1. Introduction
Having a look to the pharmaceutical compounds on the market, a large part of them is characterized by poor organoleptic properties [1], which make them very unpleasant if not hidden with different taste masking agents or pharmaceutical technologies. Taste is crucial for patient acceptability and compliance, in particular with pediatric patients. European Medicine Agency Paediatric Investigation Plan (PIP) guidelines stress the particular relevance of taste masking and palatability testing in the development of oral treatment for children [2]. It is well known that Praziquantel has a very bitter and disgusting taste [3, 4, 5, 6], which makes its administration even more complicated than the large dose needed, inducing nausea [7], or leading to vomiting or gagging if chewed, especially in children, the main involved patients [8]. Moreover, Meyer and collaborators reported an investigation on PZQ taste, revealing a less bitter taste of the active enantiomer (R-PZQ) respect to the racemic form [3], which is not surprising since the majority of the taste experiences are conditioned by the stereochemistry of the system involved. Different taste-masking technologies have been tried on PZQ, such as microencapsulation or drug active coating [9, 10], solid lipid extrusion [6], multiparticulates [11], fluid bed wet granulation technology [12]. Among all the possible techniques in drug taste masking, the formation of solid dispersions can represent a possible valid option, as it was reported for dimenhydrinate with polyvinyl acetate phthalate or artemether with mono amino glycyrrhizate pentahydrate [13]. Also, the possibility of creating cocrystals between API and a sweetening agent was reported for hydrochlorothiazide with sucralose by solubilization and subsequent crystallization [14], for many pharmaceutical APIs and saccharine using the supercritical fluid enhanced atomization process [15] or by Wang and collaborators for theophylline:acesulfame cocrystal by liquid-assisted manual grinding [16]. Nevertheless, no literature data were found on the neat mechanochemical processing of API with sweetening agents to create advantageous solid dispersions, which is the aim of this research. Conversely, previous researches involved natural sugars processing in ball mills to perform several chemical reactions driven by the mechanical energy [17, 18]. Further previous studies attested the propensity of polyols to be subjected to physical transformations upon neat grinding [19]. Also, it is well known that, as reported for sugar-based confections, the sweeteners can be subjected to phase transitions upon processing or during storage. At the same time, both synthetic sweeteners, such as aspartame, and natural sugars/polyols, e.g. maltitol, mannitol, sorbitol were found to be efficient co-formers in co-amorphous systems [20, 21]. It is then likely that the mechanochemical activation may favor the formation of a crystalline, partially crystalline or also amorphous solid dispersion between an API and the sweetener. This fact suggests also a possible enhancement of the biopharmaceutical performances, as a consequence. From this point of view, PZQ is sparingly soluble in water and it is classified as a BCS class II drug [22]. Therefore, the two main drawbacks of this drug, its bad taste and its poor bioavailability, owing to the high therapeutic dose, could be accomplished at the same time by combining mechanochemistry and the addition of a proper sweetener. In this preliminary report, six different sweeteners were selected to be ground with PZQ: four of natural origin (xylitol, mannitol, maltitol and sorbitol) and two artificial sweeteners (aspartame and sucralose). All of them are currently used both in the pharmaceutical and food industry as sweetening agents and as alternative to sucrose for dietary requirements [23, 24].
2. Materials and methods
2.1. Materials
Praziquantel (PZQ, (11bRS)-2-(Cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4-H-(pyrazino[2,1-a]isoquinolin-4-one)) of Ph. Eur. grade was kindly donated by Fatro S.p.a. (Bologna, Italy). Mannitol (MAN), xylitol (XYL), maltitol (MAL), sorbitol (SOR), sucralose (SUC) and aspartame (ASP) were from Galeno S.r.l. (Carmignano, PO, Italy). The HiPersolv Chromanorm methanol used for the HPLC analysis was of Ph. Eur. grade and purchased from VWR Chemicals (BHD PROLABO® Milano, Italy).
