1. Introduction
Saturated and unsaturated dicarboxylic acids are an attractive tool for bridging metal nodes to create hybrid metal–organic coordination polymers, which have been extensively studied in recent decades due to their diverse architecture and potential applications in luminescence, optics, catalysis, etc. [1, 2, 3, 4, 5]. Although crystal structures of four uranium coordination polymers have been established so far with citraconate anions as bridging ligands (Figure 1, top) [6, 7, 8], there are no data on the structures of uranyl compounds that contain anions of itaconic acid (Figure 1, bottom), a structural isomer of citraconic acid. One possible reason for this is the difficulties in growing large single crystals of uranyl itaconate compounds. Structural characterization of these crystals is hindered when using in-house single-crystal XRD. This work focuses on the synthesis and study of a new uranyl itaconate complex with N,N-dimethylacetamide C4H9NO (Dma), having the chemical formula [UO2(C5H4O4)(Dma)] (I) using synchrotron radiation and Fourier Transform Infrared (FTIR) spectroscopy.
Structural formulas of citraconic (top) and itaconic (bottom) acids.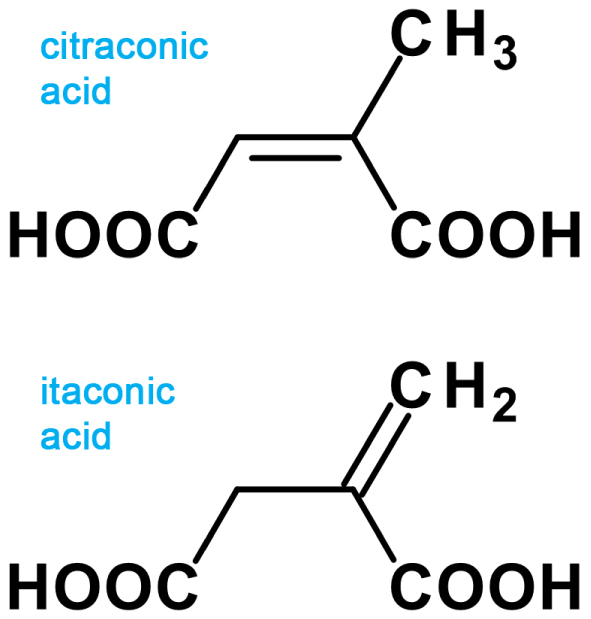
2. Material and methods
2.1. Synthesis
Caution! Compounds of U present a potential health risk owing to radioactivity. Although the uranium precursors used contain depleted uranium, standard safety measures for handling radioactive substances must be followed.
A portion of itaconic acid (0.91 g, 20.0 mmol) was partially dissolved in 2 ml of distilled water. Dma (0.91 g, 30.0 mmol), uranium(VI) oxide (0.10 g, 1.0 mmol) and 5 ml of ethyl alcohol were added to the solution. The molar ratio of uranium oxide to itaconic acid and substituted amide was 1:20:30, respectively. The bright yellow transparent solution obtained after the complete dissolution of reagents was left for crystallization at room temperature. Yellow prismatic crystals corresponding to the formula [UO2(C5H4O4)(Dma)] (I) formed after 3 days of evaporation.
2.2. X-ray diffraction analysis
X-ray diffraction analysis was carried out on a synchrotron source at the National Research Center “Kurchatov Institute”. Crystallographic details are listed in Table 1. The coordinates of the basis atoms in the structure of I, general geometrical characteristics of the structural units and an ORTEP image can be found in Supplementary Material. The coordination numbers of all atoms were determined using the method of intersecting spheres [9, 10]. The atomic coordinates were deposited into the Cambridge Crystallographic Data Centre, CCDC 1956082 [11]. The figures with fragments of crystal structures in the current paper were prepared using the VESTA 3 visualization system [12].
