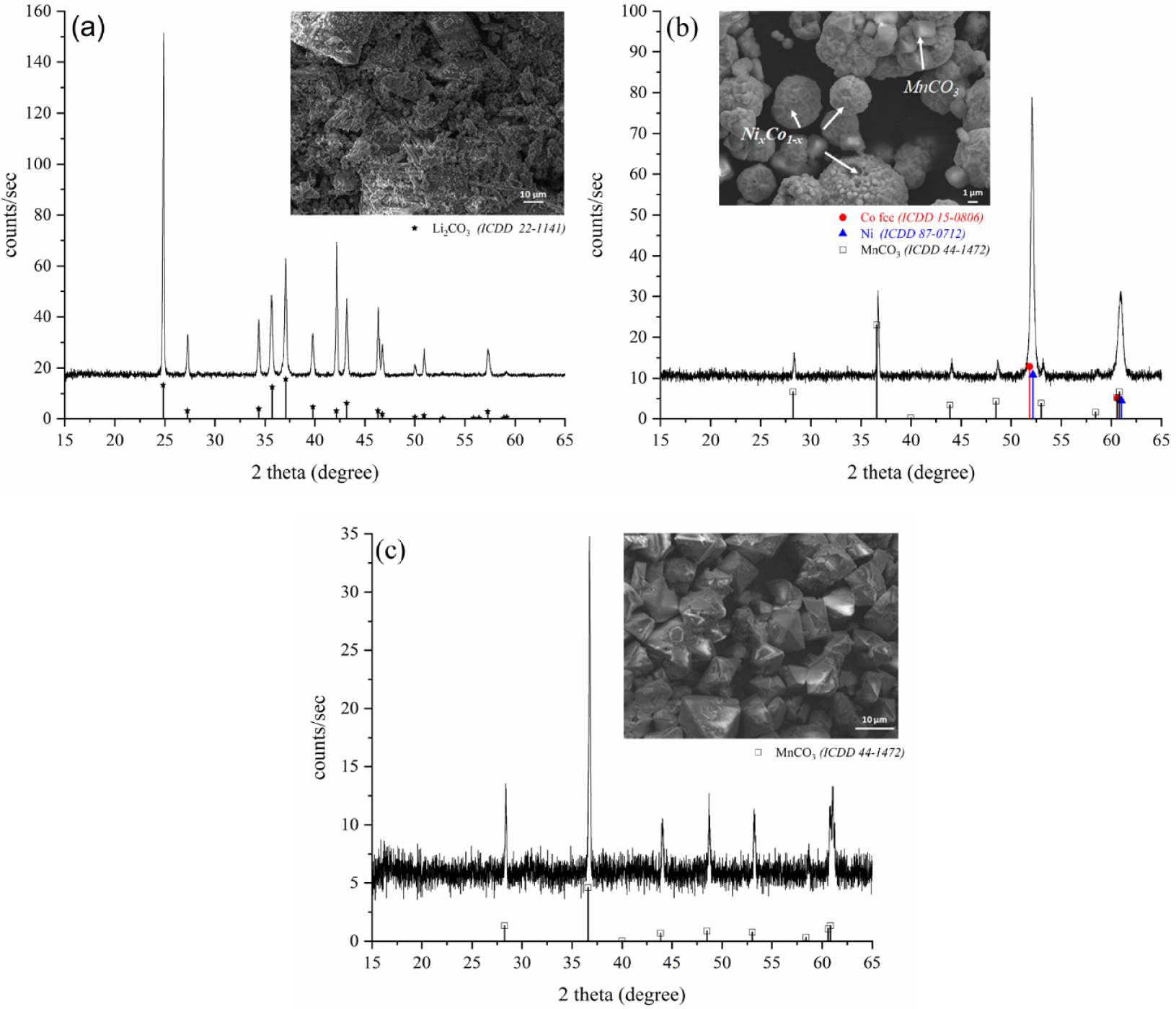
The mechanism explained in Section 3.1.1 can be completed as follows for the treatment of NMC. After extraction of Li2CO3 by its dissolution in water, the remaining powder contains MnCO3 (which is insoluble in water [32]) and the Ni–Co alloy. As the alloy is ferromagnetic, it is attracted by permanent magnet, whereas MnCO3 is not.
