1. Introduction
Enzyme immobilization has emerged as a powerful technique to enhance enzyme stability, reusability, and efficiency [1]. It represents a crucial technological advancement in industrial biotechnology, bridging the gap between the remarkable catalytic properties of enzymes and the practical demands of industrial processes. This strategy, which has evolved significantly since its first documented use in 1916 [2], has become instrumental in various industrial sectors and continues to shape the future of sustainable chemical manufacturing.
The industrial application of enzymes spans numerous sectors, demonstrating their versatility and importance in modern manufacturing processes. Key industries utilizing enzymatic processes include food processing [3], textile manufacturing [4], biodiesel production [5], biosensors [6], paper making [7], cosmetics [3], therapeutic molecules [8] and, but not limited to, chemical synthesis [9].
To understand the scale of enzyme utilization in industry, consider that glucose production alone involves the conversion of 109 tons of corn starch annually using soluble amylases. Similarly, the glucose isomerase process handles the conversion of 107 tons of glucose per year to make high-fructose corn syrup, highlighting the massive scale of these operations [1]. From the economical standpoint, the global enzyme market was valued at USD 14.0 billion in 2024 and is projected to reach USD 20.4 billion by 2029, recording a Compound Annual Growth Rate (CAGR) of 7.8% [10].
However, their successful implementation is often hindered by several inherent limitations. One major challenge is their operational instability. Enzymes are susceptible to denaturation under the harsh conditions prevalent in many industrial processes, such as extreme temperatures and pH fluctuations. Their natural structures may not withstand prolonged operational periods, leading to a decline in catalytic activity. Furthermore, the recovery of free enzymes, which act as homogeneous catalysts, presents a significant hurdle. Being water-soluble, they cannot be easily separated from the reaction mixture. Traditional recovery methods, like membrane processes, are often costly and inefficient, making continuous processing difficult. Another challenge arises from the tendency of enzymes to aggregate, particularly in hydrophobic environments or near their isoelectric point. This aggregation limits diffusion and reduces accessibility to active sites, ultimately decreasing overall activity [11].
Enzyme immobilization technology presents a compelling solution to the challenges associated with the use of free enzymes in industrial processes [12]. By anchoring enzymes onto a solid support, immobilization confers several key advantages.
Firstly, it transforms enzymes from homogeneous to heterogeneous catalysts, enabling straightforward recovery through simple filtration or centrifugation techniques. This eliminates the need for costly and inefficient downstream processing methods, significantly reducing overall process costs and improving efficiency.
Furthermore, immobilization significantly enhances enzyme stability [13]. The immobilized enzymes exhibit greater resistance to harsh environmental conditions, such as temperature fluctuations and pH changes. This is translated to an extended operational lifetime and a broader range of applicable reaction conditions.
Moreover, immobilized enzymes are ideally suited for continuous flow operations, allowing for seamless integration into packed or fluidized bed reactors. This improved process control enables more precise regulation of reaction parameters and enhances overall system efficiency [14, 15]. The choice of support material plays a crucial role in the immobilization process, affecting the properties of the resulting biocatalytic system [16]. A wide range of materials, including inorganic, organic, hybrid, and composite supports, have been explored for enzyme immobilization [16, 17]. Additionally, agroindustrial wastes have shown potential as cost effective and environmentally friendly carriers for enzyme immobilization, coconut fiber being a preferred option [18, 19].
While enzyme immobilization offers numerous benefits, the field still faces certain challenges that limit its widespread adoption since no single method is universally applicable. One key hurdle lies in the complexity and time-consuming nature of immobilization protocols [20]. Current methods often require lengthy procedures, sometimes extending up to 48 h, involving the use of hazardous chemicals such as glutaraldehyde and can result in variable protein loading capacities, hindering efficient enzyme utilization. Moreover, the recycling potential of immobilized enzymes may turn out to be limited, requiring frequent replacement and contributing to increased the operational costs.
Another challenge stems from the intrinsic limitations of support materials. Many carriers currently used are expensive and/or rely on oil-based materials, raising concerns about sustainability [21].
Furthermore, the performance of immobilized enzymes is often dependent on the specific carrier used, leading to inconsistent results. Enzyme activity retention can vary significantly, and protein loading capacity remains limited, typically reaching around 5% or 50 mg of enzyme per gram of carrier [22].
These limitations highlight the need for continued research and development to optimize immobilization techniques and develop more efficient, versatile and sustainable solutions.
