1 Introduction
The aim of this study is to investigate the possible relationship between the toxic effect of copper and the changes of some enzyme activities involved in defence mechanisms.
In plants, excess of copper can easily catalyse the generation of harmful free radicals [1] and might therefore cause oxidative stress [2]. This injurious effect may be alleviated by enzymatic reactions scavenging oxygen free radicals and including SOD, CAT and peroxidases. The activities of these enzymes are increased by copper excess treatment [3,4].
Copper can also affect membrane properties by oxidation of membrane lipids [5]. The injurious membrane effect can however be estimated from the increase of MDA, one of lipid peroxidation product [2].
Moreover, increased activities of lignifying peroxidases and PAL are known to be related to environmental injury in both biotic and abiotic stimuli [6]. In plants, lignifying peroxidases were involved in polymerization of hydroxy cinnamyl alcohols to lignin. PAL is responsible for the conversion of l-phenylalanine to trans-cinnamic acid, a key intermediate in the pathway of lignin production.
In the present study, we investigated the changes of SOD, CAT, GPX, CAPX (coniferyl alcohol peroxidase), SPX (syringaldazine peroxidase) and PAL activities in sunflower roots exposed to cupric stress.
2 Materials and methods
2.1 Plant material and growth conditions
Sunflower seeds (Helianthus annuus L.) were germinated and grown in a controlled chamber, as previously described by Mazhoudi et al. [7]. Ten-day-old seedlings, previously grown on a non-contamined nutrient medium, were treated for five days by addition of 50 μM CuSO4 on the nutrient solution.
2.2 Malondialdehyde determination
Lipid peroxidation was measured as the amount of MDA determined by the thiobarbituric acid (TBA) reaction, as described by Heath and Packer [8]. The assay was carried out according to Baccouche et al. [9].
2.3 Enzyme preparations and assays
Plant material was extracted in 50 mM potassium phosphate buffer (pH 7.0) containing 5 mM sodium ascorbate and 0.2 mM EDTA. The homogenate was centrifuged at 13 000 g for 15 min. The resulting supernatant was used for assays of CAT, SOD and peroxidases. CAT and SOD activities were determined as described by Aebi [10] and Polle et al. [11], guaiacol peroxidase and coniferyl alcohol peroxidase were assayed, respectively, according to Fielding and Hall [12] and Sato et al. [13].
For determination of PAL activity, fresh material was homogenized in 100 mM borate buffer (pH 8.8) containing 0.5 mM EDTA and 17 mM β-mercaptoethanol. The homogenate was centrifuged at 20 000 g for 20 min and the supernatant was immediately assayed for PAL activity. The reaction mixture (3 ml), containing 100 mM borate buffer (pH 8.8), 20 mM l-phenylalanine and 200 μl of extract, was incubated at 40° for 1 h. Production of cinnamic acid was measured as an increase in absorbance at 290 nm.
2.4 Electrophoretic analysis
Anionic isoperoxidases were separated on 10% polyacrylamide gel electrophoresis. The syringaldazine isoperoxidases were stained by incubation of the gel with a solution of syringaldazine as described by Tadeo and Primo-Millo [14].
2.5 Protein determination
Protein content was determined according to Bradford [15] using bovine serum albumin as standard.
2.6 Statistical analysis
The results presented are the mean values±standard errors obtained from at least five replicates. Significant differences between treated and control plants are determined using ANOVA test (P<0.05).
3 Results
3.1 Seedling growth, water content and protein level
Under 50 μM CuSO4, sunflower roots showed a reduced length (Fig. 1A) and a delay of ramification. Also, matter production and water content were reduced by 41% and 53%, respectively, in treated roots compared to the control (Fig. 1B and 1C). The amount of total protein has decreased by 53% in Cu treatment conditions (Fig. 2A).