Recovery rates of Co, Ni, Mn and Li are reported in Table 2 (respectively 79%, 80.5%, 72% and 24%). The value for cobalt is of a similar magnitude as when treating an equivalent quantity of LCO (84.1%). The purity of lithium carbonate (94.4%) is also comparable to what was obtained for LCO (90.5%). The low recovery rate for lithium means that Li+ cations remain in EG, due to the precipitation of MnCO3. These results show that, in EG, manganese carbonate precipitates preferentially and that it is therefore necessary to increase the amount of carbonate ions in order to improve the lithium recovery rate. The purity in the Ni–Co alloy (91%) is lower than for metallic cobalt in LCO, which can be explained by the presence of MnCO3 in the alloy. The magnetic separation, which was performed by a very simple method, is the reason of this low value. The purity could be considerably increased by using more suitable techniques adapted for magnetically separating powders, such as high-gradient magnetic separator (HGMS) which creates strong magnetic forces developed in association with the intense fields delivered by a superconducting magnet [33, 34].
3.3. Application of the process to a black mass
Despite the presence of graphite in addition to the active cathode material, the recycling treatment was applied to this BM to ensure that the graphite did not affect the process. It should be noted that in this case, magnetic separation was not carried out, which leads to the isolation of only two fractions instead of three. The first fraction is obtained after centrifugation and rinsing with water, and should contain the Ni–Co alloy and manganese carbonate (mixture of P2′ and P3′) if we refer to the Figure 1b. The second fraction is recovered by evaporating the rinsing water (P1) and should contain lithium carbonate. XRD characterization of these two fractions is shown in Figure 5.
XRD patterns of the mixture P2′ + P3′ (a), and P1 (b) after treating the BM extracted from a Xiaomi battery.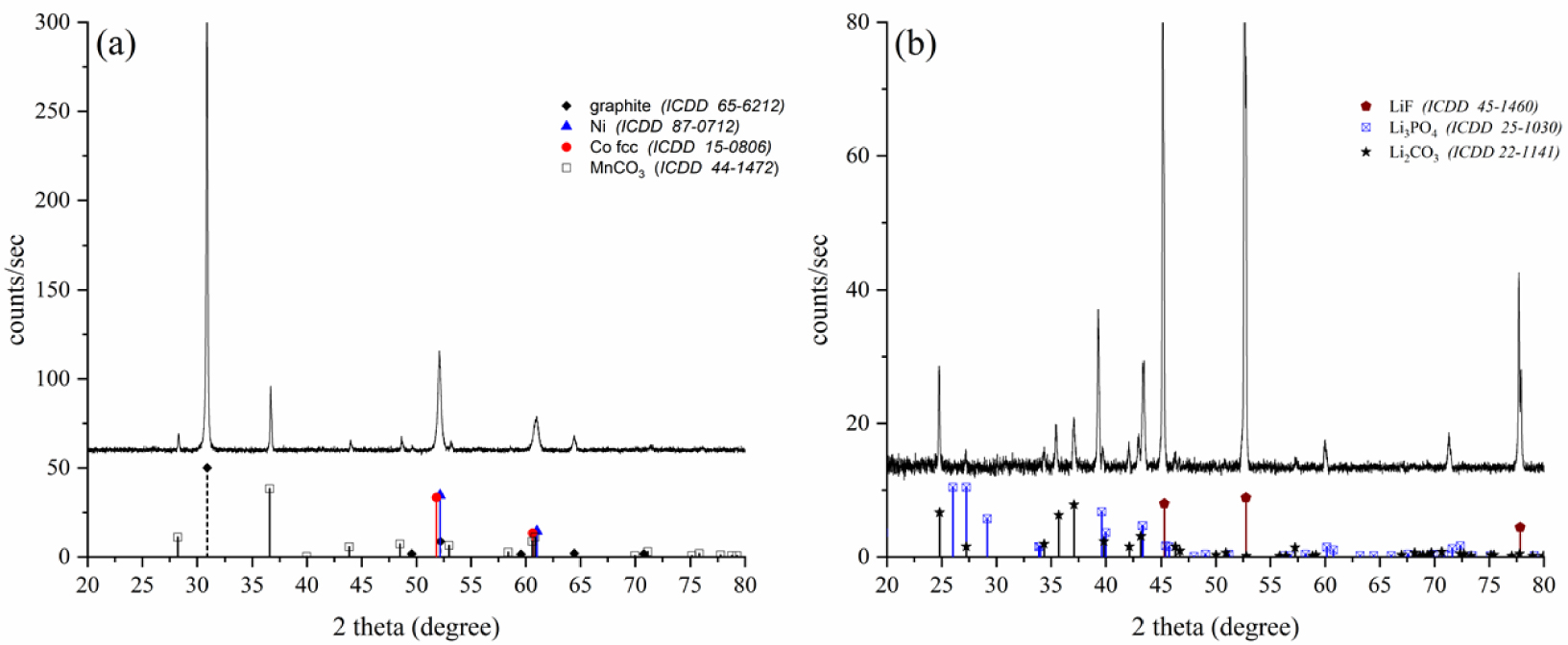
As expected, the powder fraction (P2′ + P3′) contains the alloy and MnCO3, but it also contains graphite, which obviously was not affected by the process (Figure 5a). Concerning the powder P1, it contains Li2CO3, but this phase is not the main one (Figure 5b). Another phase, identified as Li3PO4 (ICDD file No. 25-1030) is present with a higher content by regarding its diffraction peak intensities. But the main phase (by intensity) is a third compound, identified as LiF (ICDD file No. 45-1460). The formation of these two phases could be due to the presence of PVDF and/or the electrolyte in the BM before the treatment, although we did not identify these two compounds by the techniques we used. Concerning PVDF, its degradation seems unlikely at the temperature applied in the present study. Indeed, PVDF undergoes thermal decomposition only in the range 400–500 °C under atmospheric pressure [35], and under