1 Introduction
Drought stress is a global, major environmental stress that reduces crop yields and restricts plant distribution. Depending on the extent of water availability, plants can be affected by drought stress in different ways, ranging from the inhibition of normal physiological activities and cellular damage to cell death [1,2]. Therefore, many land plants have evolved structural and physiological mechanisms to protect them from mild drought stress. In most crop plants, water deficit severely reduces plant growth, yields, and crop quality due to increased osmotic and oxidative damage. Due to the dramatic increase in the human population, together with increasingly serious environmental problems, it will be difficult for world agriculture to meet worldwide food and energy requirements in the future [3]. Therefore, it is crucial to develop crop cultivars with excellent environmental stress tolerance for use in sustainable agriculture.
Potato (Solanum tuberosum L.) is one of the four staple foods worldwide. The average per capita consumption of potato is gradually increasing due to its well-known health benefits. Moreover, the potato-harvesting period is relatively short, with a high production rate. However, potato is vulnerable to several environmental stresses, including drought stress. Even short periods of drought stress can result in serious damage and cause a significant reduction in tuber yields [4]. Therefore, in view of global warming and desertification, developing a potato line tolerant to water deficit has become a major task for potato breeders. Recently, several attempts were made to create potato cultivars possessing enhanced tolerance to drought stress by overexpressing genes for osmotic metabolites [5–7], transcription factor genes in the drought-signaling pathway involved in phyto-hormone signaling or biosynthesis [8], and genes responsive to antioxidant proteins [9].
The Orange gene (Or), which is involved in carotenoid accumulation, shares high levels of sequence homology in many crop plants such as cauliflower, rice, tomato, and sweet potato, as well as the model plant Arabidopsis thaliana [10–12]. Introducing the cauliflower Or gene induced the formation of chromoplasts and increased the carotenoid contents in transgenic potato tubers [13]. Recently, overexpressing the sweet potato Or (IbOr) gene also increased the carotenoid contents in transgenic potato tubers and sweet potato storage roots [14,15]. IbOr expression in sweet potato plants rapidly increases after NaCl, PEG, and H2O2 treatment [11]. Transgenic sweet potato calli overexpressing IbOr exhibit enhanced tolerance to salt stress, with increased carotenoid contents and antioxidant activity [11]. Therefore, IbOr is not only involved in carotenoid accumulation, but it also functions in the response to multiple abiotic stresses in transgenic potato and alfalfa plants, as well as in sweet potato callus [11,14,16]. Thus, IbOr may be useful for developing valuable crops with enhanced tolerance to multiple environmental stresses, along with increased nutrient contents.
When designing an efficient expression system, it is important to select the proper promoter, such as stress-inducible, tissue-specific, or constitutively expressed promoters. We previously isolated and characterized the strong oxidative stress-inducible sweet potato peroxidase anionic 2 (SWPA2) promoter [17]. The SWPA2 promoter induces higher levels of exogenous gene expression than the 35S promoter from cauliflower 1 virus (CaMV 35S promoter) in response to various stress treatments, and was successfully applied to several transgenic plants such as poplar, potato, sweet potato, alfalfa, and rice [16,18–21]. The use of the stress-inducible SWPA2 promoter to drive transgene expression can minimize the negative effects of transgene overexpression on plant growth. Therefore, the stress-inducible SWPA2 promoter is highly suitable for generating transgenic plants with enhanced tolerance to environmental stresses.
In the present study, over a 2-year period (2013–2014), we assessed the capacity of the sweet potato IbOr genes to increase drought tolerance in transgenic potato under greenhouse conditions and determined whether negative agronomic growth qualities were associated with high levels of transgene activity. Expression of IbOr increased drought stress tolerance in transgenic potato, thereby conferring high-quality tuber production.
2 Materials and methods
2.1 Plant materials
Transgenic potato plants (S. tuberosum L. cv. Atlantic) expressing IbOr under the control of the oxidative stress-inducible SWPA2 promoter (SOR plants) were generated by Agrobacterium-mediated transformation. In a previous study [14], the introduced IbOr gene in transgenic SOR plants was confirmed by genomic and RT-PCR analysis. Transformed potato plants were maintained in vitro by sub-culturing every four weeks at 23 °C under 16-h day (4000 Lux light) and 8-h night conditions, as described by Goo et al. [22]. Ten plants of each line were then grown in 10 cm diameter pots containing commercial mineral-mixed soil in a greenhouse at approximately 28/22 °C (day/night) with daily watering; 6-week-old plants were used for water-deficient treatments.
