1 Introduction
One of the current challenges in molecular science is to prepare new compounds with combinations of two physical properties as for example magnetic interactions and electrical conductivity [1–9]. The strategy used up to now consists in assembling together conducting organic sublattice and paramagnetic inorganic sublattice in salts made of organic radical ions derived from TTF, tetrathiafulvalene and paramagnetic inorganic counter-ions. This yields in general materials with weak interactions (when they exist) between the two systems. In order to establish such interactions, we are investigating several ideas: (i) the synthesis of new materials where the conducting and magnetic systems are covalently linked through π-conjugated bridge, such as [M(hfac)2](TTF–py)2 (M = CuII, MnII, hfac = hexafluoroacetylacetonate; TTF–py = 4-(2-tetrathiafulvalenylethenyl)pyridine) [9]; (ii) the assemblies of functionalized organic donors bearing –OH or halogen (–X) functions and paramagnetic cyano complex anions, with the aim to mediate magnetic interaction through hydrogen bonding or –CN···X–interactions [5]; (iii) the assemblies of organic donors with cyano or thiocyanato paramagnetic transition metal complexes involving π ligands (see scheme). This might yield short S···S and/or –CN···X– contacts between the donors and the anions and also π–π overlap between the ligand of the anion and the π system of the donor molecule. Hence, numerous salts were obtained with this strategy and showed bulk ferrimagnetism and weak bulk ferromagnetism [8]. Recently developed tetrathiapentalene(TTP)-derived donors have high tendencies of producing metallic salts down to low temperatures, and also have potential to give molecular magnets as evidenced by an antiferromagnetic transition in β-(BDA–TTP)2FeCl4 (BDA–TTP = 2,5-bis(1’,3’-dithian-2’-ylidene)-1,3,4,6-tetrathiapentalene) [10]. We have therefore focused on this series of donors and developed a molecular magnet (BDH–TTP)[Cr(isoq)2(NCS)4], where BDH–TTP and isoq are 2,5-bis(1’,3’-dithiolan-2’-ylidene)-1,3,4,6-tetrathiapentalene and isoquinoline, respectively [11]. This salt shows a weak ferromagnetism with the transition temperature TC = 7.6 K, whose magnetic structure is characterized from single-crystal magnetic measurements as a spin-canted weak ferromagnetism derived from the single-ion anisotropy of the anions. Among the series of TTP-derived donors, DTDH–TTP (2-(1,3-dithiolan-2-ylidene)-5-(1,3-dithiol-2-ylidene)-1,3,4,6-tetrathiapentalene) is found to produce metallic salts down to low temperatures depending on the counter-anions [12]. As this molecule has a C=C double bond at one of the molecular ends and the π-electron system is more delocalised than BDH–TTP, the on-site Coulomb repulsion on the donor is reduced and the intermolecular exchange interaction is expected to be increased. In the same time, this molecule also remains C–C single bond at the other molecular end, whose flexibility increases the freedom of the packing in the crystal. There is, therefore, potential interest in studying salts containing this donor combined with paramagnetic anions in the development of new organic/inorganic hybrid molecular materials. Here we report the synthesis, X-ray crystal structure and magnetic properties of (DTDH–TTP)[Cr(isoq)2(NCS)4] (Fig. 1).

Structures of DTPH–TTP, BDH–TTP and Cr(isoq)2(NCS)4.
2 Experimental
2.1 Synthesis
The solvents were distilled under nitrogen atmosphere before use and the starting reagents were used as received. Black crystals of the title compound were obtained by galvanostatic (I = 1 μA) oxidation of DTDH–TTP [12] (8 mg) under argon atmosphere, using (isoqH)[Cr(isoq)2(NCS)4]·3 H2O [8] (100 mg) in CH2Cl2 (20 ml) as electrolytes.
2.2 Crystallographic data collection and structure determination
Single crystal of the title compound was mounted on a Nonius four-circle diffractometer equipped with a CCD camera and a graphite monochromated Mo Kα radiation source (λ = 0.710 73 Å). Effective absorption correction was performed (SCALEPACK [13]). Structure was solved with direct methods and refined with full matrix least squares method on F2 using SHELX-97 [14] programs. Crystallographic data are summarized in Table 1. Full bond lengths and bond angles, atomic coordinates and complete crystal structure results are deposited as supplementary materials.