2.2. Methods
2.2.1. Preparation of the samples
Each selected sweetener was ground in a 1:1 molar ratio with PZQ for 30 min at 25 Hz using a Retsch MM400 vibrational mill (Retsch, Germany) and two zirconium oxide jars (35 mL), each one containing two zirconium oxide balls (10 mm diameter). The sweetener:PZQ mixtures to be subjected to mechanochemical activation were prepared in a quantity of about 800 mg to get the suitable void volume in the jars and the appropriate balls-to-powder weight ratio, in order to allow a proper mechanical activation of the powder (needed to obtain an amorphous dispersion). These conditions were selected in previous research experiences dealing with the same drug [25, 26]. After the grinding procedure, the samples were collected and stored in a desiccator in the dark at ambient temperature.
For comparison purposes, simple physical mixtures of the same drug-to-sweetener equimolar ratios were prepared by manually mixing the powders in a mortar with a pestle.
2.2.2. Differential Scanning Calorimetry (DSC)
Each sample was analyzed using a Mettler DSC TA 4000 (Greifensee, Switzerland) connected to a calorimetric cell Mettler DSC20 and using STARE software version 9.30 for data analysis. Prior to analysis the instrument was calibrated with Indium, Zinc and Lead for the temperature and with Indium for the enthalpy quantification; each sample, containing about 2 mg of PZQ exactly weighted, was placed in a 40 μl aluminum pan with perforated lid and heated from 30 to 200 °C (10 °C/min) under air atmosphere. When the analyzed sample contained aspartame, the DSC was ended at 160 °C to avoid its decomposition, reported in literature by Goguta and coworkers [27].
2.2.3. Powder X-ray Diffraction (PXRD)
The samples were analyzed by powder X-ray diffraction using a Bruker AXS D5005 X-ray diffractometer with Ni-filtered Cu K(𝛼) radiation (𝜆 = 1.5418 Å). The preparation of the samples consisted in pressing about 20–30 mg of powder over a glass slide to have a flat surface. The data were collected in a 2𝜃 range of 3–35°, with steps of 0.05° every 5 s, according to previous experiences [28]. The apparatus was set at a current of 20 mA and a voltage of 40 kV for all the analyses.
2.2.4. FT-IR spectroscopy (FT-IR)
FT-IR analyses were performed using a Perkin Elmer System 2000 FT-IR on the raw samples, coground samples and the physical mixtures. Each time the sample was mixed with KBr (1:100 by wt) in an agate mortar and then pressed with a hydraulic press for 2 min at 10 Ton to obtain homogeneous and transparent discs. The analysis was conducted from 400 to 4000 cm−1 with a resolution of 4 cm−1 and total scan number of 3.
2.2.5. Water solubility
The solubility of the samples in water was analyzed by preparing saturated solutions of each samples in 20 ml deionized water, which were kept under agitation in the dark for 48 h at 25 °C. Then, the solutions were filtered (pore size 0.45 μm) and diluted 1:200 with the mobile phase prior injection in the HPLC system. The HPLC used was an Agilent HPLC-UV 1260 Infinity II with a EC-C18 Poroshell 120 A column of 4 μm and dimensions of 4.6 × 10 mm. The mobile phase was composed of 65% methanol and 35% of deionized water (MilliQ filtered) and the flux used was of 0.750 mL/min. The instrument was set at 25 °C and with a fixed wavelength of 220 nm for recording the absorbance. The external standardization method was used for the quantification of the integrated peaks. The PZQ retention peak was found at 7.7 min in a total run time of 10 min and the calibration curve obtained in the range of 0.5–10 mg/L had r2 = 0.9988. Each analysis was conducted in triplicate and the average was reported.