2. The CarboZym technology: a game changer
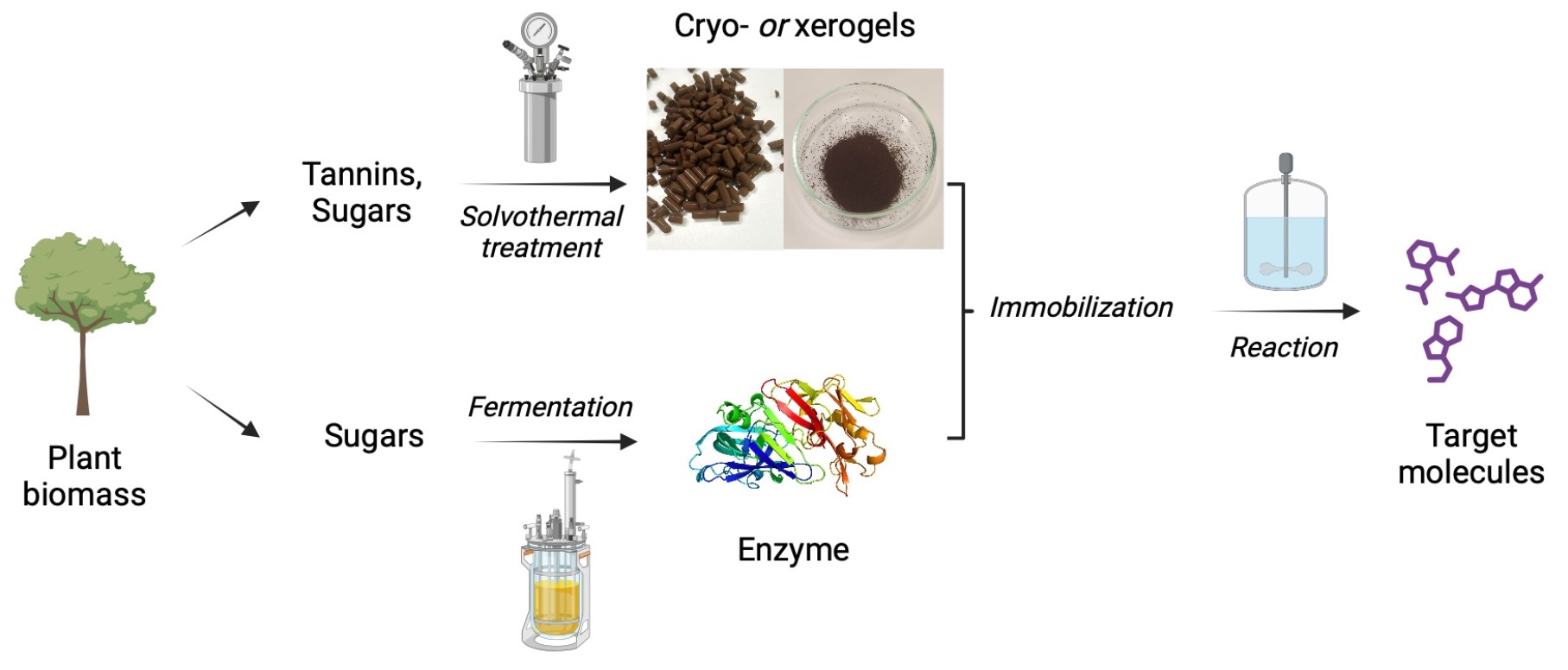
At CarboZym SAS1 , a multidisciplinary team of researchers in chemistry, bioprocess engineering, and biocatalysis collaborates to make enzyme-powered biomanufacturing both accessible and cost-effective and to pave the way for a more sustainable future by streamlining enzyme integration in processes.
2.1. Underlying principle
The CarboZym technology, which we will call “CarboZym”, marks a significant advancement in biocatalysis by patenting a biosourced, universally adaptable porous support for enzyme immobilization [23, 24]. This innovative support, derived from renewable precursors such as sugars (xylose, glucose, fructose) and phenols (phloroglucinol, catechin), leverages polycondensation mechanisms and intermolecular reactions [25]. Unique characteristics of this material include an interconnected porous structure and adjustable surface chemistry [26], enabling various types of interactions with enzymes. Relying on green chemistry principles and biodegradable raw materials, this technology meets sustainability requirements while minimizing carbon footprint, notably by revalorizing bio-waste rich in phenolic compounds (such as condensed tannins).
2.2. Material synthesis and properties
The preparation of the CarboZym support material involves a controlled synthesis process in an autoclave under solvothermal, subcritical conditions (Figure 1). The process begins with dissolving the precursors, namely a sugar and a phenol, in a water–ethanol mixture. Under solvothermal conditions, the sugars undergo dehydration: xylose is converted to furfural, while glucose and fructose generate 5-(hydroxymethyl)furfural (HMF). These heterocyclic aldehydes then quickly react with phenols such as phloroglucinol or catechin (at positions 2, 4, 6 of the aromatic ring) via intermolecular dehydration and electrophilic aromatic substitution mechanisms [25].

Description of XPh and XCat CarboZym support synthesis and chemical structure.
Initially, polymerization occurs between active sites of the phenols and the dehydrated sugars, forming oligomers that gradually assemble into three-dimensional networks through successive steps of nucleation, particle growth, and agglomeration. Within the autoclave, these steps promote the formation of a gel with interconnected pores (ranging from microporosity (<2 nm) to macroporosity (a few micrometers)) filled with liquid. This structure mimics sol–gel processes but is influenced by the composition of the precursors and the applied solvothermal conditions.
The precursor solution solidifies during the reaction in the autoclave, enabling the material to be shaped into various forms by placing a mold in the autoclave (see Figure S1.a for a macroscopic view of the shaped material). Alternatively, the material can be reduced to a powder after post-drying milling for applications in fluidized systems.
Following gel formation, two crucial steps are required to replace the interstitial liquid with air or another gas: solvent exchange through intensive washing, and drying [27]. The choice of solvent and drying method (supercritical drying, freeze drying or air drying) can significantly affect the final material properties:
- Supercritical drying is the method of choice as it yields aerogels with full preservation of the original pore texture of the wet gel. It is usually performed with carbon dioxide that presents moderate critical point temperature and pressure (31.1 °C at 1072 psi). When performed correctly, supercritical drying can largely preserve the material’s textural properties.
- Freeze-drying produces cryogels and involves solvent exchange with water or water–alcohol mixtures, freezing and ice sublimation at low pressure (to the range of a few millibars). However, ice crystal growth during the freezing stage can induce large distortion of the porous structure and a drastic loss of porosity [25].
- Convective or evaporative air-drying is a much more delicate method to use, since drying occurs by direct solvent evaporation and the gel is therefore subject to high capillary forces. It yields xerogels that often display slightly different yet functional characteristics [27].
This step allows the creation of materials with BET (Brunauer–Emmett–Teller theory)-equivalent specific surface areas ranging from 200 to 900 m2/g and pore volumes between 0.2 and 2 cm3/g, with adjustable pore sizes between 20 and 70 nm, depending on the synthesis conditions (Figure S1.b shows adsorption and desorption isotherms of XCat and XPh materials, and Figure S1.c illustrates their BJH (Barrett–Joyner–Halenda Model) pore distribution).
