1 Introduction
Dihyropyrimidinones derivatives known as “Biginelli compounds” have received significant attention because of their wide range of pharmacologically potent calcium channel blockers, antihypertensive agents and α-adrenergic antagonists, and neuropeptide Y (NPY) antagonists.
These compounds also have been found to have a broad range of biological activities such as antiviral, antitumor, cardiovascular antibacterial, anti-inflammatory and antioxidant properties [1–4]. Therefore, the preparation of these materials has attracted the significant attention of many chemists. The classical Biginelli synthesis involves three-component one-pot condensation of β-ketoester with an aldehyde and urea under strongly acidic conditions, but the reaction suffer from drawbacks such as low yields, long reaction time, and strong corrosion equipment [5]. In order to improve the efficiency of these reactions, many efforts including various solid-phase modifications and use of a variety of Lewis acid catalysts have been devoted. These catalytic systems involve ytterbium(III) resin [6], Mn(OAc)3.2H2O [7], trifluoroacetic acid (TFA) [8], boric acid [9], CeCl3.7H2O [10], Cu(OTf)2 [11], lithium bromide [12], silica/sulfuric acid [13], indium (III) bromide [14], lanthanum chloride [15], vanadium(III) chloride [16], ionic liquids BMImPF6 and BMImBF4 [17], montmorillonite-KSF [18], zinc triflate [19], I2 [20], NBS [21], bismuth triflate [22], LiClO4 [23], NH4Cl [24], zirconium(IV) chloride [25], indium(III) bromide [26], heteropolyacid Ag3PW12O40 [27], supported polyoxometal [28], benzyltriethylammonium chloride [29], Y(OAc)3 [30], AlCl3:ZnCl2 (3:1) [31], etidronic acid [32], silica-chloride [5] and -SmCl3.6H2O [33].
Among support systems, zeolite Y is an appropriate candidate for the immobilization of HAP because it produces heterogeneous catalysts that are recoverable and reusable.
In a continuation of our previous work on heterogeneous catalysts [34–36], we wish to report a cheap, recoverable and efficiency catalytic system for the synthesis of dihydropyrimidinone from aldehyde in the presence of supported polyoxometal on external zeolite surface as a solid acid catalyst.
2 Experimental
Polyoxometalates including molybdophosphoric acid, tangestophosphoric acid and tangestosilisic acid, denoted as molybdophosphoric acid (MPA), tangestophosphoric acid (TPA) and tangestosilisic acid (TSA), respectively, were purchased from Merck and purified by extraction with dimethyl ether from aqueous solution and then dried under reduced pressure. Other materials were of the commercial reagent grade and used without further purification. The NaY-zeolite was purchased from Sigma-Aldrich Chemical Company. Melting points were recorded on a Barnstead Electrothermal 9200 apparatus and are uncorrected. All of the reactions were performed under magnetically stirring and the progress of the reactions was monitored by thin layer chromatography (TLC). All products were known compounds and identified by comparing their physical data to their authentic samples.
2.1 General procedure for the preparation of supported polyoxometalate
The catalysts were prepared by the incipient wetness method [28]. The framework of Y zeolite involves aluminum atoms that show basicity. Thus, they may decompose as a heteropolyacid structure [37]. Therefore, Y zeolite was modified by acid treatment. In the typical procedure, aqueous NaY suspension was prepared (8 wt%) and dealuminated by additing HClO4 to suspension of zeolite in water following 0.5 mM heteropolyacid (HPA) addition [38]. The mixture of HPA was supported on dealuminated zeolite (HY), stirred for 24 h followed by drying at 383 K and calcined for 4 h at 250 °C to obtain the support catalyst.
2.2 General procedure for the synthesis of dihydropyrimidinones
In a typical procedure, ethyl acetoacetate (1 mmol), aldehyde (1 mmol) and urea (1.5 mmol) were mixed with HPA supported on Y zeolite (8 wt% NaY + 0.5 mM HPA) and refluxed in the presence of acetonitrile (10 mL) as a solvent for an appropriate time. The progress of reaction was monitored by TLC. At the end of reaction, heteropoly acid (HPA) supported on HY filtered off and washed with hot water and ethanol to remove urea from the surface of the catalyst. Then, the catalyst dried and was maintained for new runs. The filtrate was concentrated and the crude product was recrystallized from ethanol to afford the pure product. Products were identified by comparison with melting points of the authentic compounds.
In addition, the Biginelli reaction was performed in the presence of zeolite-encapsulated HPA for comparison. Synthesis of this catalyst is discussed in our previous works [34–36].