2.2 Genomic PCR and RT-PCR
Genomic DNA from regenerated plants was isolated with a GeneAll Exgene™ Plant SV kit (Seoul, Korea) as described by the manufacturer. PCR was carried out with purified genomic DNA and primer sets for the IbOr and bar genes. To purify total RNA from the plants, leaves or tubers were macerated in liquid N2, and Trizol reagent (Invitrogen, CA, USA) was used to extract the RNA, as described in the manufacturer's protocol. The RNA was further purified with an RNeasy Plant Mini kit (Qiagen, Germany), followed by cDNA synthesis with a kit (Toyobo, ReverTra Ace-αE-, Osaka, Japan). PCR and RT-PCR products were fractionated in a 1.5% agarose gel. The sequences of the primers used for amplification of the IbOr and bar genes were previously described [14].
2.3 Drought stress treatment
For dehydration treatment, the potato plants were irrigated with similar quantities of water through trays placed underneath the pots for 60 days, followed by withholding water for 15 days. The plants were then watered and allowed to recover from drought conditions. Tests for visible damage caused by dehydration were repeated in triplicate. Survival rates and wilting grades caused by dehydration conditions were evaluated after treatment and expressed as percentage survival rate and wilting grade level.
2.4 Wilting grade assay
The wilting grades were scored after drought stress treatment using visual grading scale from 0 to 5; where 0, is no symptom of wilted leaves; 1, less than 20% wilted; 2, 20–40% wilted; 3, 40–60% wilted; 4, 60–80% wilted; 5, more than 80% wilted.
2.5 Determination of PSII photosynthetic efficiency
PSII photosynthetic efficiency in leaves was estimated by chlorophyll (Chl) fluorescence determination of photochemical yield (Fv/Fm), which represents the maximal yield of the photochemical reaction in PSII, using a portable Chl fluorescence meter (Handy PEA, Hansatech, England).
2.6 Tuber analyses
Transgenic potato plants used for tuber yield analysis were in vitro propagated and transferred to soil, followed by growth in a greenhouse for a total of 3 months under a natural day length photoperiod at 25 ± 3 °C with weekly fertilization. Tubers were harvested and photographed, and tuber yield was evaluated as the:
- • number of tubers per plant;
- • individual weight of each tuber;
- • and total tuber yield (weight) per plant.
For both types of analysis, three independent experiments were conducted with five individual plants assessed for each transgenic line or wild-type control per experiment; the plants were arranged in a randomized design for each experiment.
2.7 Analysis of relative water content
The degree of drought stress was assessed by the relative water content (RWC) of leaves after withholding water. The RWC was estimated as described by Ma et al. [23]. RWC was measured in the third or fourth leaves from the top of the plant.
2.8 Statistical analyses
Data were analyzed by one-way analysis of variance (ANOVA). The subsequent multiple comparisons were examined based on the least significant difference (LSD) test. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 12), and statistical significance was set at P < 0.05 and P < 0.01.
3 Results
3.1 Selection of transgenic IbOr potato lines for tuber analysis
We screened PCR-positive transgenic lines via genomic and RT-PCR analysis to identify lines actively expressing the IbOr transgene before transfer to greenhouse conditions (Fig. 1A and B). We obtained five to ten independent transgenic lines displaying detectable levels of IbOr transgene expression (data not shown). From this subset, we selected two independent lines that expressed the transgene at similar levels for use in all subsequent analyses. As potatoes are grown for their tubers, we determined if the negative pleiotropic effects of ectopic IbOr expression also affected tuber shape and yields. The SOR transgenic plants exhibited inhibited tuber formation compared with non-transgenic (NT) potato plants (Fig. 1C).

Genomic PCR, RT-PCR, and tuber formation in transgenic potato lines SOR1 and SOR2 bearing IbOr. A. Genomic DNA was purified from selected transgenic potato plants, and PCR was carried out with primer sets for IbOr or the bar gene. B. Total RNA was purified from selected transgenic potato lines, and RT-PCR was carried out with primer sets for IbOr and actin. C. Tuber formation in transgenic potato lines. Genomic PCR and RT-PCR products were fractionated on a 1.5% agarose gel. M: size markers; NT: non-transgenic parental line, Atlantic cultivar (negative control); SOR: SWPA2-IbOr transgenic potato; P: positive control.