2.3. Technology description
The flexibility of CarboZym material synthesis offers optimization possibilities tailored to industrial needs. For instance, by selecting catechin as the phenol and xylose as the sugar, the final material (designated XCat) retains unaltered catechol functions, allowing chelation with metal ions (see chemical structure in Figure 1). This option enables binding of His-tagged proteins to the material through metal complex formation [28, 29]. In the presence of an ion such as nickel, cobalt, or iron, these catechol groups form stable bonds with His-tagged enzymes, ensuring robust fixation and precise enzyme orientation on the support (Figure 2b). This binding also facilitates enzyme recycling by simply washing out the bound enzyme under mild conditions that disrupt the His–Nickel interaction, by using imidazole, which is essential for continuous-flow or industrial processes where the catalytic lifespan of the enzyme is crucial.

(a) Protein confinement in the CarboZym support; (b) Protein bonding through formation of a stable XCat–Fe3+–histidine complex; (c) Weak bonding of protein through electrostatic interaction, Van der Waals forces, π–π stacking and/or hydrophobic interaction.
The CarboZym material also exhibits compatibility with non-His-tag enzymes, which interact through weak bonds such as hydrophobic interactions or hydrogen bons, enabled by functional groups present on the porous surface. Thus, an XPh-type material (derived from xylose and phloroglucinol, Figure 1) would be suitable for non-His-tagged proteins as it is more likely to interact with a protein via hydrophobic interactions given its less oxygen-rich surface chemistry compared to XCat. The material’s adaptability in terms of surface and chemistry makes it compatible with a wide range of enzymes, rendering this technology potentially universal.
The enzymatic immobilization protocol is simplified compared to conventional methods (such as cross-linking, entrapment, covalent attachment) (Figure 2a), where the enzyme self-confines directly within the material meso- and macroporosity via physisorption and/or chemisorption. At the molecular scale (Figure 2b), the immobilization of His-tagged enzymes appears to occur through metal complexation with catechol groups in XCat gels, while non-His-tag enzymes bind to the material through electrostatic interactions (via lysine, histidine, or arginine residues), van der Waals forces, π–π stacking and/or hydrophobic interactions (e.g., between proline residue and polyphenol ring) [30, 31, 32].
The sugars and phenols used in the synthesis of CarboZym supports enhance its biosourced aspect. Catechin, for example, is a monomer of condensed tannins, demonstrating the feasibility of using bio-waste containing these compounds to design the gel. Revalorizing this waste (such as wood or seed extracts) in the material’s production not only reduces environmental impact but also allows for sustainable and economical precursor sourcing [33, 34].
2.4. Green chemistry and industrial relevance
From an industrial standpoint, enzyme immobilization on CarboZym carrier material offers several major advantages over the use of free enzyme use. First, with the ability to immobilize up to 50 wt% of enzyme on the CarboZym material enables reusability, significantly lowering the cost per usage cycle. Moreover, this technology allows enzyme utilization in continuous mode, meeting the growing industrial demand for flow optimization. However, technical challenges remain in enzyme immobilization, such as activity loss due to diffusion limitations or demanding attachment conditions.
CarboZym addresses limitations of traditional immobilization technologies. The binding process is fast and spontaneous, without requiring costly activation steps. The material’s interconnected pores enhance mass transfer, minimizing activity losses from diffusion constraints. Additionally, enzyme reuse is assured, making this approach compatible with moderately active enzymes, thereby avoiding costly enzyme engineering optimizations. Finally, the biosourced nature and green chemistry principles underlying this technology strengthen its positioning in sustainable industrial applications.
3. Illustrative case studies
3.1. Tailoring Candida antarctica lipase B (CALB) performance through surface chemistry
Lipases are enzymes that catalyze the hydrolysis of ester bonds in lipid molecules [35]. Their natural function is the breakdown of fats, but they are also widely used in synthetic processes such as esterification and transesterification, especially in non-aqueous environments [36, 37]. These enzymes are favored in biocatalysis because of their broad substrate specificity, regioselectivity, and stereoselectivity, making them valuable tools in the pharmaceutical, food, and chemical industries. Lipases work at the interface between aqueous and organic phases, and their activity is often enhanced by this interfacial activation. One of the most prominent lipases in industrial applications is Candida antarctica lipase B (CALB). This enzyme stands out due to its high stereoselectivity and substrate flexibility, making it a key player in asymmetric synthesis, particularly for secondary alcohols. CALB’s robust catalytic performance under various conditions, including in organic solvents, at wide temperature and pH range, contributes to its broad use in reactions such as polymerization, hydrolysis, and esterification.
Despite its numerous benefits, CALB’s cost can be a significantly limiting factor for large-scale industrial applications. To address this, immobilization of CALB is often employed to enable enzyme reuse and thus lower the overall production cost. However, the commercial version of CALB, such as Novozyme 435, is already immobilized on a resin. Despite its widespread use, one of the challenges associated with this preparation is leaching—the gradual loss of enzyme from the support during reactions. This leaching can diminish the enzyme’s activity over time, reducing the expected cost savings from immobilization [38].
The immobilization of CALB was carried out on two versions of CarboZym support to form biocatalysts i-CALB-XCat and i-CALB-XPh. Such biocatalysts were implemented in the hydrolysis of p-nitrophenyl butyrate (pNPB) as a model reaction (see Figure 3 caption and supporting information for more detailed protocol). Conversion ratio and cumulative leaching on 5 cycles associated are displayed on Figure 3 for both biocatalysts. In both cases, the enzyme is still active after 5 cycles but their remaining activity is different, averaging 20 and 44% for i-CALB-XCat and i-CALB-XPh, respectively. One explanation probably is linked to the fact that the cumulative leaching over 5 cycles is almost 2 times greater for XCat than for XPh support. According to these observations, XPh support is a more suitable material for CALB immobilization as it is more difficult to remove the enzyme from the material probably due to a stronger interaction. Our hypothesis here is that the CALB interacts with the carbon-based support through hydrophobic interactions which are stronger with XPh than XCat.