Crystal data and structure refinement
| Empirical formula | C32H20CrN6S12 |
| Formula weight | 925.26 |
| Temperature (K) | 293(2) |
| Radiation, λ/Å | Mo Kα, 0.710 73 |
| Crystal system | Monoclinic |
| Space group | C2/c (# 15) |
| a (Å) | 16.0836(5) |
| b (Å) | 19.2448(6) |
| c (Å) | 12.6829(6) |
| α (°) | 90 |
| β (°) | 95.669(1)) |
| γ (°) | 90 |
| V (Å3) | 3906.5(2) |
| Z | 4 |
| dcalc (g cm– 3) | 1.573 |
| μ (mm– 1) | 0.969 |
| Reflections collected | 8792 |
| Independent reflections | 4476 |
| [I > 2 σ(I)] | 2899 |
| Final R1a, wR2b | 0.0515, 0.1248 |
2.3 Physical property measurements
Magnetic susceptibility was measured using a Quantum Design MPMS-5 SQUID magnetometer down to 1.8 K with an applied field of 0.1 T. Non-oriented microcrystalline sample (2.8 mg) was wrapped with an aluminium foil, whose contribution was subtracted from the observed data.
3 Results and discussion
3.1 Crystal structure
Selected bond distances and bond angles are given in Table 2. ORTEP drawing [15] of the molecular structure with the atomic numbering scheme is shown in Fig. 2.
Selected bond lengths (Å) and angles (°)
| Cr–N(1) | 1.988(3) | S(5)–C(14) | 1.731(4) |
| Cr–N(2) | 1.987(3) | S(5)–C(16) | 1.742(6) |
| Cr–N(3) | 2.085(3) | S(6)–C(14) | 1.739(4) |
| S(4)–C(12) | 1.729(4) | S(6)–C(15) | 1.771(5) |
| S(4)i–C(13) | 1.749(4) | C(1)–S(1) | 1.612(4) |
| S(3)–C(12) | 1.729(4) | C(2)–S(2) | 1.614(4) |
| S(3)–C(13) | 1.736(4) | C(12)–C(12)i | 1.358(7) |
| C(15)–C(16) | 1.379(8) | ||
| N(1)–Cr–N(2) | 90.0(1) | C(14)–S(5)–C(16) | 95.5(2) |
| N(1)–Cr–N(3) | 90.5(1) | C(14)–S(6)–C(15) | 95.6(2) |
| N(2)–Cr–N(3) | 89.6(1) | C(1)–N(1)–Cr | 176.5(3) |
| C(12)–S(3)–C(13) | 93.8(2) | C(2)–N(2)–Cr | 172.2(3) |
| C(12)–S(4)–C(13)i | 93.4(2) |
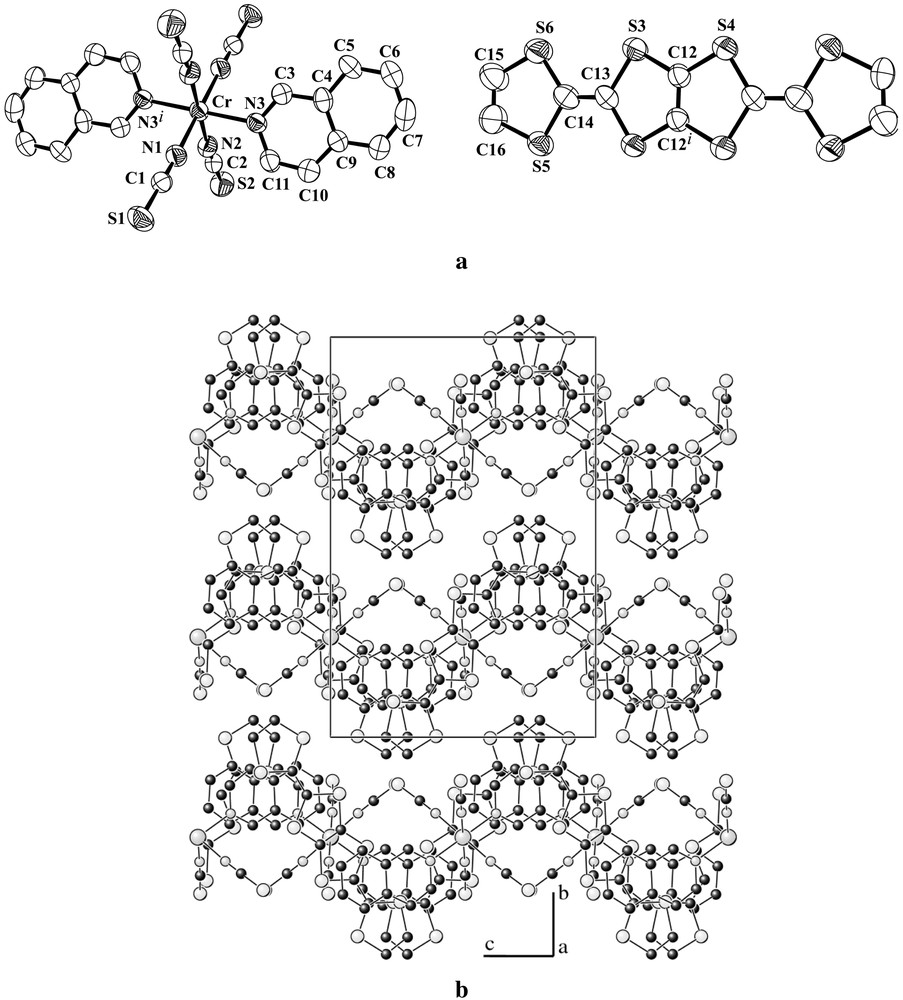
(a) ORTEP diagram with 50% probability level and atom numbering scheme; (b) projection of the crystal structure onto the bc plane, showing the π overlap between the isoquinoline ligands and the DTDH–TTP molecules.