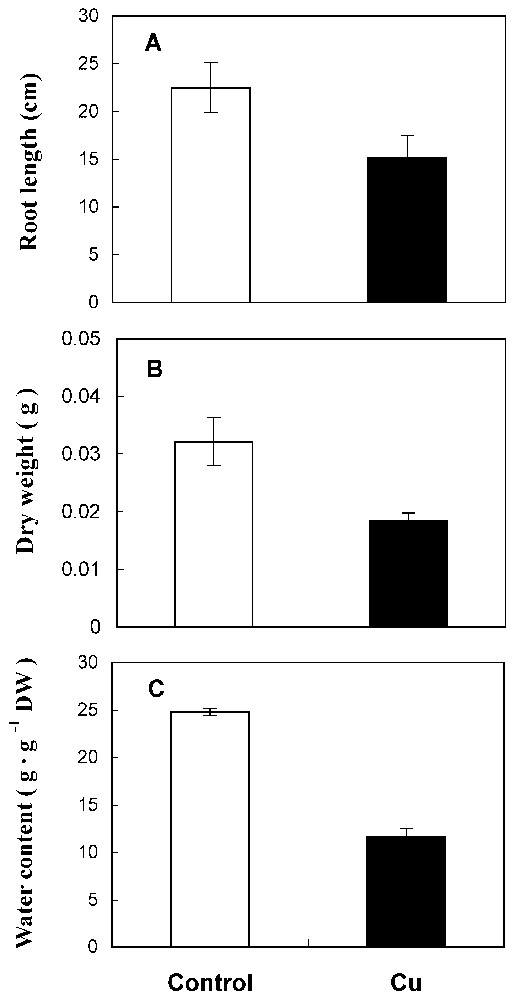
Root length (A), dry weight (B) and water content (C) from 10-day-old sunflower seedlings grown in control nutrient medium (□) or supplemented with 50 μM CuSO4 (■) for five days. The values given are the means of ten experiments. Standard errors are indicated by vertical bars.
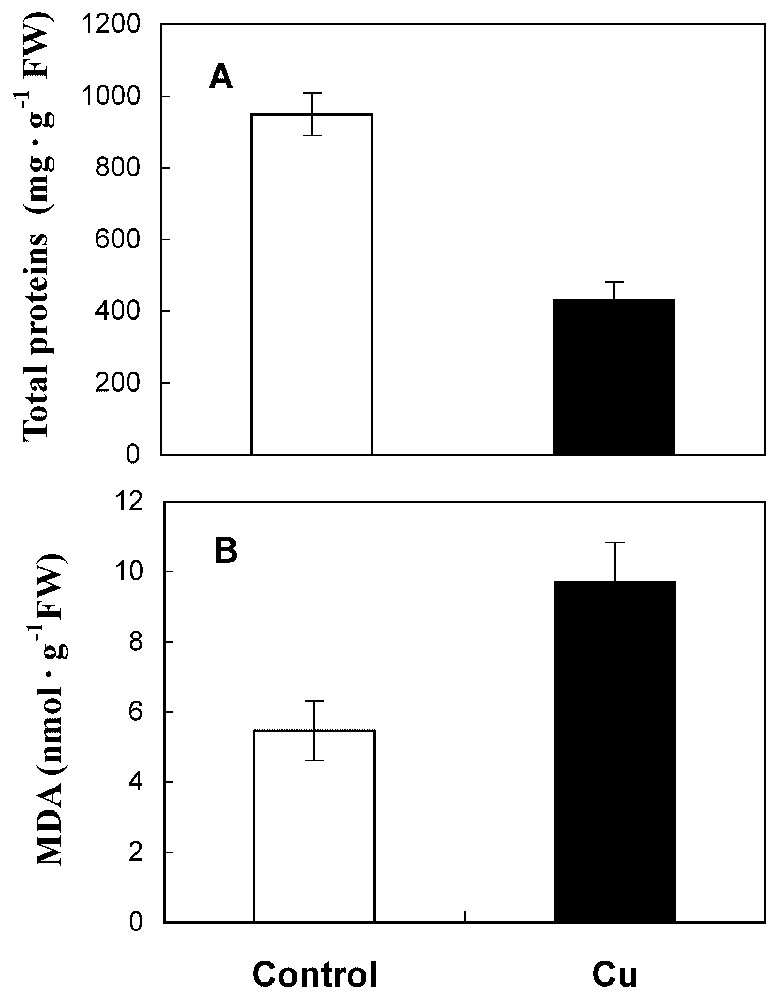
Total protein content (A) and MDA level (B) in roots from 10-day-old sunflower seedlings grown in control nutrient medium (□) or supplemented with 50 μM CuSO4 (■) for five days. The values given are the means of five experiments. Standard errors are indicated by vertical bars.