Crystallographic data, experimental parameters and results of refinement of [UO2(C5H4O4)(Dma)] (I)
| Crystal system, space group, Z | Monoclinic, P21∕n,4 |
| a, Å | 8.3235(17) |
| b, Å | 12.213(2) |
| c, Å | 12.911(3) |
| β, deg | 100.71(3) |
| V , Å3 | 1289.6(5) |
| Dx, g∕cm3 | 2.499 |
| Radiation (λ; Å) | Synchrotron; 0.9699 |
| μ, mm−1 | 13.035 |
| Temperature, K | 100 |
| Crystal dimensions, mm | 0.07 × 0.05 × 0.03 |
| The registration of absorption; Tmin; Tmax | Multi-scan; 0.462; 0.696 |
| θmax, deg | 38.435 |
| h, k, lrange | − 7⩽h⩽9 |
| − 15⩽k⩽15 | |
| − 13⩽l⩽16 | |
| Reflection number: collected/unique | 8042/2629; 0.0870/1760 |
| (N1); Rint∕with I > 2σ(I) (N2) | |
| F (000) | 888 |
| Parameters refined | 167 |
| Uncertainty values: | |
| wR2 on N1 | 0.1783 |
| R1 on N2 | 0.0688 |
| S | 1.005 |
| 𝛥ρmin∕𝛥ρmax, e∕Å3 | − 2.181∕2.927 |
2.3. FTIR spectroscopy
The FTIR spectrum of compound I was recorded as a KBr pellet at room temperature in the range of 500 −−3000 cm−1 on a PerkinElmer Spectrum 100 FT-IR spectrometer. The assignment of absorption bands was performed according to the literature data [13, 14, 15] and the Spectral Database for Organic Compounds [16] (Table 2). The FTIR spectrum of compound I exhibits characteristic absorption bands corresponding to vibrations of and ions and Dma molecules. The antisymmetric valence vibration of the group (ν3) appears at 931 and 918 cm−1. The decrease in the vibration frequency ν(C=O) of Dma in the FTIR spectrum of the title compound (1609 cm−1) as compared to free molecules (1655 cm−1 [17]) indicates that the amide is coordinated to the uranyl cation.
Assignment of absorption bands in FTIR spectrum of [UO2(C5H4O4)(Dma)] (I)
| Wavenumber, cm−1* | Assignment |
|---|---|
| 2926 w. | ν(CH) |
| 1704 v.s. | ν(C=C), ν(C=O) |
| 1609 s. | ν(C=C), ν(C=O) |
| 1567 v.s. | νas(COO) |
| 1528 v.s. | νas(COO) |
| 1509 v.s. | νas(COO) |
| 1444 v.s. | δas(CH3), νs(COO) |
| 1404 v.s. | δas(CH3), νs(COO) |
| 1385 s. | δs(CH3) |
| 1310 m. | δ(CH) |
| 1247 m. | δ(CH), ν(C−N) |
| 1217 m. | δ(CH), ν(C−N) |
| 1168 m. | δ(CH), ν(C−N) |
| 1044 m. | δ(CH), ν(C−N) |
| 1027 m. | δ(CH), ν(C−N) |
| 987 w. | δ(CH) |
| 965 m. | δ(CH) |
| 931 v.s. | |
| 918 v.s. | |
| 883 m. | δ(CH) |
| 828 m. | δ(CH) |
| 753 m. | δ(CH) |
| 655 m. | δ(CH), δ(COO) |
| 625 m. | δ(COO) |
| 607 m. | δ(COO) |
| 555 m. | δ(COO) |
*Band intensities: v.s. – very strong, s. – strong, m. – medium, w. – weak.
3. Results and discussion
3.1. Description of the crystal structure
The structure of I contains one independent uranium atom with a coordination number of 7 and a coordination polyhedron in the form of a pentagonal bipyramid UO7. The axial positions of the coordination polyhedron are occupied by the uranyl oxygen atoms. Among the five equatorial oxygen atoms, four belong to three quadridentate bridging chelate itaconate ions (coordination mode Q21-4) and the remaining oxygen atom is related to the monodentate terminal molecule of Dma. The designations of coordination modes are given in accordance with the nomenclature from ref. [18].
The uranyl cation in the structure of I has the following characteristics: the U=O1 and U=O2 distances are equal to 1.752(13) Å and 1.774(12) Å, respectively, and the OUO angle is equal to 177.6(5)°. The uranium atom Voronoi–Dirichlet polyhedron (VDP) is a pentagonal prism with a volume of 9.11 Å3. This value of VDP volume is in good agreement with the average value of 9.2(2) Å3, established for U(VI) atoms surrounded by oxygen atoms [19].