The title compound is isostructural to the previously reported salt (BDH–TTP)[Cr(isoq)2(NCS)4] [11]. The asymmetric unit contains half of an anion and half of a DTDH–TTP molecule, both lying on inversion centres in special positions, (1/4, 1/4, 0) and (1/4, 1/4, 1/2), respectively. The Cr–N (of NCS) distances (average value 1.988(3)Å) are slightly shorter than those to isoquinoline (Cr–N3 = 2.085(3) Å), hence the CrN6 coordination octahedra is axially distorted. As the organic molecule on the inversion centre is asymmetric, it namely contains one single C–C bond in one side and a double C=C bond in the other side, and lies on the inversion centre, this unit should be orientationally disordered. However, we did not detect this disorder from the X-ray analysis. From the 1:1 stoichiometry, the charge on the donor molecule is assumed equal to +1. As shown in Fig 2b, the cations and anions form mixed zigzag layers within the bc-plane, which are then stacked along the b-direction. The layer consists of one-dimensional chains elongated to the c-direction (Fig. 3). The S···S distances of the short intermolecular contacts within the chain (S2···S3: 3.350(2), S2···S4: 3.419(2), S1···S5: 3.420(2) Å) are remarkably shorter than the van der Waals distance (3.70 Å) [16]. The adjacent chains are then connected with a π–π overlap of isoquinoline moieties (interplanar separation: d = 3.64 Å) between the anions, and short S···S contact (S5···S5 = 3.621(3) Å) between the donors. In the [101] direction, the neighbouring anions are related by the c-glide plane, and therefore the orientation of these anions is different; the angle between the molecular axes (defined as isoq–Cr–isoq axes) is 7.7(3)°. The difference in the orientation of the units in the [101] direction can be seen more clearly considering the DTDH–TTP donors that are orthogonal to each other.