3.2 Lipid peroxidation
An increase in the level of lipid peroxidation products, measured as thiobarbituric acid reactive metabolites, was observed in sunflower roots after copper treatment. The MDA content was increased by 22% in treated roots compared to the control (Fig. 2B).
3.3 Enzyme activities
The catalase and guaiacol peroxidase activities were significantly enhanced by copper treatment (Fig. 3A and 3B). By contrast, data showed a notable reduction of superoxide dismutase activity in treated roots compared with control (Fig. 3C).
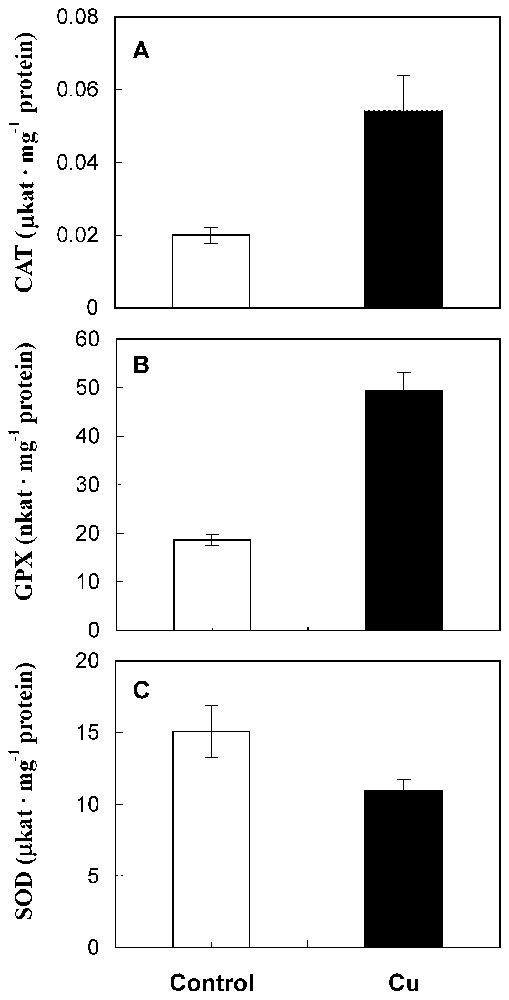
CAT (A), GPX (B) and SOD (C) activities in roots from 10-day-old sunflower seedlings grown in control nutrient medium (□) or supplemented with 50 μM CuSO4 (■) for five days. The values given are the means of five experiments. Standard errors are indicated by vertical bars.
Fig. 4A showed that coniferyl alcohol peroxidase activity was strongly enhanced (245% increase over the control). In the same way, PAL activity has increased in treated roots with respect to control (Fig. 4B).