Each itaconate anion in the structure of I uses all four of its oxygen atoms to form bonds with uranium atoms (this fact is denoted by the symbol Q – quadridentate – in the notation of their coordination mode Q21-4). In particular, it binds in the monodentate mode with two U atoms and in the bidentate chelate mode with one U atom.
The angle between the plane of the Dma molecule and the equatorial plane of the uranyl ion is approximately equal to 49°. The main structural units of I are the layers of the [UO2(C5H4O4)(Dma)] composition (Figure 2, top), which have the crystal chemical formula [18]. These layers form a family of lattice planes with Miller indices (). Such layers are interconnected into a supramolecular three-dimensional framework via intermolecular noncovalent interactions (Figure 2, bottom). The method of molecular VDP (MVDP) was applied to quantify the contribution (𝛥AZ, %) of various types of interactions into the supramolecular binding of structural units in I [20, 21, 22]. According to the obtained data (Table 3), hydrogen bonds (H/O contacts, Table 4) and dispersion interactions (H/H contacts) make the greatest contribution to the formation of the supramolecular structure of compound I – the values of 𝛥HO and 𝛥HH are equal to 39.0% and 47.8%, respectively.
In order to quantify the influence of the position of the double C=C bond on the characteristics of packing of neutral complexes, a comparative analysis of the title compound I with the earlier reported uranyl citraconate compound (II), which corresponds to the same chemical formula [UO2(C5H4O4)(Dma)] [8], was carried out with the use of the MVDP method. The structure of compound II also contains neutral layers of the composition [UO2(C5H4O4)(Dma)] with the crystal chemical formula . The uranium atom in the structure of II has the same coordination number of 7 and a similar coordination polyhedron in the form of a pentagonal bipyramid as in compound I.
According to our calculations, at the intermolecular level (Table 3), compound I exhibits C/C and H/N interactions, which are not manifested in compound II. At the intramolecular level (Table 5), the two compounds differ in the interactions C/N, C/U, H/U and N/N. The change in the position of the double C=C bond in the carbon skeleton of itaconic and citraconic acids leads to an increase in mobility of one of the carboxylate groups of the itaconic acid molecule and, as a consequence, an increase in the number of noncovalent interactions in the structure of crystals of the title compound in comparison with the uranyl citraconate complex II (5 and 3 types of intermolecular contacts in compounds I and II, respectively).
3.2. Analysis of coordination modes of itaconate ions
To summarize the available information on the structure and coordination modes of itaconate ions, the crystal chemical analysis of all itaconate-containing compounds from the CSD (Cambridge Structural Database, version 5.40 with August 2019 update) was performed [11]. The following criteria were employed for the formation of the subset of compounds [23]:
(1) The crystal structure contains itaconate ions bound to the metal coordination center.
(2) The bond valence S (S = v∕CN, where v is the valence of the coordination center and CN is its coordination number in the crystal structure) of metal–oxygen bonds between metals and itaconate ions is ⩾0.25.
(3) The crystal structure is determined with a nonzero R1 value, which does not exceed 0.1.
(4) The crystal structure does not exhibit disorder.
The stated requirements were satisfied by the data on the crystal structures of 31 coordination compounds containing 46 crystallographically independent itaconate ions. Cu, Ca, Zn, Cd, Co, Ni, Pt, La, Gd, Tb, Dy and Ho serve as coordination centers in these compounds.