Results for immobilized CALB (a) Batch mode: conversion ratio and cumulative leaching for XPh and XCat support ([buffer: 20 mM Tris-HCl + 1 wt% genapol; pH 7] [immobilization conditions: 10 mg XCat or XPh support—10% (w/w) protein loading—800 rpm; 4 °C]; [reaction conditions: 15 mM pNPB—25 °C]); (b) Continuous mode: conversion ratio and STY ([buffer: same as batch mode] [immobilization conditions: 65 mg support—10% (w/w) protein loading—0.1 ml/min; 4 °C]; [reaction conditions: same as batch mode; circulation mode—5 mM p-NPB—13.3 ml at 0.5 ml/min—5 cycles]).
The i-CALB-XPh biocatalyst was then implemented in a fluidized bed continuous flow system (see Supplementary Information) to prove the implementation of CarboZym technology in such industry-approved reactors. In continuous mode, the substrate solution was flowed (without re-circulation) through the thermostatted column at 0.5 ml/min with negligible protein loss and achieving space–time yields (STY) close to 7 g⋅L−1⋅h−1 (for the p-NPB hydrolysis). These results demonstrate significant progress toward achieving the industry-standard metrics required to ensure process viability [39].
3.2. GsOYE-catalyzed reduction of 2-cyclohexenone to cyclohexanone
Ene-reductases (EREDs) are a class of oxidoreductase enzymes that catalyze the asymmetric reduction of activated alkenes to saturated products, typically using nicotinamide cofactors such as NAD(P)H as electron donors. Among the ene-reductases, the old yellow enzyme (OYE) family is one of the most well-studied and widely applied groups. OYE enzymes are flavin mononucleotide (FMN)-dependent oxidoreductases known for their ability to catalyze the reduction of a wide variety of α,β-unsaturated carbonyl compounds, including ketones, aldehydes, and nitroalkenes [40, 41]. OYE enzymes exhibit broad substrate specificity and high stereoselectivity can be obtained, making them valuable for applications in asymmetric synthesis. One notable application is the reduction of cyclohexenone to cyclohexanone, a key reaction in the synthesis of fine chemicals and pharmaceutical intermediates. In this reaction, the α,β-unsaturated bond in cyclohexenone is reduced to the corresponding cyclohexanone with excellent stereoselectivity, highlighting the utility of ene-reductases for producing optically pure compounds. The versatility and stability of OYE enzymes, coupled with their ability to perform reductions under mild conditions, have made them a focal point in efforts to develop greener and more sustainable chemical processes. Ongoing research and enzyme engineering have expanded the substrate scope and improved the efficiency of OYEs, making them a powerful tool for modern synthetic applications [42].
The GsOYE is a newly identified ene-reductase from Galdieria sulphuraria alga [41]. It features properties that fulfill the specifications required for industrial applications, such as high catalytic activity and robustness (thermostability, wide range pH optimum, cosolvent tolerance) as well as high-yield production/purification.
GsOYE was expressed in E. coli with an N-terminal His-tag. Fe-XCat CarboZym support was then chosen to immobilize the protein via coordination with iron bound to XCat material. Such i-GsOYE-Fe-XCat biocatalyst was implemented to catalyze the reduction of cyclohexenone to cyclohexanone (see Figure 4 and Supplementary Information for protocol details). As described in Figure 4a, the biocatalyst was used in batch mode and displays a conversion ratio ∼3.5 times higher than the free enzyme from cycle 1 onwards. It suggests that the enzyme activity can be enhanced upon immobilization. This may be caused by the fact that the immobilized state promotes a more catalytically favored conformation compared to its free form. Reuse of i-GsOYE-Fe-XCat biocatalyst showed that the enzyme can remain active over five cycles (with a mean remaining activity around 32%) resulting in a Total Turnover Number (TTN) of 5868 ± 758 compared with 860 ± 338 for the free enzyme.

Results for immobilized GsOYE. (a) Batch mode: conversion ratio and cumulative leaching ([buffer: 200 mM Tris-HCl + 50 mM NaCl + 2 vol% DMSO; pH 7.5] [immobilization conditions: 25 mg Fe-XCat support—10% (w/w) protein loading—800 rpm; 4 °C]; [reaction conditions: 50 mM cyclohexenone—75 mM NADH—30 °C]); (b) Continuous mode: conversion ratio and STY ([buffer: same asbatch mode] [immobilization conditions: 50 mg Fe-XCat support—10% (w/w) protein loading—0.1 ml/min; 4 °C]; [reaction conditions: same as batch mode; recirculation mode—120 ml at 1 ml/min—5 cycles]). Masquer
Results for immobilized GsOYE. (a) Batch mode: conversion ratio and cumulative leaching ([buffer: 200 mM Tris-HCl + 50 mM NaCl + 2 vol% DMSO; pH 7.5] [immobilization conditions: 25 mg Fe-XCat support—10% (w/w) protein loading—800 rpm; 4 °C]; [Lire la suite
Continuous mode operation was also tested, where the reaction mixture was recirculated through a packed column filled with i-GsOYE-Fe-XCat (Figure 4b). Over the course of two hours, GsOYE consistently reduced cyclohexenone, with steadily increasing conversion rate and TON. It was also shown that after stopping the process and storing it overnight at 4 °C, the continuous reaction can be restarted and regained an efficiency similar to that before storage. The low leaching and robust activity under continuous conditions position this system as an effective biocatalyst for scalable processes. It should also be noted that the leaching measured in batch and continuous mode remains very low (<1%), probably thanks to the stable binding provided by the support/His-tag interaction.