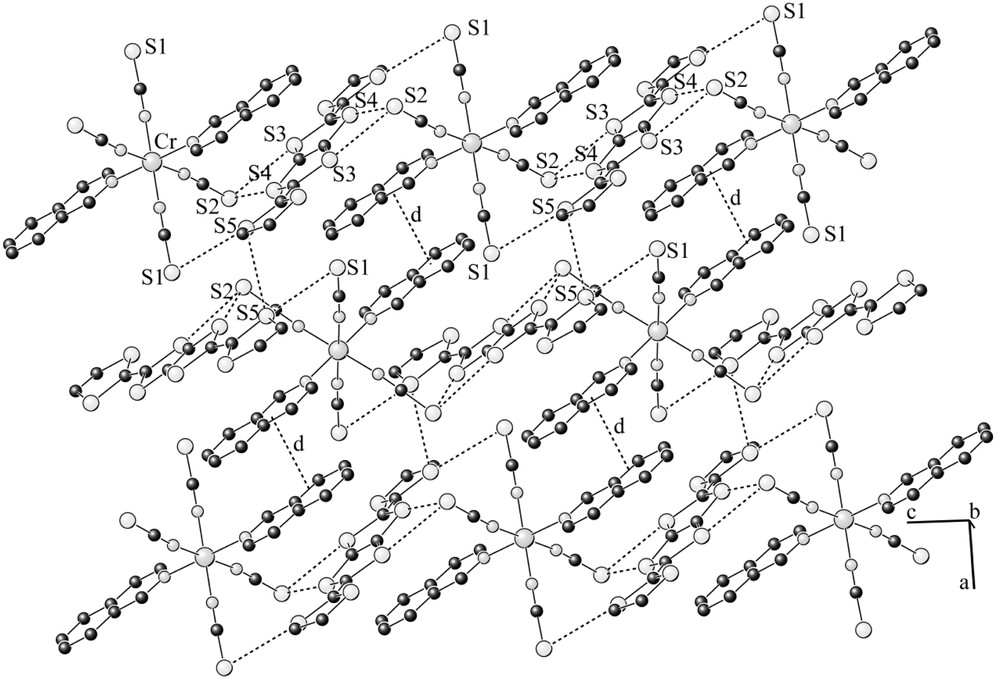
Projection of the mixed layer onto the ac plane showing the mixed chain of anion and donor; dotted lines indicate the anion–anion overlap (interplanar separation: d = 3.64 Å) and intermolecular S···S contacts (S2···S3: 3.350(2), S2···S4: 3.419(2), S1···S5: 3.420(2), S5···S5: 3.621(2) Å).
3.2 Magnetic properties
Fig. 4 shows the temperature dependence of χ T and χ–1 measured at 0.1 T, where χ is the molar magnetic susceptibility for non-oriented microcrystalline sample after subtracting core diamagnetism contribution (= –4.67 × 10–4 emu mol–1). The susceptibility obeys the Curie–Weiss law above ca. 50 K with the Curie constant C = 2.35 emu K mol–1 and the Weiss temperature Θ = –20 K. The Curie constant is close to the corresponding spin-only value (2.25 emu K mol–1) for non-interacting anion (S = 3/2) and donor (S = 1/2) spins. Below 50 K, χ T decreases as the temperature decreases, showing the antiferromagnetic coupling between the anion and donor spins. At TC = 8.7 K, χ T shows a steep increase due to a magnetic transition. The inset in Fig. 4 presents the field-cooled magnetization (FCM) measured at 0.1 T, and the remanent magnetization (RM) observed after the external field of 0.1 T is removed at 1.8 K. Below TC = 8.7 K, the FCM steeply increases and the RM also emerges, showing the presence of the spontaneous magnetization. When comparing the present DTDH–TTP salt with the isomorphous (BDH–TTP)[Cr(isoq)2(NCS)4] [11], the magnetic transition temperature is slightly increased, whereas the Weiss temperature remains in the same value. Since a one-dimensional system does not take place in a magnetic ordering in the finite temperature, the magnetic transition is realized by the inter-chain interactions and the transition temperature is governed by their strength. The S···S contacts between the donors in the adjacent chains (S5···S5: 3.621(2) Å) are slightly shorter than in the BDH–TTP salt (3.639(2)Å). This decrease of the inter-chain S···S distance between donors may be responsible to the increase of TC in the present DTDH–TTP salt. In the same time, the HOMO of DTDH–TTP calculated using a semi-empirical PM5 Hamiltonian [17] (Fig. 5) is more delocalised to the outer sulphur atoms (S5 and S6 in Fig. 2a) compared to BDH–TTP due to the presence of the terminal C=C double bond. As a result, inter-chain exchange interactions between donors are increased, which also works to elevate TC of this compound.