2.2.6. Intrinsic dissolution rate (IDR)
The IDR is a measure of the rate of dissolution of an active pharmaceutical ingredient where the conditions of surface area, temperature, stirring speed, medium are all kept constant. For the intrinsic dissolution rate determinations, about 150 mg of the samples were inserted in the sample holder and pressed using a manual hydraulic press (Perkin Elmer) for 1 min at 1 ton. The sample surface area obtained was of 0.785 cm2 and the entire sample holder with the compressed powder was immersed in a vessel containing 900 ml of distilled water kept at 37 °C. The system used was a Hanson Research SR8 Plus dissolution test station and the paddles were positioned at 3.5 cm from the tablet surface, with a rotation speed of 100 rpm. About 2 ml of the dissolution medium were withdrawn every ten minutes till 60 min and immediately replaced with an equal amount of thermo-stated distilled water. The aliquots were then diluted 1:20 with the mobile phase and analyzed by HPLC using the same above-mentioned method. The analyses were performed in triplicate and for each point the mean, with SD (%), was computed. The amount of the dissolved drug per unit area over time was indicated by the slope of the curves, obtained through a linear regression method.
2.2.7. Physical stability
Coground samples were placed in desiccators over calcium chloride and stored at 25 °C in the dark. The samples were regularly analyzed by DSC over a period of one year to detect potential recrystallization of the drug.
3. Results
The grinding of PZQ with the 6 sweeteners (selected among the most commonly used sweeteners in pharmaceutical formulations) was very successful, since after only 30 min of process at 25 Hz highly disordered solid dispersions were formed. As reported in Figure 1, the PXRD spectra of the coground