The single layer in the structure of [UO2(C5H4O4)(C4H9NO)] (I) on the () projection (top) and the arrangement of layers relative to each other (view along the layers, bottom). Uranium, oxygen, nitrogen, carbon and hydrogen atoms/coordination polyhedra are depicted by yellow, red, blue, gray and pink, respectively.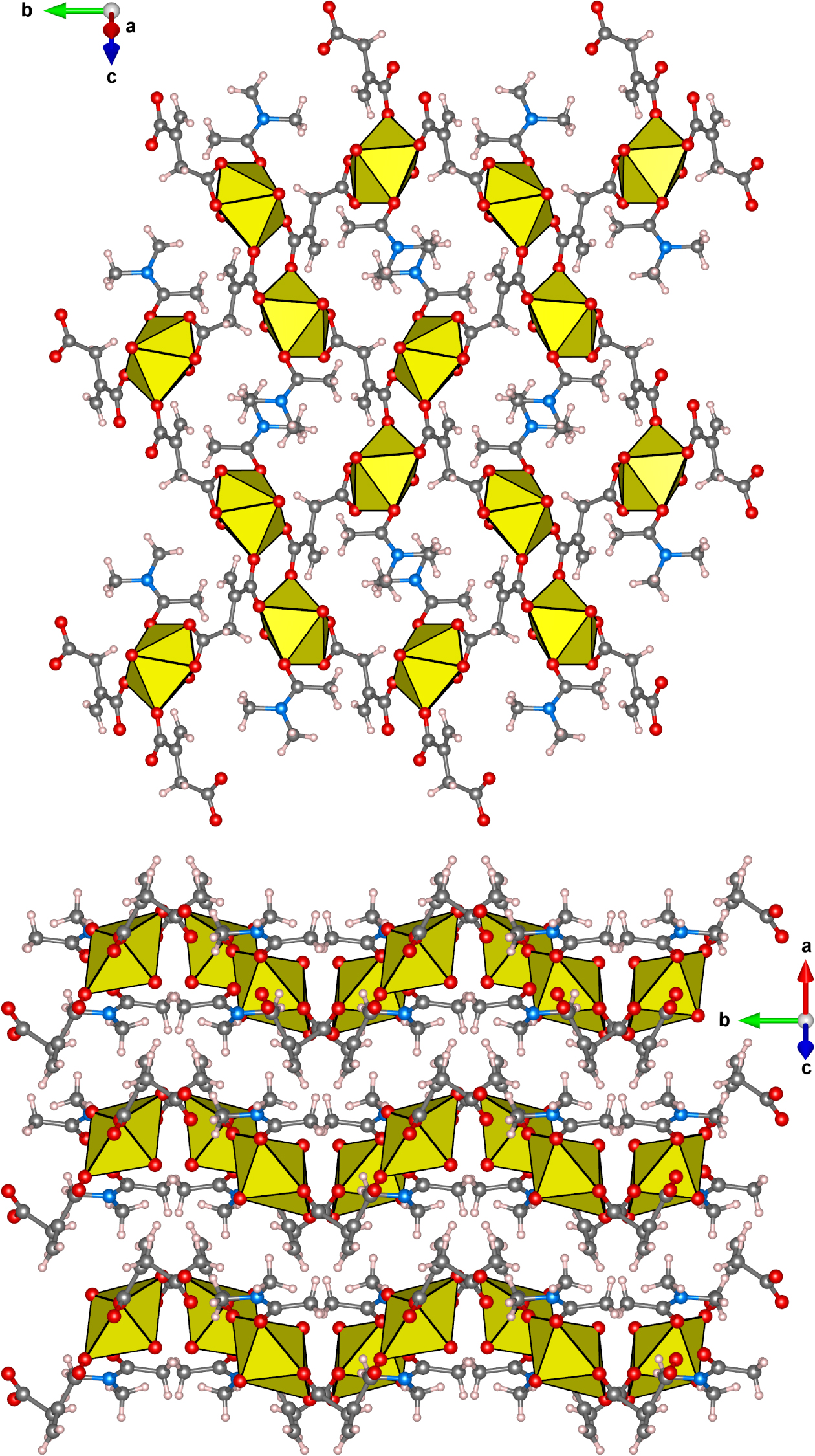
Characteristics of intermolecular noncovalent interactions in structures of compound I and isomeric complex of uranyl citraconate with Dma (II)*
| Contact A∕Z | N | d, Å | SAZ, Å2 | 𝛥AZ, % |
|---|---|---|---|---|
| [UO2(C5H4O4)(C4H9NO)] (I), itaconate | ||||
| H/H | 43 | 2.429–4.454 | 66.62 | 47.8 |
| H/C | 20 | 2.833–4.052 | 16.76 | 12.0 |
| C/C | 5 | 3.932–4.167 | 0.42 | 0.3 |
| H/N | 2 | 3.464–3.464 | 1.36 | 1.0 |
| H/O | 32 | 2.613–4.405 | 54.33 | 39.0 |
| Sum | 102 | 2.429–4.454 | 139.49 | 100.0 |
| [UO2(C5H4O4)(C4H9NO)] (II), citraconate | ||||
| H/H | 26 | 2.481–4.256 | 39.38 | 37.5 |
| H/C | 26 | 2.744–4.193 | 13.92 | 13.2 |
| H/O | 26 | 2.606–4.245 | 51.79 | 49.3 |
| Sum | 78 | 2.481–4.256 | 105.08 | 100.0 |
* N is the total number of all faces of VDPs, corresponding to intermolecular interactions; d is the range of the corresponding interatomic distances A∕Z; SAZ is the total area of all faces of a given type in the VDPs of atoms contained in one formula unit of a compound; 𝛥AZ is the partial contribution of the corresponding noncovalent A∕Z contacts to the total sum (the bottom line) for the molecular VDP.