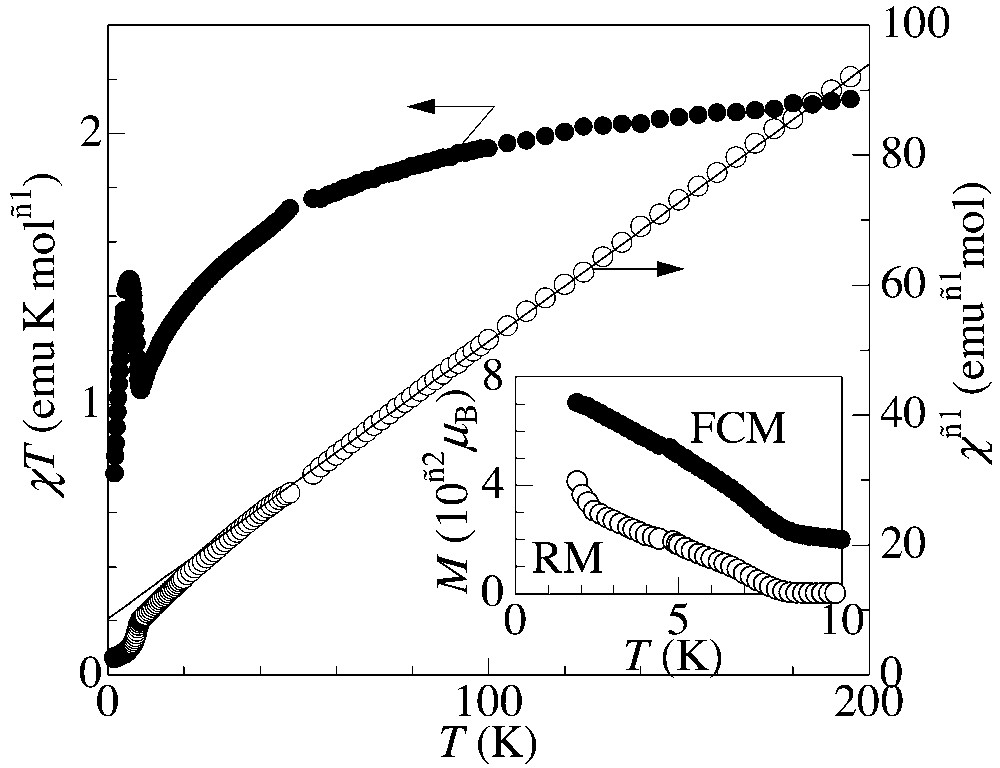
Temperature dependence of χ T (filled circles) and χ–1 (open circles), where χ is the molar paramagnetic susceptibility. Solid line is the Curie–Weiss fitting (C = 2.25 emu K mol–1, Θ = –20 K). Inset: temperature dependence of the field-cooled (•) and remanent magnetization (○).