3.3. Immobilized transaminase for efficient pyruvate conversion
Transaminases (TAs) are widely recognized for their role in catalyzing the transfer of amino groups from amine donors to acceptor molecules, such as ketones or aldehydes, facilitating the formation of valuable chiral amines [43]. These enzymes are particularly attractive for green chemistry applications in industries such as pharmaceuticals and agrochemicals, where they are employed for the synthesis of enantiopure compounds [44, 45]. The demand for such biocatalysts is driven by their high selectivity, operational stability, and ability to operate under mild conditions, reducing the need for toxic chemicals and harsh environments. One example is the transaminase from Thermomicrobium roseum (UniProt accession number B9L0N2), classified as an ω-transaminase. This class of transaminases typically operates with both primary amines and ketones, which makes them versatile tools in biotransformations. These enzymes belong to a family of enzymes dependent on pyridoxal-5′-phosphate (PLP) as a cofactor [46]. PLP plays a critical role in the catalytic mechanism, acting as an intermediate carrier of the amino group during the transamination process. B9L0N2 demonstrates excellent activity in the asymmetric synthesis of a variety of chiral amines. This enzyme shows high efficiency in catalyzing the conversion of substrates like pyruvate and racemic amines, making it a valuable tool in producing pharmaceutical intermediates [47].
The advantages of using B9L0N2 include its ability to perform reactions under mild conditions and with minimal by-products, as well as its compatibility with continuous processing, which enhances its potential industrial applications. Its robustness, combined with low substrate inhibition and tolerance to organic solvents, makes it highly versatile for use in different biotechnological settings.
Similarly to GsOYE, B9L0N2 was immobilized on Fe-XCat support to form a i-B9L0N2-Fe-XCat biocatalyst through Fe3+/His-tag coordination. The transamination of pyruvate in the presence of racemic α-methylbenzylamine was chosen as a model reaction to evaluate the efficiency of such a biocatalyst both in batch and continuous mode. The results of these assays are displayed on Figures 5a and 5b, respectively.

Results for immobilized B9L0N2. (a) Batch mode: conversion ratio and cumulative leaching ([buffer: 50 mM Tris-HCl + 300 mM NaCl + 10 wt% glycerol; pH 7.5] [immobilization conditions: 25 mg Fe-XCat support—10% (w/w) protein loading—800 rpm; 4 °C]; [reaction conditions: 25 mM pyruvate, 25 mM racemic α-methylbenzylamine, 0.1 mM PLP—25 °C]); (b) Continuous mode: conversion ratio ([buffer: same as batch mode] [immobilization conditions: 70 mg Fe-XCat support—5% (w/w) protein loading—0.1 ml/min; 4 °C]; [reaction conditions: same as batch mode; circulation mode—0.1 ml/min—5 hours]). Masquer
Results for immobilized B9L0N2. (a) Batch mode: conversion ratio and cumulative leaching ([buffer: 50 mM Tris-HCl + 300 mM NaCl + 10 wt% glycerol; pH 7.5] [immobilization conditions: 25 mg Fe-XCat support—10% (w/w) protein loading—800 rpm; 4 °C]; [reaction ... Lire la suite
The batch test results demonstrate that the enzyme activity remains highly stable across six consecutive cycles, with a relative residual activity of approximately 92% from the first to the sixth cycle. Under these conditions, the cumulative turnover number over these cycles is increased by a factor of about 8.75 compared to the enzyme in its free form, indicating that immobilizing B9L0N2 can reduce enzyme-related costs proportionally. Given the robust stability of the immobilized biocatalyst, this factor has the potential to be enhanced further by extending the number of cycles, underscoring the practicality of utilizing CarboZym technology for this application.
Additionally, performance in continuous mode (without recirculation) showed similarly high stability, with a consistent conversion rate maintained over a five-hour period. Importantly, minimal enzyme leaching was observed in both batch and continuous setups (<1%), supporting the durability of the immobilization. These findings demonstrate, as a proof of concept, that CarboZym technology offers significant benefits for the industrial application of transaminases like B9L0N2, enabling efficient and cost-effective biocatalysis.
4. Future prospects and industry applications
4.1. Enzyme co-immobilization: mimicking nature’s metabolic efficiency
Enzyme co-immobilization represents a sophisticated approach that bridges the gap between nature’s complex metabolic networks and industrial requirements [48, 49]. This strategy involves the immobilization of multiple enzymes onto a single support material. It allows running enzymatic cascades into one-pot reaction systems creating an artificial microenvironment where sequential enzymatic transformations can occur efficiently within a single reaction vessel. By mimicking the intricate choreography of natural metabolic pathways, this strategy offers a powerful solution to the limitations of traditional single-enzyme approaches.
4.1.1. Fundamental principles and advantages
In living cells, metabolic pathways operate through precisely organized spatial arrangements of enzymes. This natural organization enables rapid substrate channeling, where intermediates are efficiently transferred between consecutive enzymes [50]. Enzyme co-immobilization seeks to recreate this efficiency in artificial systems by positioning multiple enzymes on a single support material, eliminating the need for intermediate isolation or purification [51].
Traditional single-enzyme approaches face significant limitations when handling complex multistep reactions. Each enzymatic step typically requires separate reaction vessels, leading to increased complexity, cost, and logistical challenges. Moreover, intermediates produced in one step may degrade before being utilized in subsequent reactions, resulting in reduced yields and increased purification demands [52].
Co-immobilization elegantly addresses these challenges by creating a concentrated microenvironment where enzymes work in concert. The close proximity of enzymes facilitates direct transfer of intermediates, minimizing diffusion limitations and byproduct formation. This spatial arrangement promotes faster reaction rates and shields enzymes from harsh conditions, enhancing their operational stability and lifespan [53].
4.1.2. Design and technical considerations
Successfully implementing enzyme co-immobilization requires careful attention to multiple factors. The choice of support material is crucial, as it must provide sufficient surface area and appropriate chemical functionality while maintaining individual enzyme activities. The material should offer mechanical stability and enable efficient mass transfer of substrates and products.
Several strategies have emerged for enzyme co-immobilization, each with distinct advantages. Entrapment within porous matrices, such as alginate beads or hydrogels, offers a simple and cost-effective approach but may face diffusion limitations [54]. Covalent attachment through chemical bonds provides stable immobilization and precise control over enzyme positioning but requires careful selection of coupling chemistry to preserve activity [55].