Parameters of hydrogen bonds in the structure of I*
| C–H⋯O | d(C⋯O), Å | d(C−H), Å | d(H⋯O), Å | Angle (C–H⋯O), deg | 𝛺(H⋯O), % | RF** |
|---|---|---|---|---|---|---|
| C5–H3⋯O6 | 3.329 | 0.95 | 2.61 | 133.1 | 11.51 | 6 |
| C5–H4⋯O1 | 3.473 | 0.95 | 2.61 | 150.1 | 12.52 | 0 |
| C8–H8⋯O2 | 3.477 | 0.98 | 2.61 | 147.5 | 14.78 | 20 |
| C9–H11⋯O1 | 3.690 | 0.98 | 2.91 | 136.9 | 11.04 | 13 |
| C9–H12⋯O5 | 3.465 | 0.98 | 2.50 | 170.0 | 14.51 | 18 |
* Allowance was made for contacts with C–H⋯O angle >130°, d(H⋯O) < 3 Å and 𝛺(H⋯O) > 10%.
** The rank of face (RF) indicates the minimal number of covalent bonds connecting atoms whose VDPs have a common face. RF = 1 for chemical bonds, RF > 1 for intramolecular noncovalent contacts and RF = 0 for intermolecular contacts.
The occurrence of 17 coordination modes of itaconate ions in the crystal structures of coordination compounds present in the CSD.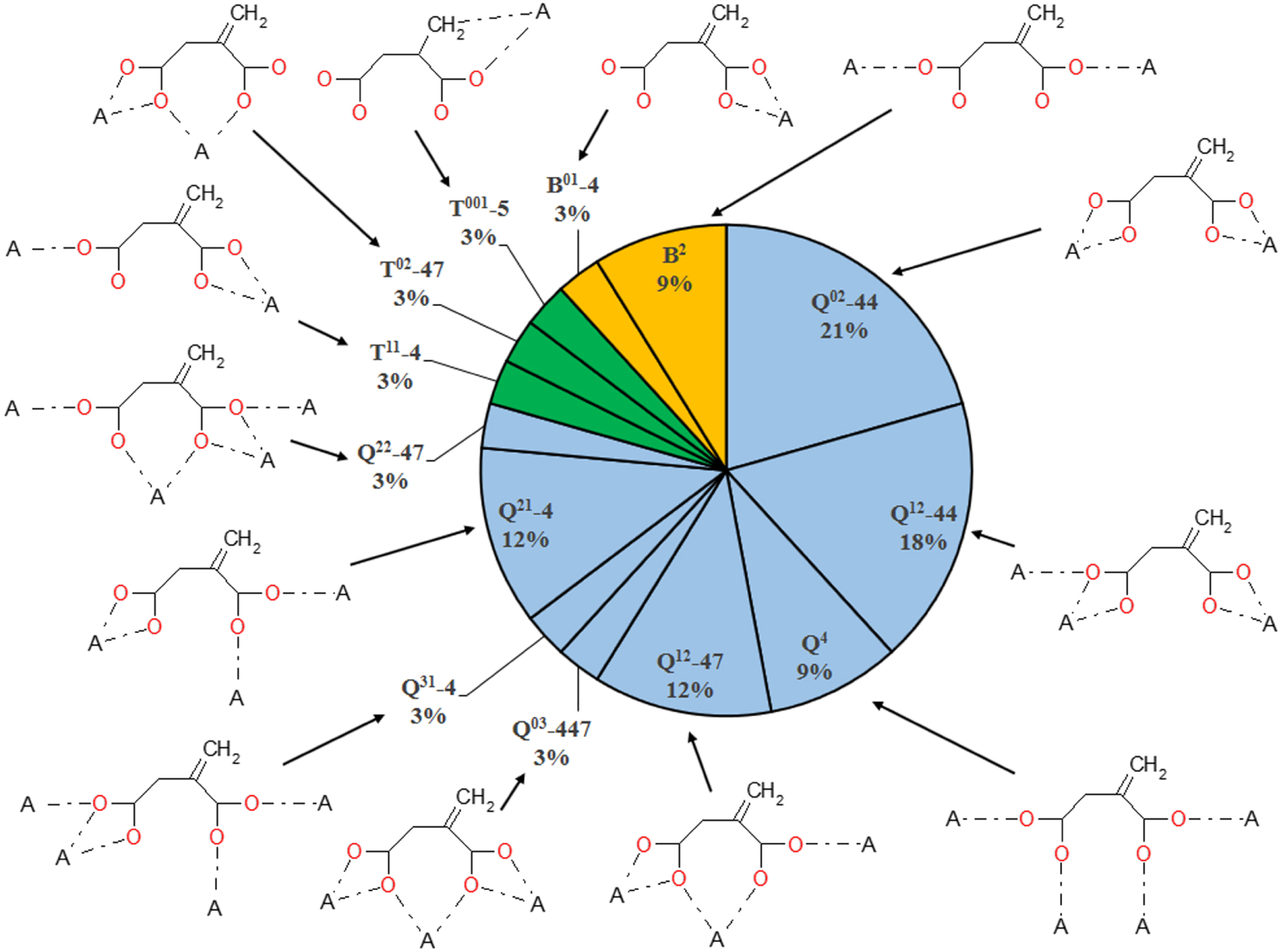
In the investigated compounds, itaconate ions exhibit 17 different coordination modes (Figure 3). Seven of them are the most common and are observed for 78% of itaconate ions: Q02-44, Q12-44, Q4, Q12-47, Q21-4, T3 and B2. The identified coordination modes show that itaconate ions are able to act as terminal (B01-4 and T001-35) and bridging (Q02-44, Q12-44, Q4, Q12-47, Q21-4, Q31-4, Q03-447, Q22-47, Q21-7, T11-4, T02-47, T3, T21-4, T11-7 and B2) ligands and are able to coordinate to the central atom without the formation of cycles (B2, Q4 and T3) or with the formation of four- to seven-membered cycles (B01-4, Q02-44, Q12-44, Q12-47, Q21-4, Q31-4, Q03-447, Q22-47, Q21-7, T11-4, T02-47, T21-4 and T11-7). Among the 46 studied itaconate ions, 25 ions form four-membered cycles and 3 ions form seven-membered cycles, while 7 ions form simultaneously both four- and seven-membered cycles (itaconate ions with coordination modes Q12-47, Q03-447, Q22-47 and T02-47). In addition to the oxygen atoms of two carboxylate groups, itaconate ions were found to coordinate through the olefin group to the Pt atom (coordination mode T001-35 with the formation of a five-membered cycle) in the single known coordination compound of platinum(II) itaconate with 2,2-diaminomethylpropane [24].