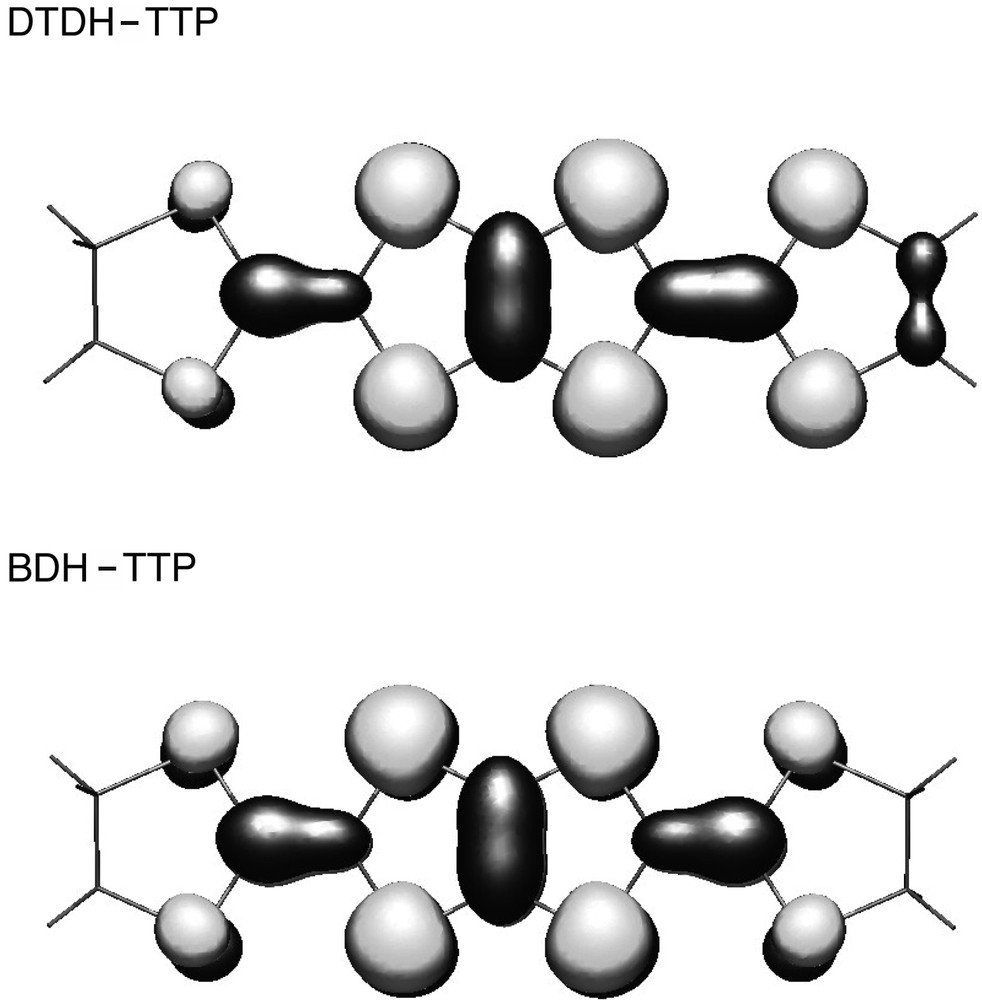
The HOMOs of DTDH–TTP (top) and BDH–TTP (bottom), calculated using a semi-empirical method using PM5 Hamiltonian.
Fig. 6 shows the magnetization curves at T = 2 K measured on non-oriented crystalline samples. As the field increases, the magnetization tends to reach the value of 2 μB, showing that all anion spins (S = 3/2) and donor spins (S = 1/2) are aligned anti-parallel to form ferrimagnetic chains. On the magnetization curve, a broad anomaly is observed at B = 1 T, which is more clearly shown as a peak in the dM/dB vs. B plot (lower panel of Fig. 6). From the analogy to the previous result on the BDH–TTP salt [11], this anomaly should be assigned to a spin-flop transition. Around B = 0 T, a hysteresis loop is observed, which is consistent with the presence of the remanent magnetization. Although the present magnetic data are based on the non-oriented sample, it is reasonable to assume that the spontaneous magnetization is parallel to the b-axis direction (perpendicular to the two-dimensional sheets of the donors and anions), since this salt is isostructural to (BDH–TTP)[Cr(isoq)2(NCS)4] whose magnetic structure has been clarified from the single-crystalline data. From this hysteresis loop, we can estimate the remanent magnetization along the b-axis from the hysteresis loop at where is the average value of the direction cosine. The spin-canting angle is therefore estimated at sin–1(0.10 μB/2 μB) = 2.9°, which is same as the corresponding value of (BDH–TTP)[Cr(isoq)2(NCS)4]. This accordance in the canting angle for both compounds is reasonable, since the spin canting is derived from the single-ion anisotropy caused by the uniaxial distortion of CrN6 coordination octahedron and canting of these anisotropy axes, similarly in both BDH–TTP and DTDH–TTP salts.