3.2 IbOr transgenic potato exhibits increased drought stress tolerance
To evaluate the drought stress tolerance of SOR plants, 1-month-old NT and SOR plants were subjected to water deficit for 15 days. Before withholding the water supply, the plants were irrigated with similar quantities of water for 2 months; no obvious differences were observed between SOR and NT plants. After 15 days of withholding water, we observed severe wilting of the NT plants, while the SOR plants exhibited less wilting. When the plants were re-watered after drought stress treatment, all SOR lines recovered successfully, with only a few withered leaves, whereas NT plants were almost dead and failed to recover from dehydration conditions (Fig. 2A). After a 15-day water-deficient period, the survival rate of NT was 35%, whereas that of the SOR2 lines was approximately 80% (Fig. 2B). The transgenic plants also exhibited lower wilting severity levels compared with control plants (Fig. 2C). These results indicate that the presence of IbOr increases drought stress tolerance in SOR plants.
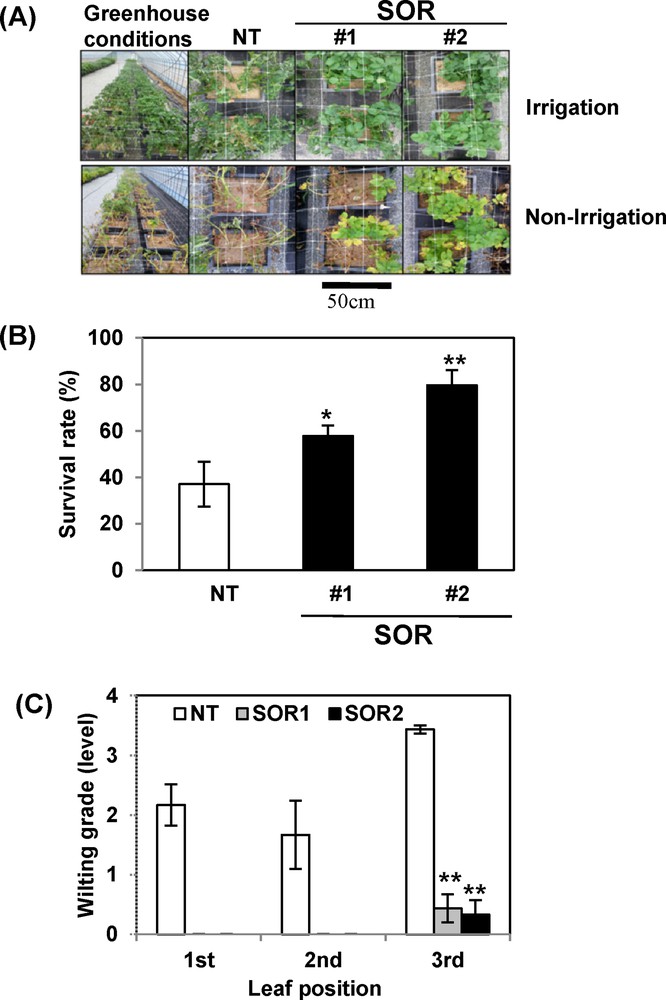
Drought stress analysis of transgenic potato l lines SOR1 and SOR2. A. Visible damage appeared on plants after drought stress treatment. Photographs were taken after 2 weeks of withholding water. B. Survival rate (%) of transgenic lines after 14 days of withholding water. C. The wilting grade was measured in the first fully expanded leaf from the shoot tip. Data represent the average of three replicates. Statistical significance of differences between the control and treatment groups was determined by one-way ANOVA with LSD post hoc test (*P < 0.05; **P < 0.01).
3.3 IbOr transgenic potato exhibits minimal negative effects on tuber yield after drought stress
While the SOR lines were capable of tuber production, and despite the beneficial gain in drought tolerance, the significant decreases in tuber number and mass in these lines would be agronomically unacceptable (Fig. 3). Expressing a transgene under the control of an abiotic stress-inducible promoter minimizes the negative effects of the transgene on plant growth [24]. Importantly, after drought stress treatments, the SOR transgenic lines retained tuber-formation capacity, and the number and mass of tubers in these lines were similar or greater than those of NT plants (Fig. 3).