Histogram of angles between the planes of two carboxylate groups in itaconate ions in the crystal structures of coordination compounds present in the CSD.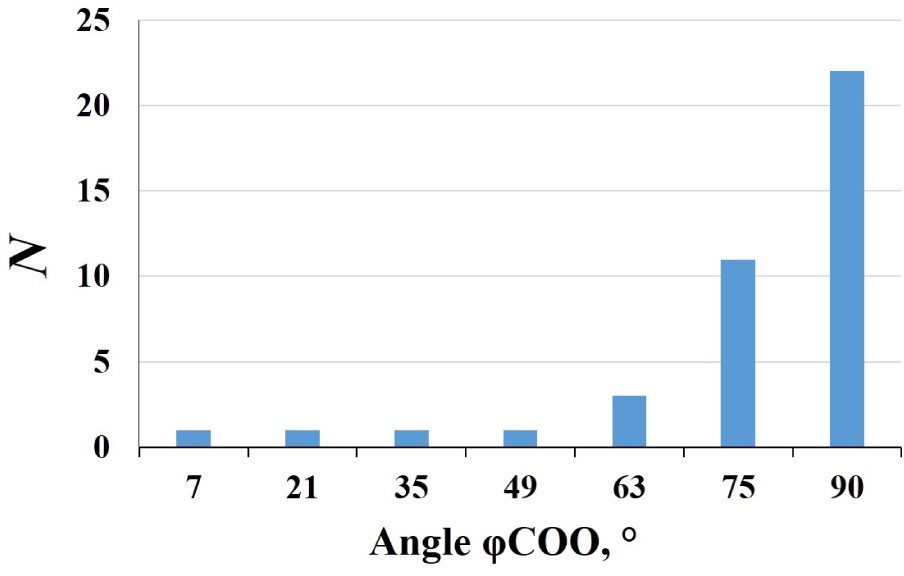
Characteristics of intramolecular noncovalent interactions in the structures of compared compounds*
| Contact A∕Z | N | d, Å | SAZ, Å2 | 𝛥AZ, % |
|---|---|---|---|---|
| [UO2(C5H4O4)(C4H9NO)] (I), itaconate | ||||
| H/H | 31 | 1.599–4.353 | 65.66 | 30.6 |
| H/C | 33 | 1.916–3.770 | 20.12 | 9.4 |
| C/C | 2 | 2.441–2.556 | 0.33 | 0.2 |
| H/N | 11 | 2.003–3.701 | 10.08 | 4.7 |
| N/N | 1 | 3.657–3.657 | 0.18 | 0.1 |
| H/O | 40 | 2.346–4.373 | 79.62 | 37.1 |
| C/O | 12 | 2.356–3.710 | 4.94 | 2.3 |
| N/O | 1 | 2.263–2.263 | 0.09 | 0.04 |
| O/O | 20 | 2.190–4.277 | 33.68 | 15.7 |
| H/U | 1 | 3.429–3.429 | <0.01 | <0.01 |
| Sum | 152 | 1.599–4.373 | 214.70 | 100.0 |
| [UO2(C5H4O4)(C4H9NO)] (II), citraconate | ||||
| H/H | 43 | 1.599–4.460 | 85.18 | 36.2 |
| H/C | 26 | 1.964–3.845 | 19.98 | 8.5 |
| C/C | 5 | 2.480–3.783 | 0.39 | 0.2 |
| H/N | 10 | 2.011–3.990 | 11.12 | 4.7 |
| H/O | 41 | 2.477–4.345 | 74.15 | 31.5 |
| C/O | 16 | 2.340–3.727 | 7.45 | 3.2 |
| N/O | 2 | 2.230–3.675 | 0.13 | 0.1 |
| O/O | 21 | 2.201–4.335 | 37.12 | 15.8 |
| C/U | 1 | 2.813–2.813 | <0.01 | <0.01 |
| C/N | 1 | 2.439–2.439 | 0.01 | 0.01 |
| Sum | 166 | 1.599–4.460 | 235.53 | 100.0 |
* See footnote to Table 3.