samples were quite broad and a significant halo pattern is visible: the intensity of the characteristic reflections of the sweeteners were very reduced, comparing to their starting highly crystalline nature, while PZQ peaks have completely disappeared. In fact, even though the starting PZQ (anhydrous Form A) possesses a highly crystalline character, its characteristic peaks (a very intense peak at 4.01° of 2𝜃, followed by reflections at 6.3, 8.0–8.2, 14.7, 15.7, 16.4, 18.5, 19.3, 20.1 and 22.6° of 2𝜃) are no longer recognizable after 30 min of cogrinding with the sweeteners. This fact was not attributable to a dilution effect since in the PXRD patterns of the physical mixtures (likewise prepared in equimolar ratio) both the drug and the sweeteners reflections were well visible. According to our experience, this amorphization ability towards praziquantel is quite peculiar: a similar magnitude of crystallinity-degree reduction is usually obtained after 4 h of grinding in analogous operating conditions [25]. An exception of this trend was found in the coground sample with XYL, which will be described separately.
PXRD patterns of the binary PZQ systems with mannitol, maltitol, aspartame, xylitol, sucralose and sorbitol. Each frame compares PZQ (black), the sweetener (blue), the coground (pink), corresponding physical mixture (green) and, only in the case of XYL coground, PZQ Form B (red).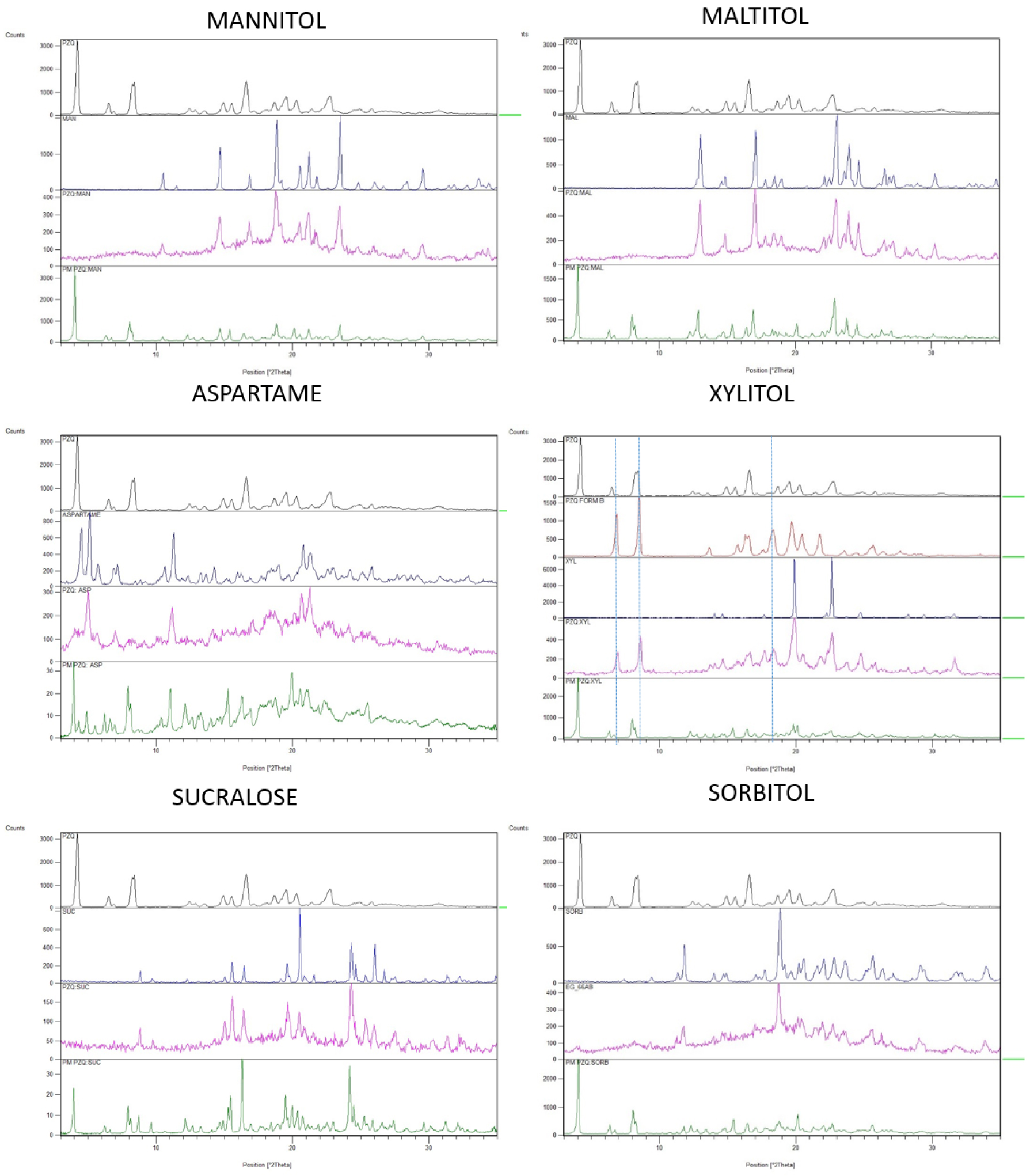
The DSC traces confirmed previous findings (Figure 2). In the physical mixtures the melting peaks of the sweeteners and of PZQ were found in the same temperature range of the raw materials, indeed with a slightly reduced enthalpy due to a dilution effect. Besides, in the coground samples the sweetener event(s) is(are) still detectable in all cases (with remarkable temperature shifts), whereas PZQ endothermal melting event (originally at 141.73 °C) was absent or barely visible. Once again PZQ:XYL coground had a separate behavior.