supercritical conditions in water it decomposes only above 290 °C [11]. Furthermore, it was shown that the PVDF present on cathodes was not degraded when treated in ethylene glycol at a temperature close to its boiling point (197 °C) [36]. A recent study shows that the conversion of LiPF6 into LiF and Li3PO4 is possible by water extraction using a hydrothermal way [37]. In this study, the authors show that the interest of treating LiPF6 lies in the fact that this compound is an environmental pollutant, and that producing LiF and Li3PO4 is economically profitable. The possibility that the process developed in the present study could be another way of treating LiPF6 needs to be supported by additional characterisation, particularly spectroscopic, in order to clearly highlight the presence of the electrolyte before treatment.
4. Conclusions
A new method for recycling lithium-ion battery cathodes has been developed. This method enables the extraction and the separation of valuable elements (cobalt, nickel, lithium, manganese) without the need for high temperatures or the consumption of acids or bases. The solvent used, with low environmental impact, is reusable. Its continuous reuse allows for the accumulation of lithium as lithium carbonate, which partially dissolves during treatment, until EG becomes saturated, thereby increasing the lithium recovery rate.
Furthermore, it has been shown that recovery rates, especially for cobalt, are significantly improved when processing large quantities of active cathode materials. A magnetic separation step is necessary when the processed material contains cobalt, nickel, and manganese simultaneously. This step strongly influences the purities of the Ni–Co alloy and manganese carbonate collected after treatment. The use of more efficient magnetic separation devices than the one used in this study could greatly enhance these purities.
The application of this treatment to NMC-type black mass from a battery has demonstrated that the presence of graphite does not affect this process. Additionally, it has been shown that the recovered powder also contains LiF. Further investigations need to be conducted to determine if this compound originates from the decomposition of LiPF6 present in the electrolyte.
Declaration of interests
The authors do not work for, advise, own shares in, or receive funds from any organization that could benefit from this article, and have declared no affiliations other than their research organizations.
Funding
The authors would like to thank the ESP (Energy and Propulsion Systems) Carnot Institute for their financial support in the context of the NeoBATT project. This work was additionally supported by the European Union and the Normandy region as part of the operational program FEDER/FSE 2014-2020 through the RECYLION project, and by the CNRS (French National Center for Scientific Research) in the frame of the prematuration CARY-ION project.





 CC-BY 4.0
CC-BY 4.0