Physical adsorption relies on non-covalent interactions like hydrogen bonding and electrostatic forces, offering a gentler alternative that better preserves enzyme activity and allows for enzyme replacement when needed [21]. Surface modification techniques enable precise control over enzyme orientation and density through the creation of specific binding sites on the support material.
The ratio of different enzymes in the co-immobilized system requires careful optimization to prevent bottlenecks and ensure smooth reaction progression [56]. Unlike natural systems with tightly regulated enzyme expression, artificial systems need balanced enzyme activities to match sequential reaction rates.
4.1.3. Applications across industries
The versatility of enzyme co-immobilization has led to its adoption across various sectors. In pharmaceutical production, co-immobilized enzymes enable the synthesis of complex drug molecules with high selectivity and purity, including the production of chiral intermediates and the biotransformation of drug precursors [57]. The food and beverage industry utilizes this technology for producing modified sweeteners, flavorings, and other additives, while also enabling the bioconversion of food waste into valuable products [58]. In the biofuel sector, co-immobilized enzyme systems show promise in converting complex biomass into useful products, particularly in the degradation of cellulosic materials and the production of biodiesel from vegetable oils [59]. Environmental applications include bioremediation of contaminated soils and water, degradation of toxic pollutants, and the production of bioplastics from renewable resources [60].
4.1.4. Current challenges and limitations
Despite its potential, enzyme co-immobilization faces several challenges. The complexity of optimizing multiple parameters simultaneously makes system design and implementation demanding. Mass transfer limitations become more significant in co-immobilized enzymes, requiring careful consideration of substrate and product diffusion through the support material [61].
The stability of these systems can be compromised if any single enzyme in the cascade fails, potentially necessitating complete system replacement. Economic viability often depends on achieving sufficient operational stability and reusability to justify the initial investment in expensive support materials and complex immobilization procedures [62].
4.1.5. Future perspectives and innovations
The future of enzyme co-immobilization lies in developing more sophisticated systems that better mimic natural metabolic pathways. An elegant study by Korman et al. designed a homogeneous system comprising 27 enzymes for the conversion of glucose into monoterpenes such as limonene, pinene and sabinene with high yields [63]. Co-immobilization of this entire synthetic biochemistry platform would probably improve its stability and the total turnover numbers reached paving the way to challenge cell-based systems.
Advanced materials science and nanotechnology could offer promising solutions for creating structured support materials with precise control over enzyme positioning [64].
The field continues to evolve through computational modeling and artificial intelligence, which could accelerate system optimization by predicting optimal enzyme arrangements and ratios [65]. As our understanding of enzyme interactions and immobilization techniques advances, co-immobilized systems will likely play an increasingly important role in enabling sustainable and efficient production processes across diverse industries [66].
The versatility of CarboZym’s immobilization carriers was shown to be an asset to successfully perform the simultaneous co-immobilization of up to six different enzymes on the same carrier. We are currently demonstrating this concept with a simplified enzymatic cascade allowing the access to terpenoids starting from isopentenol [67, 68, 69, 70]. The six overexpressed and purified enzymes were mixed, and XPh was added to co-immobilize them. This immobilized preparation successfully catalyzed the conversion of isopentenol to limonene, demonstrating the successful co-immobilization of the six enzymes and their catalytic capabilities. This promising study is currently being investigated by CarboZym and a proof of concept will be published in the near future.
4.2. Enzymatic cofactor recycling
Enzymatic cofactor recycling represents a crucial aspect of industrial biocatalysis, particularly for reactions involving nicotinamide cofactors (NADH/NADPH) and adenosine triphosphate (ATP) [71, 72]. These essential molecules serve as electron carriers and energy sources in numerous biochemical transformations, but their high cost and stoichiometric consumption pose significant challenges for industrial applications.
This overview examines the importance of cofactor recycling systems, their implementation strategies, and their integration with enzyme immobilization technologies.
4.2.1. Fundamental principles
Nicotinamide cofactors play vital roles in oxidation–reduction reactions, serving as electron carriers in numerous enzymatic transformations. NAD(P)H acts as a reducing agent in many valuable synthetic processes, including the production of chiral alcohols, amino acids, and other fine chemicals [73]. ATP, on the other hand, functions as an energy carrier, driving thermodynamically unfavorable reactions and enabling phosphorylation processes essential for many biotransformations [74].
The economic feasibility of enzymatic processes heavily depends on efficient cofactor recycling systems. Without recycling, the stoichiometric use of these expensive cofactors would make most industrial applications prohibitively expensive. For instance, the cost of NADH and NADPH can represent a significant portion of the overall process costs. Similarly, ATP’s high cost and inherent instability necessitate efficient regeneration systems for practical applications [75].
4.2.2. Technical approaches to cofactor recycling
NAD(P)H recycling
Several strategies have been developed for NAD(P)H regeneration, each with distinct advantages [71]. The enzyme-coupled approach employs secondary enzymes such as formate dehydrogenase, glucose dehydrogenase, or alcohol dehydrogenase to regenerate the reduced cofactor. These systems typically use inexpensive sacrificial substrates like formate, glucose, or isopropanol to drive the regeneration of NAD(P)H.
Substrate-coupled recycling represents an elegant alternative where a single enzyme catalyzes both the main reaction and cofactor regeneration. This approach simplifies the reaction system but requires careful optimization to balance the main reaction and regeneration rates.
ATP recycling
ATP regeneration systems often utilize phosphoryl transfer enzymes such as acetate kinase or pyruvate kinase, coupled with high-energy phosphate donors like acetyl phosphate or phosphoenolpyruvate. These systems can achieve multiple recycling cycles, though their efficiency is typically lower than NAD(P)H regeneration systems due to ATP’s complex chemistry and stability issues [71].