DSC curves of PZQ:sweetener equimolar coground (purple), corresponding physical mixture (green), sweetener (blue) and commercially available PZQ (black).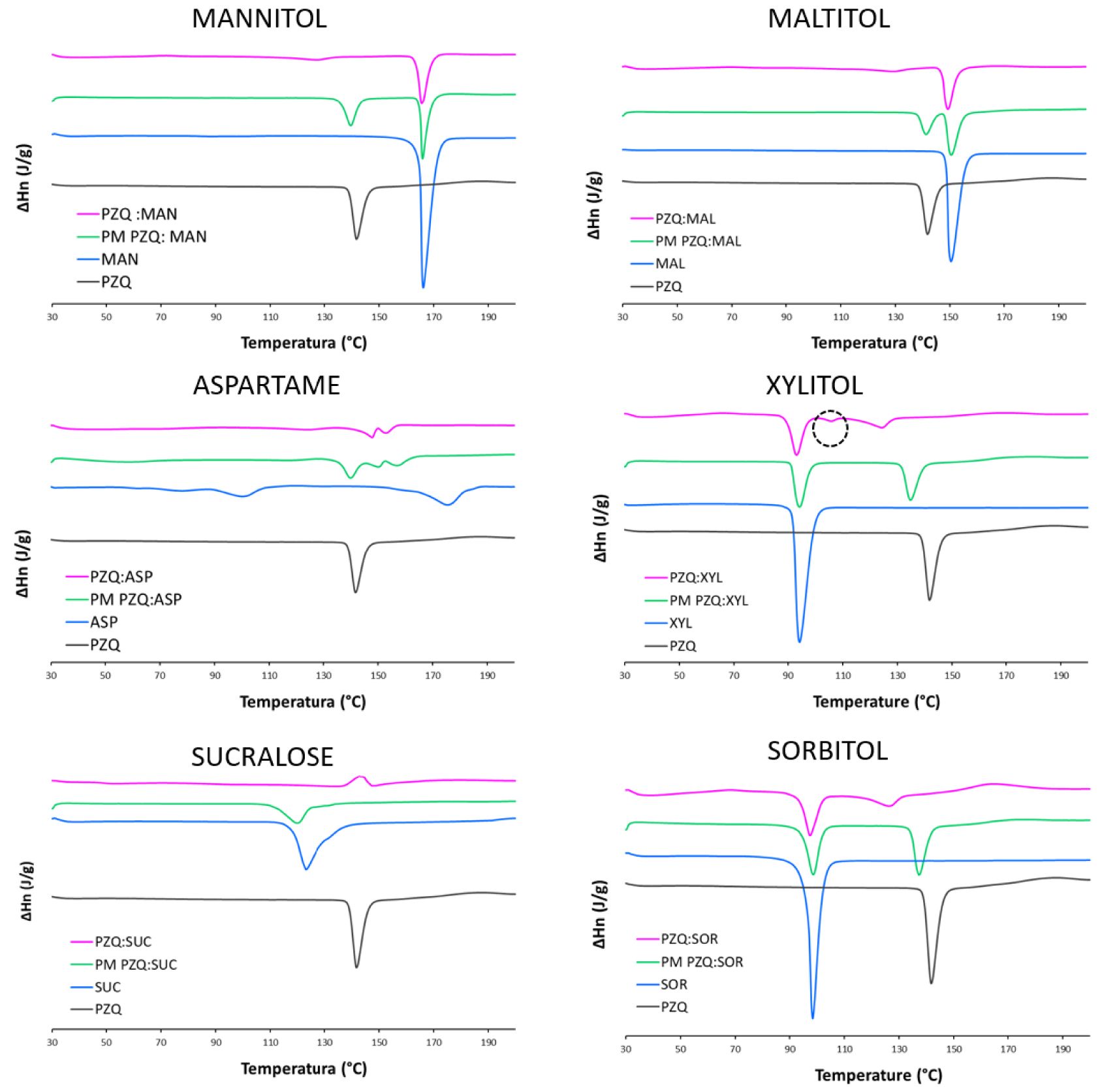
FT-IR was performed to further understand whether molecular interactions occur between the drug and the sweeteners. The FT-IR spectra of PZQ dispersions, the corresponding physical mixtures and the individual components are shown in Figure 3 (for the sake of brevity only PZQ:SOR and PZQ:XYL are presented, while the remaining ones are reported in Figure S1). As a typical feature of amorphous samples, in the FT-IR spectra of the coground samples a general tendency of slightly broader peaks when compared to crystalline physical mixtures of the raw crystalline ingredients was noticed, [29]. Furthermore, the two nearly overlapped peaks at 1647 and 1624 cm−1 of original PZQ (due to the stretching vibrations of the heterocyclic carbonyl and to that of the carbonyl group joined to the cyclohexyl), anticipated at higher frequencies by a typical shoulder (ranging about 1668 cm−1) [30], were replaced in the coground spectra by only one broad peak. This behavior corresponds to that seen in a previous study dealing with polymer-based amorphous solid dispersions with PZQ [25], thus confirming once again their amorphous character and underlying the importance on the crystal packing of the two carbonyl groups (no longer distinguishable in the amorphous dispersion spectra). Conversely, the simple equimolar physical mixture of the components presented the same 2 carbonyl stretching vibrations of the original drug (as visible in the frame), indicating the absence of intermolecular interaction between PZQ and excipients.