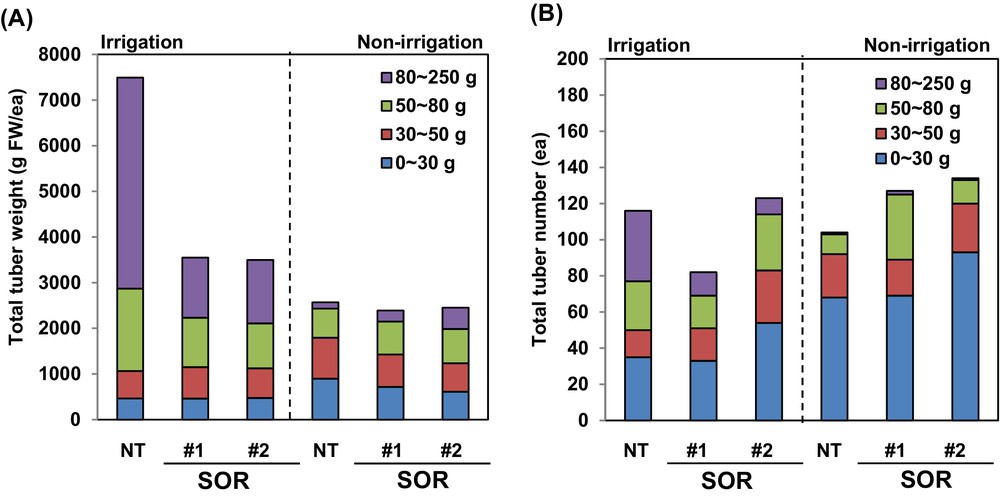
Tuber productivity-related features of transgenic potato lines SOR1 and SOR2 under greenhouse conditions. A. Total weight of harvested potato tubers under normal or dehydration conditions. B. Total number of harvested potato tubers under normal or dehydration conditions. Data are means of 40 individual plants per line.
3.4 Analysis of drought tolerance and tuber production in the transgenic plants in 2014
In 2013, we selected the SOR2 line with the highest level of IbOr expression as well as increased tuber production and drought stress tolerance for subsequent analysis. In 2014, to assess the effects of drought stress tolerance on the SOR2 plants, soil-grown potato plants were not watered for 12 days, followed by watering for 6 days for recovery (Fig. 4A). Consistent with the results from 2013, more bleaching and a greater increase in wilting levels were observed in NT plants than in the transgenic plants (Fig. 4B). Additionally, the transgenic plants exhibited higher levels of PSII efficiency than NT plants (Fig. 4C). SOR2 plants also exhibited slightly higher levels of RWC than NT plants during time courses (Fig. 4D).
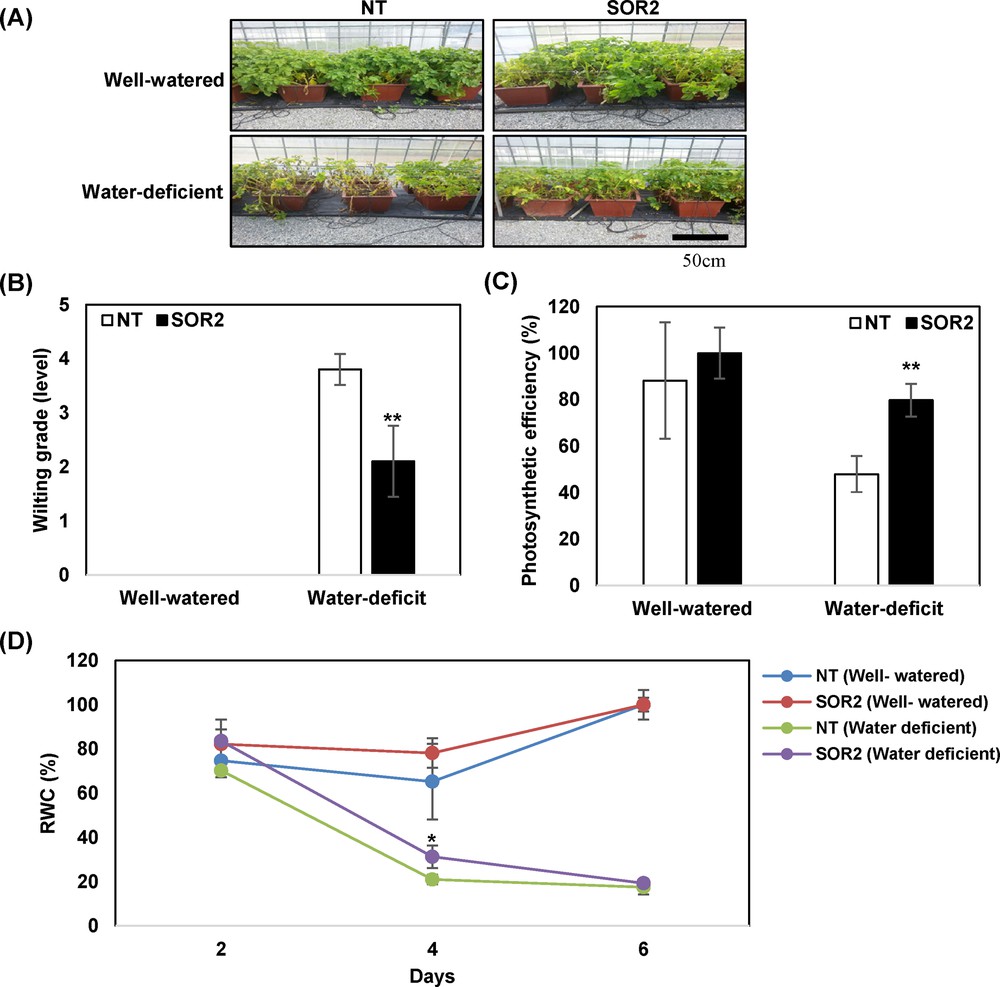
Drought stress analysis of transgenic SOR2 lines in 2014. A. Visible damage appeared on plants after drought stress treatment. Photographs were taken after two weeks of withholding water. B. The wilting grade was measured in the first fully expanded leaf from the shoot tip. C. Effects of drought stress