4.2.3. Integration with enzyme immobilization
The combination of cofactor recycling with enzyme immobilization technologies offers promising solutions for industrial applications [76]. Co-immobilization of main and recycling enzymes can create efficient microenvironments where cofactors are rapidly regenerated and reused. This spatial organization mimics natural metabolic pathways and can significantly enhance the overall process efficiency [77].
Various strategies have been developed for immobilizing both enzymes and cofactors. Cofactor tethering involves the chemical modification of cofactors to enable their attachment to support materials, while maintaining their catalytic function. This approach can reduce cofactor loss but may affect the kinetics of enzymatic reactions due to diffusion limitations [78].
4.2.4. Applications and industrial impact
Cofactor recycling systems have enabled numerous industrial applications. In the pharmaceutical industry, NAD(P)H-dependent reactions are crucial for the synthesis of chiral alcohols and amines which are key building blocks for many active pharmaceutical ingredients. The integration of efficient recycling systems has made these processes economically viable at industrial scales [79].
ATP-dependent processes find applications in the production of phosphorylated compounds, including nucleotides and sugar phosphates. These molecules serve as important intermediates in various biochemical pathways and have applications in both pharmaceutical and food industries.
The combination of cofactor recycling with enzyme immobilization has led to the development of continuous flow processes, where immobilized enzyme systems can operate for extended periods with minimal cofactor loss. These systems offer improved productivity and reduced operational costs compared to batch processes [80].
4.2.5. Current challenges
Despite significant progress, several challenges remain in the field of cofactor recycling. The stability of cofactors under industrial conditions remains a concern, particularly for ATP, which is susceptible to hydrolysis. The development of more stable cofactor analogs or protective strategies represents an active area of research [81, 82]. Mass transfer limitations in immobilized systems can affect the efficiency of cofactor recycling, particularly when multiple enzymes and cofactors are involved. The design of carrier materials with optimal porosity and surface chemistry is crucial for addressing these limitations [83].
4.2.6. Future perspectives
The successful conversion of isopentenol into limonene with six co-immobilized enzymes (see Section 4.1.5) also exemplifies the recycling of ATP. Indeed isopentenol is successively phosphorylated into isopentenyl phosphate (IP) and isopentenyl diphosphate (IPP) involving the concomitant consumption of two molecules of ATP yielding 2 × ADP. In this case, ATP regeneration is catalyzed by an acetate kinase (one of the six co-immobilized enzymes) using acetyl phosphate as a phosphate donor [8].
The continued development of innovative solutions that address current limitations while maintaining economic viability will shape the future of this field, enabling more sustainable and efficient production processes across various industries.
4.3. Scaling up hydrothermal carbonization: from laboratory to industrial reality
The CarboZym technology is based on the hydrothermal carbonization, or more accurately the solvothermal carbonization, of sugar in the presence of phenolic compounds. Hydrothermal carbonization (HTC) is a thermochemical process that transforms organic matter into valuable carbon-based materials under subcritical conditions, i.e., at relatively high temperature and pressure [84]. HTC has demonstrated its efficacy at the laboratory scale and holds immense potential for sustainable material production [84, 85]. However, translating this technology to industrial scale presents a unique set of challenges and opportunities that have been partially addressed in the literature for wet biomass wastes [86] and sewage sludges [87]. Several companies have developed HTC plants at pilot and industrial scales, such as C-Green (OxyPower HTC™ plant in Finland), TerraNova Energy, Ingelia, CarboREM (CarboREM C-700 Plant installed in Italy), and HTC-Cycle. Technical, economic, and environmental considerations are crucial for successfully scaling up HTC processes for porous carbon-based material production [84]. Even though CarboZym technology involves partially purified precursors that are soluble and therefore easier to process, the scale-up remains challenging and necessitates a meticulous approach to reactor design and operational parameters.
4.3.1. Technical hurdles and engineering solutions
Reactor design and operation: navigating heat and pressure
As reactor volume increases, the efficient transfer of heat becomes paramount. Maintaining uniform temperature distribution within the reaction medium is critical for consistent product quality and requires careful consideration of heating systems and mixing mechanisms. Efficient external jackets, internal heating elements, and robust mixing systems, such as impellers or recirculation loops, are essential for achieving homogeneous heating. In a recent industry perspective on zeolite manufacturing, Maurer and Parvulescu from BASF discussed heat distribution heterogeneities within high volume synthesis reactors (10–20 m3) [88]. A computational fluid analysis showed that even for well-stirred and heated reactor gradients, mixing flows and temperature distributions could occur during the hydrothermal treatment [88].
Pressure management is another crucial aspect. HTC processes typically operate under autogenous pressure, ranging from 10 to 40 bar, demanding robust reactor construction materials capable of withstanding these conditions. Corrosion resistance is equally important, given the acidic nature of the reaction medium (pH is usually below 3 at the end of reaction). Selecting materials like stainless steel alloys or specialized corrosion-resistant coatings is vital for reactor longevity and safety. However, scaling up the synthesis of porous materials under these conditions is not insurmountable; the commercial scale production of zeolites [88, 89], in particular Zeolite A (LTA), is a proof. Large-volume reactors of 10–20 m3 have been reported for manufacturing zeolites at commercial scale using conventional batch technologies. Process intensification via continuous flow technology is currently developed by Arkema at pilot scale [90].
Process control and monitoring: ensuring consistency and quality
Maintaining consistent product quality at industrial scale requires sophisticated control systems. Continuous monitoring of key parameters, including temperature, pressure, residence time, and slurry concentration, is essential. Implementing in-line sensors and automated control systems allows for real-time adjustments, minimizing deviations from optimal operating conditions and ensuring product uniformity. Data acquisition and analysis can provide valuable insights into process performance, enabling continuous improvement and optimization.