FT-IR spectra of coground system (green), corresponding physical mixture (blue), sweetener (purple), PZQ Form B (red, if present) and PZQ Form A (black). Frames highlight diagnostic regions.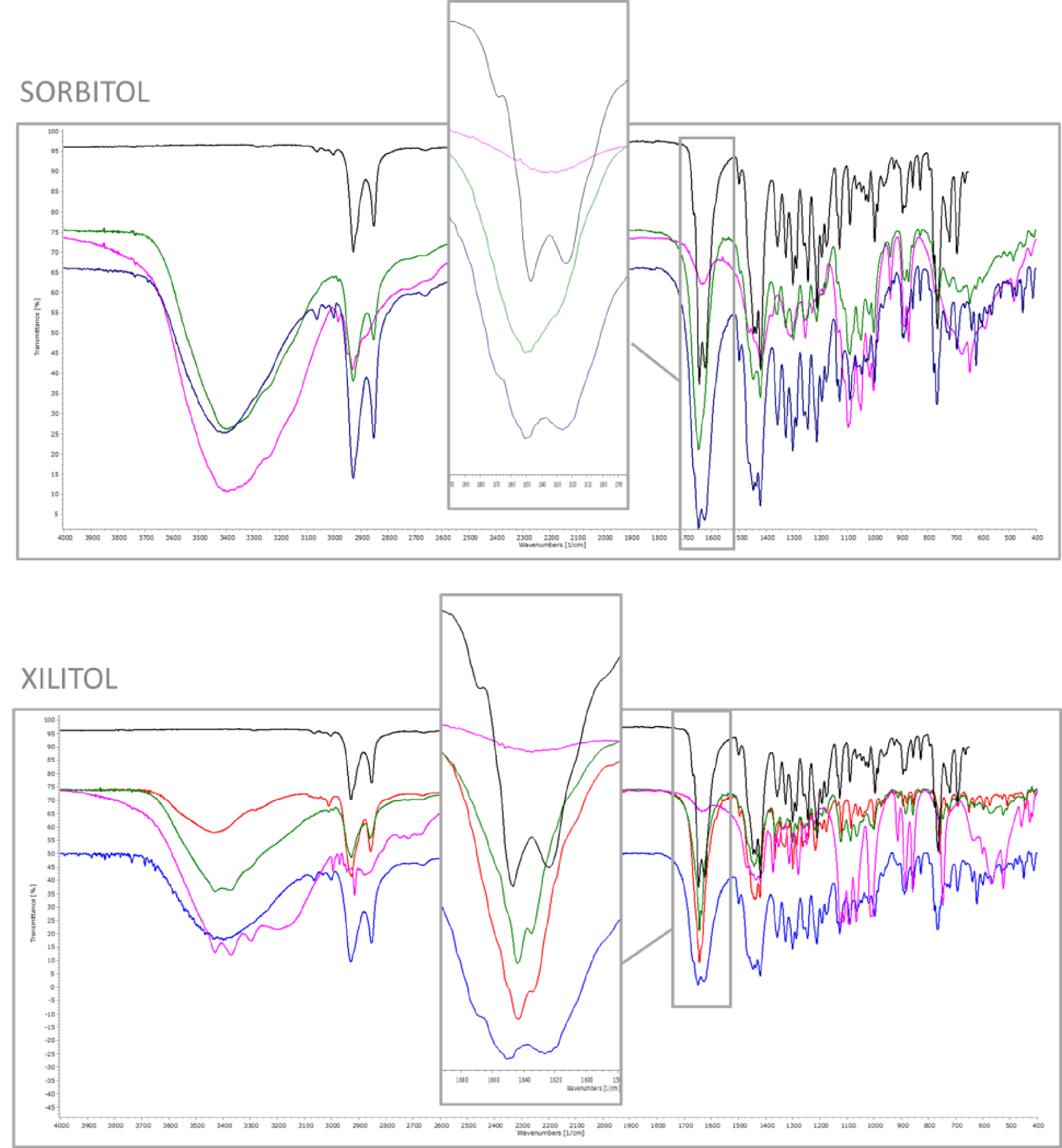
Unlike the other systems, in the PZQ:XYL coground system evidences of the formation of anhydrous polymorphic Form B of PZQ were found. In the PXRD spectrum of the PZQ:XYL coground sample (reported in Figure 1), dominated by a pronounced amorphous character, the residual reflections were identified as the ones of PZQ Form B (e.g. 16.9, 17.7, 23.6 and 27.5° of 2𝜃) [31], together with the ones of the sweetener. In the thermal analysis (Figure 2) PZQ melting was seen at about 106 °C and 123 °C, with very small endotherms, that can be identified as PZQ Form B and commercial Form A, respectively. The melting peak of the recrystallized Form A at 123.42 °C, largely inferior to its usual melting point, can be referred to a reduction of the PZQ crystal size with a Gibbs–Thompson effect (also compatible with the recorded PXRD pattern) and to an interaction at the solid state with the excipient, already visible in physical mixture (see PZQ m.p. downshift in the green curve of Figure 2).