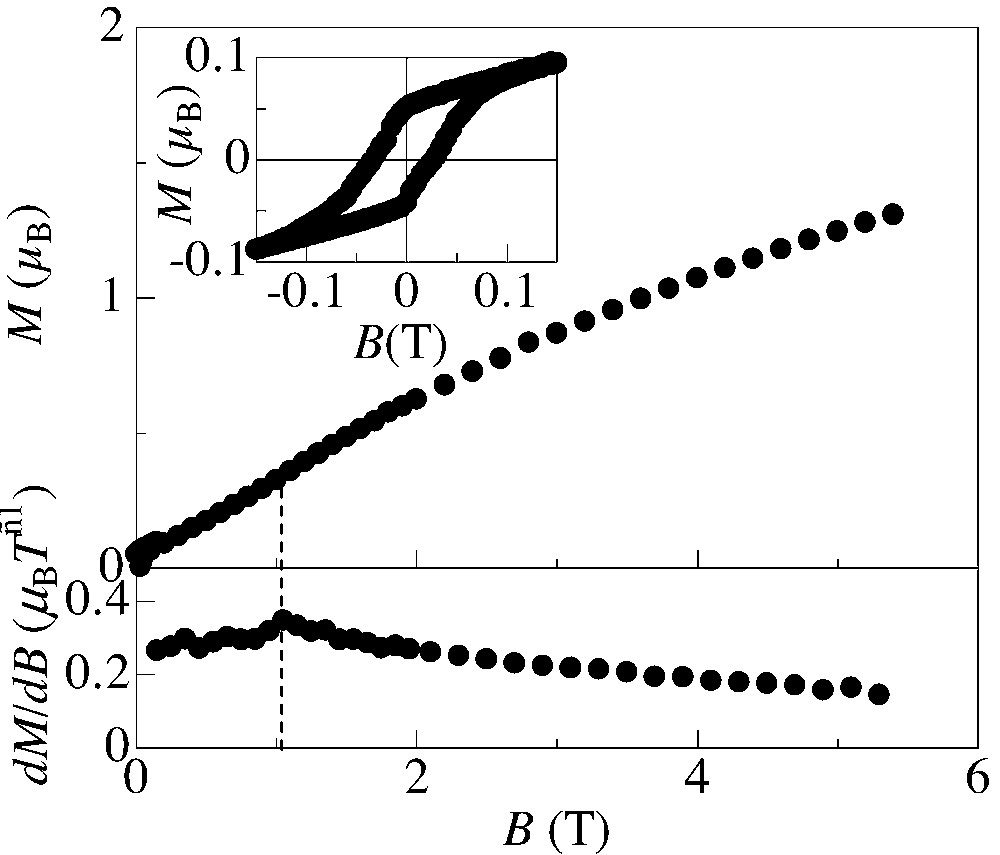
Field dependence of the magnetization M (top) and dM/dB (bottom) measured at 2 K. Insets: hysteresis loop around B = 0 T.
4 Conclusion
A new molecular weak ferromagnet was prepared and characterized. This compound contains mixed chains of anions and radical cations that give rise to a ferromagnetic spin structure. These ferrimagnetic chains interact antiferromagnetically with each other through π···π overlap between isoquinoline ligands of neighbouring anions and S···S contacts between donor molecules, but since these chains are not related by lattice translation, a canting angle is observed between them leading to a weak canted ferromagnetic long-range ordering. The title compound (DHDT–TTP)[Cr(isoq)2(NCS)4] is isostructural to BDH–TTP salt with the same anion but show higher TC (8.7 K instead of 7.6 K).
5 Supplementary Material
The supplementary material has been sent to the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, as supplementary material CCDC 195693 and can be obtained by contacting the CCDC (quoting the article details and the corresponding SUP number).
Acknowledgements
We are grateful for financial support from CNRS, CNRS–JSPS exchange program No. 9473 and NEDO research project 00MB4, and Grant-in-Aids (Nos. 12046231, 14540530) from the Ministry of Education, Science, Sports, and Culture, Japan. S.F. also thanks the French and Algerian Ministries of Education for a PhD fellowship.