Feed handling, product recovery, washing and drying: streamlining the process
Scaling up feed handling systems presents unique challenges. Solutions have been proposed for the continuous flow synthesis of zeolites with in-line mixing of the precursors and in-line seeding of the synthesis gel solution [90]. Efficient downstream processing is also essential. As mentioned above (Section 2.2), two crucial downstream processing steps are required to handle wet gels and slurries after HTC, e.g., solvent exchange through intensive washing, and drying. These steps will be inspired by downstream processing of hydrochar and zeolite slurries adopted in pilot plants, but also by other cryo- and aerogel technologies such as Starbon(R), a spin out from the University of York’s Green Chemistry Centre of Excellence [91]. Besides, efficient solid–liquid separation techniques are essential for recovering the HTC-produced carbon-based material. Filtration methods, such as membrane filtration or pressure filtration, may be employed depending on the desired particle size and purity.
4.3.2. Economic viability: balancing costs and returns
The economic viability of scaled-up HTC processes hinges on several key factors.
Raw material supply: securing a steady stream
Securing a consistent supply of suitable biomass feedstock at competitive prices is paramount. The scale-up strategy must consider feedstock availability, transportation costs, and potential seasonal variations in supply. Diversifying feedstock sources and establishing strong partnerships with biomass suppliers can mitigate supply chain risks.
Operating costs: optimizing efficiency and minimizing expenses
Energy consumption, particularly for heating and potential activation processes, significantly impacts operating costs. Implementing heat recovery systems, optimizing process parameters, and exploring alternative energy sources, such as biomass-derived fuels, can contribute to improved energy efficiency and reduced operational costs.
Capital investment: weighing the initial outlay
The initial capital investment required for HTC facilities is substantial. Careful financial planning, thorough market analysis, and a robust business plan are essential for assessing the return on investment.
Modular design approaches, where production capacity can be incrementally increased, offer flexibility in adapting to changing market demands and minimizing initial capital requirements.
4.3.3. Overcoming challenges: ensuring sustainability and consistency
Product consistency: maintaining quality at scale
Maintaining consistent product quality across large production volumes is a key challenge. Implementing robust quality control systems, standardized operating procedures, and rigorous testing protocols are essential for ensuring that the HTC-produced carbon-based materials meet the required specifications.
Environmental impact: minimizing the footprint
Managing process emissions and wastewater treatment at industrial scale requires careful consideration. The development of closed-loop systems for water recycling and emission control becomes increasingly important. Implementing strategies for capturing and utilizing process emissions, such as carbon dioxide sequestration or biogas production, can further enhance the environmental sustainability of HTC processes.
4.3.4. Future prospects: innovation driving advancement
The future of HTC scale-up lies in continuous innovation and technological advancements.
Advanced process control: harnessing the power of data
The integration of artificial intelligence and machine learning can enable more sophisticated process control and optimization, leading to improved product consistency, reduced operating costs, and enhanced process efficiency.
Sustainable processing: embracing circular economy principles
Integrating renewable energy sources, such as solar or wind power, can reduce the carbon footprint of HTC processes. Implementing circular economy principles, such as utilizing the HTC-derived soluble byproduct as biofuels or platform molecules for biorefinery schemes, can further enhance the sustainability and economic viability of HTC.
4.3.5. Conclusion: a promising path forward
Scaling up HTC for porous carbon-based material production presents a complex yet promising opportunity for industrial development. Success requires a multifaceted approach, addressing technical, economic, and environmental considerations. By embracing innovation, sustainability, and a commitment to responsible development, HTC has the potential to become a key technology for producing high-quality carbon-based materials, contributing to a more sustainable and resource-efficient future [84, 85]. CarboZym has already reached key milestones, producing several hundred grams of support materials per week at laboratory scale with a high degree of homogeneity and reproducibility.
5. Conclusion
This report highlights the transformative potential of CarboZym technology through three compelling case studies. CarboZym’s carbon-based, biomass-derived carriers offer a unique advantage in biomanufacturing by minimizing reliance on petrochemicals and aligning with the industry’s growing demand for low-carbon solutions.
The simple immobilization procedure, demonstrated with industrially relevant enzymes (CALB, GsOYE, and ω-TA B9L0N2), proved quick and highly effective, achieving 95–100% immobilization within approximately two hours. This success underscores the versatility of CarboZym’s applicability in green chemistry and sustainable biocatalysis. Each immobilized enzyme exhibited distinct catalytic enhancements, showcasing the platform’s adaptability to diverse enzymatic systems.
Immobilized CALB demonstrated exceptional stability and reusability, maintaining industrially relevant conversion rates over multiple cycles with minimal enzyme leaching. GsOYE exhibited excellent efficiency and stability in both batch and continuous flow systems, achieving excellent turnover numbers in continuous mode. Immobilized ω-TA B9L0N2 achieved significant cost reductions through repeated use, retaining nearly full activity.
By combining high catalytic performance, low leaching, enhanced reusability, and a sustainable footprint, CarboZym’s immobilization platform addresses key challenges facing biomanufacturing.
The broader implications of this technology extend far beyond individual applications. As the biomanufacturing industry strives for greater sustainability and efficiency, CarboZym emerges as a crucial enabler of this transition. Successful scale-up and adoption of CarboZym-based systems can significantly reduce costs, accelerating the shift towards greener, more circular industrial practices.
In conclusion, the CarboZym technology represents a transformative approach to enzyme immobilization, poised to reshape the future of sustainable biomanufacturing. By seamlessly integrating high-performance catalysis, operational robustness, and environmental responsibility, CarboZym paves the way for a more economically and ecologically viable industrial biotechnology landscape, unlocking new avenues for innovation and growth.
Declaration of interests
JD, NB and CM are declared inventors on patents WO2024153593A1, WO2024153594A1 used by CarboZym. JD, NB and QH are cofounders of the CarboZym SAS.
Funding
This project was supported by Univ. Montpellier (LabMUSE Chimie 2021), Région Occitanie (Pre-maturation 2021) and SATT AxLR (Pre-maturation 2021, Maturation 2023).
1 CarboZym SAS, a CNRS spin-off established in November 2024, has set itself the mission to transform chemical manufacturing through the power of enzymes.





 CC-BY 4.0
CC-BY 4.0