Also, the FT-IR spectra of the ground PZQ:XYL sample (shown in Figure 3) presented the same vibration peaks as the anhydrous polymorphic Form B, confirming once again its identity by the superimposable shape and position of the asymmetric stretching of the PZQ carbonyl groups at 1641 and 1633 cm−1. As already said, these frequencies represent the symmetric stretching of the heterocyclic carbonyl and of the carbonyl near to the cyclohexyl group, respectively. The difference between the frequencies of the carbonyl groups was reported to be index of their spatial disposition: when in the syn conformation, the difference is higher, as in the case of raw PZQ (1651 and 1626 cm−1), while in PZQ Form B is lower, indicating the presence of anti conformers, as it was also reported in literature [30]. Thus, the discriminating capability of FT-IR analysis towards PZQ polymorphic forms also in mixtures with excipients was here attested. The presence of PZQ Form B is a particularly interesting achievement, since so far, this anhydrous form was obtained by means of simple one-component long grinding only [31, 32].
After having characterized the solid state of the binary systems, the coground samples were tested for their biopharmaceutical properties, looking towards the second aim of the research. As reported in Figure 4, all the tested samples had a 2-fold enhanced PZQ water solubility at 25 °C (after 48 h in the dark) comparing to that of commercially available PZQ Form A (dotted line in Figure 4).
Praziquantel water solubility at 25 °C of the coground systems with the 6 sweeteners, compared to PZQ Form B and commercial PZQ (Form A).
PZQ:ASP and PZQ:SUC were selected for further intrinsic dissolution analyses in the light of the best solubility results and in consideration of their reported high sweetening powers (being 180–200 times higher than sucrose for ASP, and from 100 to 300 times for SUC [33]). In addition, PZQ:XYL coground was analyzed for the above-mentioned peculiar characteristic amongst the coground (i.e. presence of PZQ Form B). To exclude the effects of experimental variables (such as particle size, stirring pattern, etc.) intrinsic dissolution rate (IDR) of both original PZQ and cogrounds were determined, also including Form B as a matter of comparison. This is a standardized kinetic method defined as the dissolution rate of pure substances, after compaction, in constant surface conditions. As shown in Figure 5, IDR of the coground systems, were higher of a factor of 2.5 than commercial PZQ. In fact, values of 0.083 ± 0.023 mg/cm2/min and 0.0535 ± 0.01 mg/cm2/min were found for PZQ:SUC and PZQ:ASP, respectively, while the raw drug showed an intrinsic dissolution rate of 0.030 ± 0.031 mg/cm2/min. This feature could be attributed not only to the hydrotropism of the sweeteners, but also to the dramatic change in the solid state of the coground in comparison to that of pure API. Moreover the superimposable intrinsic dissolution trends of PZQ:XYL and Form B (0.068 ± 0.010 mg/cm2/min and 0.062 ± 0.001, respectively) confirmed the PZQ polymorphic transition in presence of xylitol. The observed sucralose dissolution enhancement was particularly remarkable.