3 Results and discussion
Catalysts were prepared by the impregnation of HPA in an acidic suspension with zeolite as a support. The zeolite structure shows basicity because of aluminum atoms so that it causes the decomposition of HPA. Therefore, Y zeolite was dealuminated by adding perchloric acid according to the reported procedure [38]. The reaction of aldehydes with acetoacetate and urea in the presence of HPA supported on dealuminated zeolite produced corresponding dihyropyrimidinone compounds (Scheme 1). Several solvents were used with 4-Nitro benzaldehyde as a model substrate and zeolite supported-MPA as a model catalyst at reflux conditions. The best results were obtained with acetonitrile (Table 1). Under this condition, a wide range of aromatic aldehyde bearing electron-withdrawing or electron-donating groups was reacted to obtain the corresponding compounds in good to excellent yield (Table 2). Zeolite-encapsulated HPA was used in these reactions for comparison. The result proved in the presence of zeolite-encapsulated HPA, the yield of dihyropyrimidinone is very negligible that proved dihyropyrimidinone molecules cannot be formed in the supercage of zeolite because of the large size of these molecules. Therefore, the supported system was chosen as the catalytic system. The reusability of catalyst was investigated using 3-nitrobenzaldehyde (1 mmol) as a model substrate in the presence of recovered catalyst (0.7 mol%) and acetonitrile (10 mL) as solvent under reflux conditions. At the end of the reaction, the catalyst was filtered and washed by hot water and ethanol to remove unreacted species. Then, the catalyst was activated by drying at 120 °C for 3 h and was used for new runs. No appreciable loss in the catalytic activity was detected, which means that HPA is still present on the support system (Table 3).
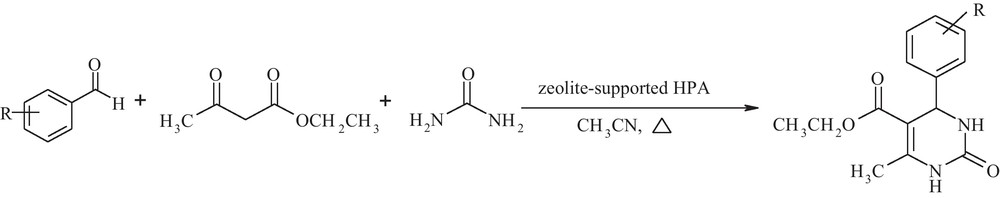
Synthesis of conventional dihydropyrimidinones in the presence of zeolite-supported heteropolyacid under reflux condition.
Synthesis of ethyl-6-methyl-4-(4-nitrophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxilate in the presence of zeolite-supported molybdophosphoric acid in various solvent after 6 h under reflux conditions.a
| Yieldb (%) | Solvent | Entry |
| 55 | Ethylacetate | 1 |
| 25 | dichloromethane | 2 |
| 45 | Chloroform | 3 |
| 74 | Methanol | 4 |
| 97 | Acetonitrile | 5 |
a Reaction conditions: 4-Nitro benzaldehyde (1 mmol), acetoacetate (1 mmol), urea (1.5 mmol), solvent (10 mL), catalyst (0.7 mol%).
b Isolated yield.
Synthesis of 3,4-dihydropyrimidin-2 (1H)-ones, catalyzed by zeolite-supported heteropolyacid.
| Entry | Substrate | MPA-supported HY | TPA-supported HY | TSA-supported HY | Melting point | ||||
| Time (h) | Yielda (%) | Time (h) | Yielda (%) | Time (h) | Yielda (%) | Foundb | Reported | ||
| 1 | 10 | 94 | 7 | 65 | 11 | 72 | 223 | 220 [7] | |
| 2 | 8.5 | 95 | 9.5 | 95 | 10.5 | 94 | 225–227 | 227–228 [14] | |
| 3 | 6 | 97 | 5 | 80 | 6 | 92 | 208–210 | 209–211 [13] | |
| 4 | 6.5 | 95 | 6.5 | 95 | 8.5 | 92 | 213–215 | 214–216 [26] | |
| 5 | 7.5 | 93 | 7.5 | 95 | 8.5 | 93 | 206 | 207–208 [30] | |
| 6 | 7.5 | 90 | 92 | 4.5 | 8.5 | 90 | 213 | 215–216 [7] | |
| 7 | 7.5 | 95 | 8 | 80 | 12 | 97 | 198 | 199–202 [17] | |
| 8 | 11 | 70 | 5 | 70 | 11 | 70 | 157–159 | 156–158 [30] | |
| 9 | 8 | 90 | 9.5 | 93 | 10 | 80 | 217–218 | 215–216 [7] | |
| 10 | 7.5 | 80 | 6 | 90 | 5.5 | 90 | 198–199 | 199–201 [28] |
a Isolated yield.
b Compounds are known and were characterized by their physical and spectral data.
The results obtained in the reusability of zeolite-supported molybdophosphoric acid catalyst in the synthesis of ethyl-6-methyl-4-(4-nitrophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxilate after 6 h.
| Yielda (%) | Run |
| 97 | 1 |
| 95 | 2 |
| 94 | 3 |
| 95 | 4 |
| 90 | 5 |
a Isolated yield.
4 Conclusion
In conclusion, in this paper, we reported a mild and efficient protocol for the synthesis of dihydroprimidinone compounds that utilizes a multicomponent coupling reaction catalyzed by HPA supported on Y zeolite in refluxing acetonirtile. In the presence of zeolite-encapsulated HPA, the yield of reaction is very negligible due to the size of dihydropyrimidinone compounds being larger than the supercage of zeolite. Then, support system was chosen as the catalytic system. The catalyst was recoverable and reused for several times without losing effective catalytic activity.