on photo-inhibition, as measured by Fv/Fm values (%). D. RWC after dehydration treatment. Data represent the average of three replicates. Statistical significance of differences between the control and treatment groups was determined by one-way ANOVA with LSD post hoc test (*P < 0.05; **P < 0.01).
We also examined harvested potato tubers, finding that the transgenic lines retained tuber-formation capacity and produced tubers in similar numbers and with similar masses to those of NT plants under both control and drought stress treatment (Fig. 5). Interestingly, during drought stress, the SOR2 plants produced higher marketable tuber (over 80 g) yields than NT plants. These results indicate that expressing the IbOr transgene can lead to significant gains in yield and drought tolerance in potato, thereby increasing the quality of this crop by affecting these agronomically important traits.
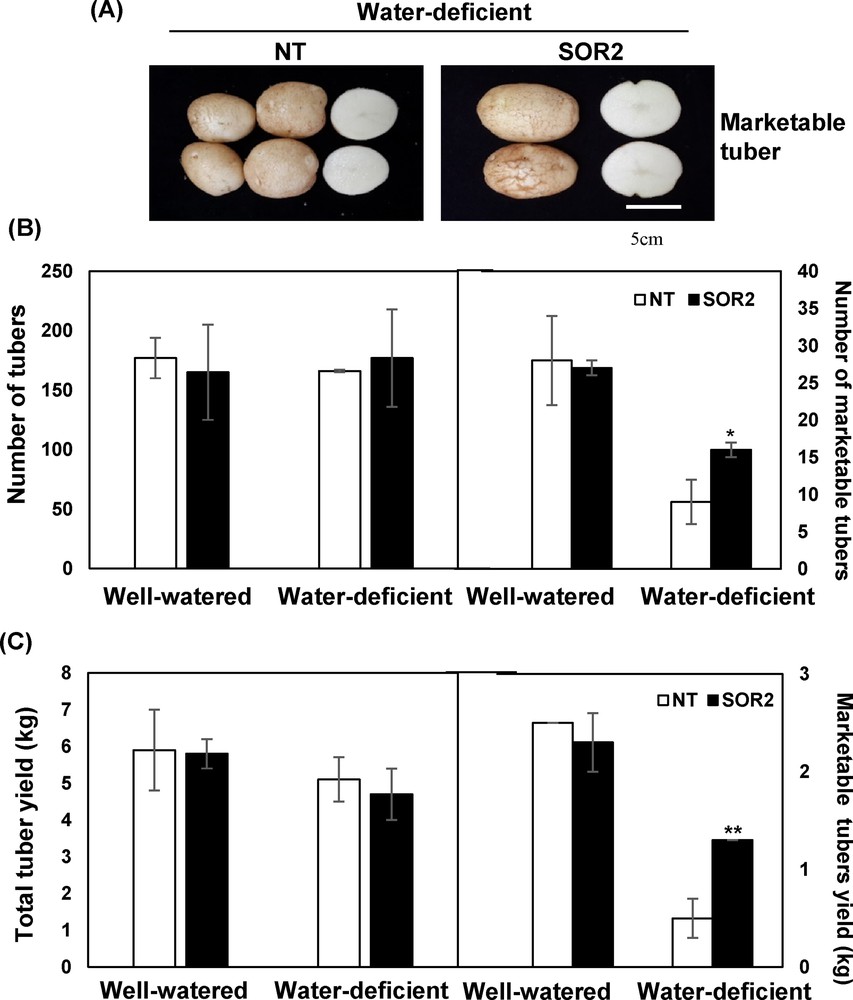
Tuber productivity in transgenic SOR2 lines in 2014. A. Visible marketable tuber formation in potato plants under water-deficient conditions. B. Total number of harvested potato tubers and number of marketable tubers (over 80 g) under normal and dehydration conditions. B. Yields of all harvested potato tubers and marketable tubers (over 80 g) under normal and dehydration conditions. Data represent the average of 40 plants. Statistical significance of differences between the control and treatment groups was determined by one-way ANOVA with LSD post hoc test (*P < 0.05; **P < 0.01).