The closest homologue of itaconic acid is maleic acid. For the maleic acid, it was found in ref. [23] that the double C=C bond leads to the rigid geometry of the maleate ions in the crystal structures. The displacement of C atoms from the plane of the carbon skeleton in all maleate ions does not exceed 0.05 Å, and the mean torsion angle C–C=C–C is equal to 1(2)°. Two carboxylate groups of the maleate ions in the structures are preferably orthogonal to each other. Analysis of itaconate-containing compounds from the CSD shows that the aforementioned trend in the mutual arrangement of the two maleate carboxylate groups is also observed for itaconate ions (Figure 4). The two COO groups of itaconate ions in the structures of the investigated complexes show a tendency toward a mutually perpendicular arrangement. The bonding of one of the carboxylate groups with the sp3 hybridized carbon atom can be noted as a peculiarity of the structure of the itaconic acid molecule. Due to this feature, the carbon skeleton of this acid becomes more flexible: the methylene fragment together with one of the carboxylate groups can rotate with respect to the double bond and the other COO group (Figure 1, bottom). In the structures of 31 studied compounds, the angle between the planar fragment containing the double C=C bond and the C atom of the nearest carboxylate group from one side and the bond between the sp3 hybridized carbon atom and the C atom of the carboxylate group from the other side varies from 2° to 71° (Figure 5). This fact confirms the lability of the carbon skeleton of itaconate ions as ligands.
The angle between the planar fragment containing the C=C double bond and the C atom of the nearest carboxylate group from one side and the bond between the sp3 hybridized carbon atom and the C atom of the carboxylate group from the other side in the itaconate ion.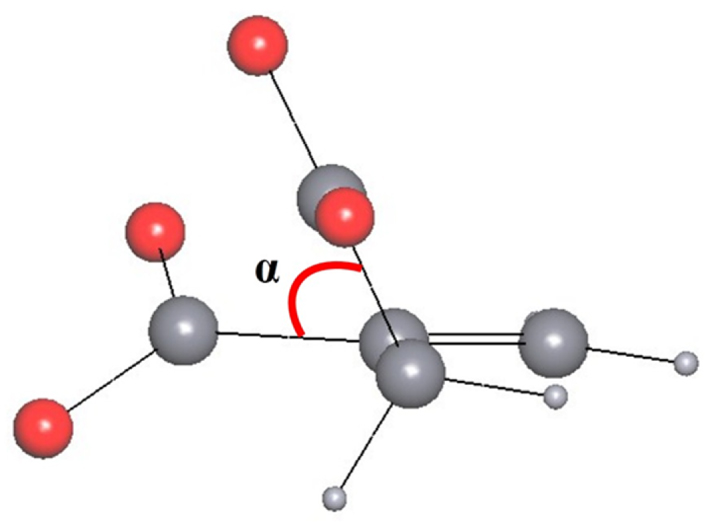
4. Conclusion
The synthesis, FTIR spectrum and the crystal structure of a new uranyl complex (I) with itaconate ions are reported. The crystal structure of I is constructed of layers with the composition [UO2(C5H4O4)(Dma)]. Itaconate ions realize quadridentate bridging and chelating coordination mode Q21-4 with the formation of four-membered U-containing cycles.
A comparative analysis of noncovalent interactions in the structures of the synthesized compound (I) and the isomeric uranyl citraconate complex with dimethylacetamide (II) was performed. According to our calculations, at the intermolecular level, compound I exhibits C/C and H/N interactions, which are not manifested in compound II. At the intramolecular level, the two compounds differ in the C/N, C/U, H/U and N/N interactions.
The coordination modes of itaconate ions in all the crystal structures of coordination compounds from the CSD were analyzed. It was shown that the itaconate ions adopts 17 different coordination modes, seven of which are the most abundant: Q02-44, Q12-44, Q4, Q12-47, Q21-4, T3 and B2.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This project was financially supported by the base part of the government mandate of Ministry of Science and Higher Education of the Russian Federation (project numbers 4.5037.2017/8.9 and 0777-2017-0008). YVZ is indebted to Ministry of Science and Higher Education of the Russian Federation (project AAAA-A19-119020890025-3). The contribution of the Center for Molecular Composition Studies of INEOS RAS is gratefully acknowledged.
Supplementary data
Supporting information for this article is available on the journal’s website under https://doi.org/10.5802/crchim.8 or from the author. It contains experimental details and randomization protocols.
CCDC-1956082 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre http://www.ccdc.cam.ac.uk/.




 CC-BY 4.0
CC-BY 4.0