Praziquantel intrinsic dissolution profiles from selected sweet binary systems (PZQ:SUC, PZQ:ASP and PZQ: XYL) compared to commercial PZQ (Form A) and PZQ Form B.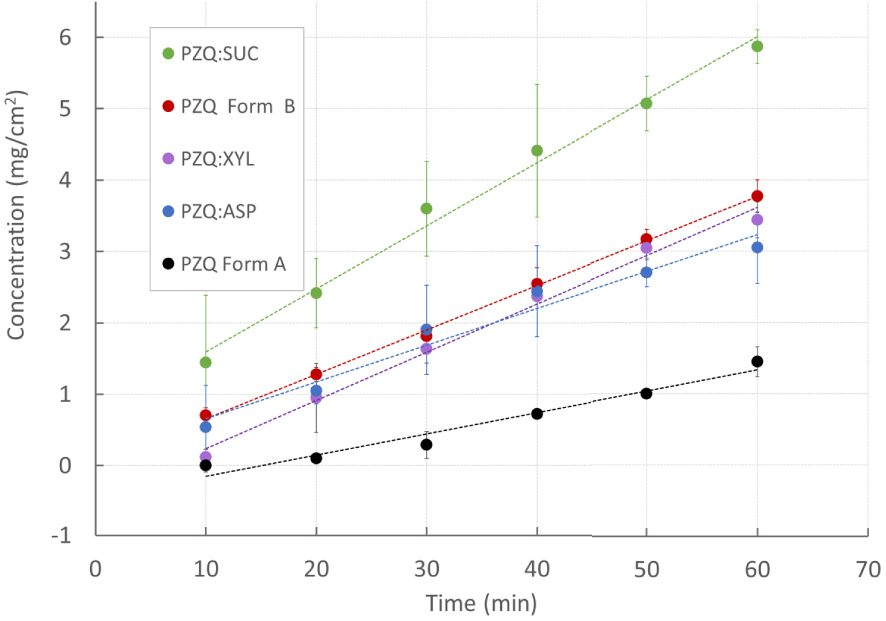
Since the main disadvantage of using amorphous drug formulations is their poor and largely unpredictable physical stability and the risk of an amorphous–crystalline conversion during manufacturing, storage and administration [34], the physical stability of the equimolar mixtures was evaluated over a period of one year. From Figure S2, depicting the DSC curves, it can be concluded that sweeteners contribute to the stabilization of the amorphous state of the drug, since recrystallization in the original PZQ Form A was not found. Again, a different behavior was noticed for the PZQ:XYL coground, where a slight upward shift can be noticed in the barely visible melting point of the drug, probably attributable to a slow recrystallization from PZQ Form B towards the original PZQ Form A.
4. Conclusions
The grinding of Praziquantel with the selected sweeteners (sorbitol, maltitol, xylitol, mannitol, aspartame and sucralose) allowed to obtain really interesting products, of prevalent amorphous character (physically stable for at least one year) and with a net increase in solubility and intrinsic dissolution when compared to the commercial Praziquantel. All these promising characteristics put in light the possibility of ameliorate PZQ dosage forms, very likely to have a better taste and biopharmaceutical properties compared to the actual administered form. These products can be cheap and highly useful premixes in the formulation of oral dosage forms for Praziquantel. Among the sweeteners, in this preliminary report the performance of sucralose appears largely superior: this coground will be characterized for taste sensory properties as well as for verification of the maintenance of anthelmintic activity in future researches.
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
Conceptualization, BP; methodology, BA, BP, GZ and DZ; software, DZ; validation and formal analysis, BA and NP; investigation, DZ; data curation, DZ; writing—original draft preparation, DZ; writing—review and editing, BP, GZ, BA and NP; resources, BA and BP; visualization, DZ; supervision, BP; project administration, BP; all DZ contributions were in 2015–2018.
Funding
This research received no external funding.
Acknowledgments
BP thanks Professor V. Lughi and the staff of the Department of Engineering and Architecture, University of Trieste, Italy for their precious cooperation and Fatro S.p.A. for the gift of the raw praziquantel.




 CC-BY 4.0
CC-BY 4.0