4 Discussion
We previously developed transgenic potato plants expressing the sweet potato orange (IbOr) gene under the control of the oxidative stress-inducible SWPA2 promoter (SOR plants) and characterized the plants under MV-mediated oxidative stress and high salinity conditions [14]. In this study, we determined that expressing IbOr in potato increased drought stress tolerance under water-deficient conditions. These results demonstrate that IbOr can potentially be used to improve the agricultural traits of potato, increasing the crop's adaptability to drought stress.
Drought stress is one of the most adverse environmental stress factors affecting plant growth and productivity [25,26]. Exposure to drought stress leads to the generation of reactive oxygen species in plants, which in turn have a negative effect on cellular structure and metabolism [1,26]. One stress defense mechanism is the antioxidant defense system, which includes antioxidant enzymes and low-molecular-weight antioxidants, such as carotenoids.
Recent advances in plant biotechnology have led to the production of transgenic plants with increased tolerance to abiotic stresses as well as improved biomass or nutritional value. Expression of the Or gene, which is highly conserved in many crops, results in carotenoid accumulation and increased stress tolerance [10–16]. We recently reported that the expression of IbOr in transgenic potato plants improved their tolerance to MV-mediated oxidative and high salinity conditions [14], indicating that the use of this transgene is a highly efficient technique for crop improvement. Wang et al. [16] recently reported that the expression of IbOr in alfalfa is strongly responsive to abiotic stress and confers increased tolerance to various abiotic stresses. In the present study, potato plants expressing IbOr under the control of the stress-inducible SWPA2 promoter (SOR plants) were highly tolerant to water-deficient conditions (Figs. 2 and 4). IbOr was expressed in SOR plants under normal conditions, perhaps due to the stressful growth environment in pots and sensitivity of the SWPA2 promoter to abiotic stress conditions. These results indicate that IbOr increases the viability of SOR plants under drought stress conditions, suggesting that this gene will be useful for improving plant tolerance to multiple abiotic stresses.
Constitutive overexpression of stress-responsive genes, while leading to increased stress tolerance, is also associated with growth retardation of plants and/or reduced crop production [24,27]. Kasuga et al. [27] demonstrated that the use of a stress-inducible promoter to drive transgene expression can minimize the negative effects of overexpression on plant growth. In the present study, we assessed the capacity of IbOr to increase drought tolerance in potato, as well as whether negative agronomic growth qualities were associated with transgene activity and if the use of a stress-inducible promoter could increase drought tolerance while minimizing the negative effects of the transgene on yields. The results show that the yields and number of tubers were not significantly different between NT and IbOr plants under drought stress conditions, whereas IbOr plants had higher yields and higher numbers of marketable tubers than control plants (Fig. 5). Further characterization of IbOr plants should be performed to determine their abiotic stress tolerance under field conditions.
In summary, we found that stress-inducible expression of sweet potato IbOr increased drought tolerance in potato plants while increasing marketable tuber production. These results suggest that stress-inducible expression of the IbOr transgene may be an effective strategy for increasing drought stress tolerance in potato while significantly improving tuber production, an important agronomic trait, under unfavorable conditions. In addition, SOR plants may be useful as breeding materials for producing potato cultivars with new characteristics and for sustainable growth on marginal lands.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A1A02036323) and the Agro and Bio-industry Technology Development Program (Grant No. 314021-3-1-SB050), Ministry of Agriculture, Food and Rural Affairs, South Korea and the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01212401)” Rural Development Administration, Republic of Korea.