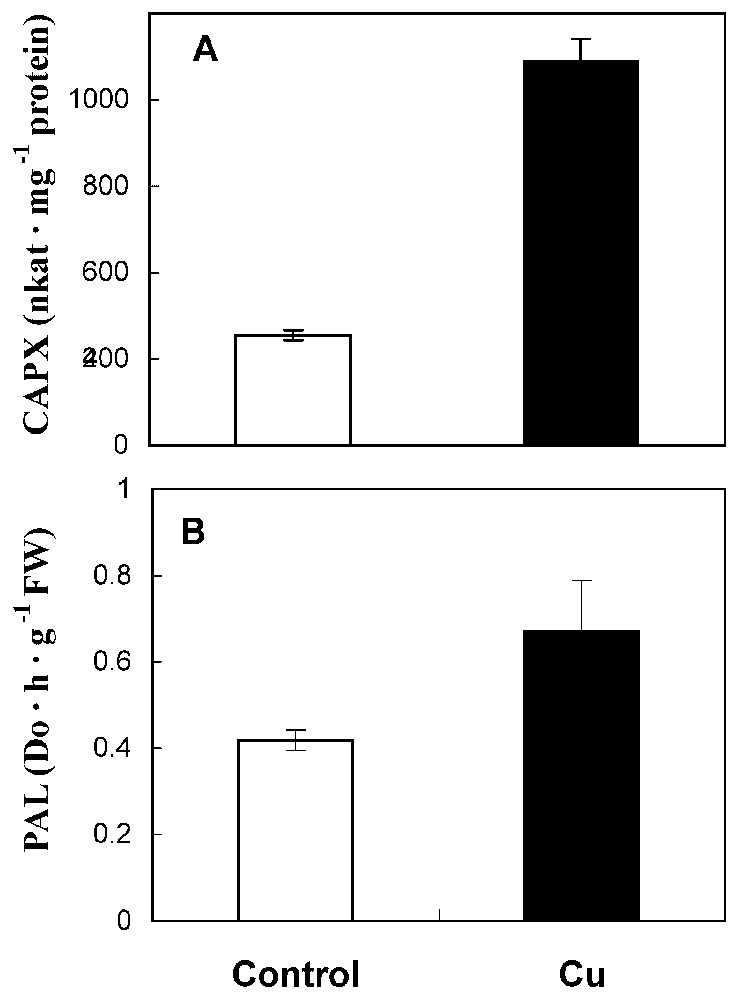
CAPX (A) and PAL (B) activities in roots from 10-day-old sunflower seedlings grown in control nutrient medium (□) or supplemented with 50 μM CuSO4 (■) for five days. The values given are the means of five experiments. Standard errors are indicated by vertical bars.
Native gel electrophoretic analysis of syringaldazine peroxidase activity in control roots showed only one isoform (A2). In treated roots, the isoform A2 was increased and another putative isoform A1 is displayed and seems to be induced, or its amount increased (Fig. 5).
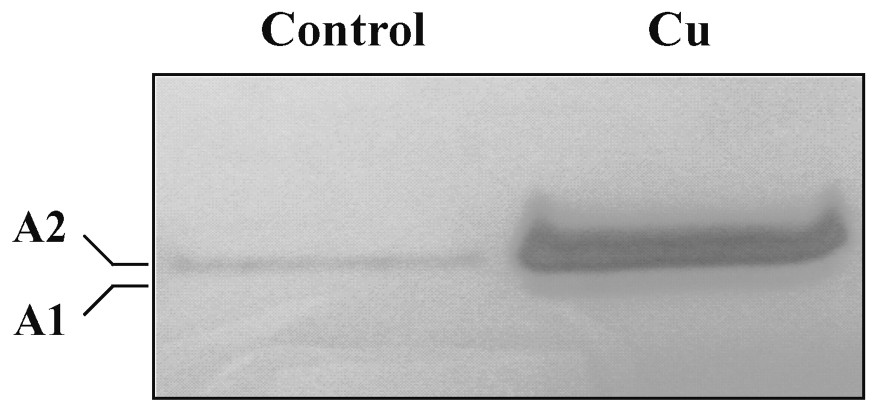
Native PAGE of syringaldazine peroxidase isozymes in root extracts from 10-day-old sunflower seedlings grown in control nutrient medium (control) or supplemented with 50 μM CuSO4 (Cu) for five days. The same protein amount (75 μg) is deposed in each lane.
4 Discussion
In the present work, we have examined the effect of copper excess on growth and several physiological processes in roots of sunflower seedlings.
A significant reduction of dry matter production, root length and water content are observed in roots exposed to copper treatment (41, 33 and 53%, respectively, Fig. 1). In fact, copper has been identified as being a powerful inhibitor at high levels [2,7,16,17]. Our findings indicate again a significant decrease in protein level by cupric stress (53%, Fig. 2A). This reduction of protein amount could be related to the ability of Cu to interfere with thiol groups of a wide range of enzymes [16] and might therefore produce disorder in protein metabolism. Moreover, cupric ion is considered as an efficient generator of toxic oxygen species that caused protein degradation [18].
Our results also show an increase in the MDA level in roots of Cu-treated sunflower seedlings compared with controls (Fig. 2B). Lipid peroxidation was already demonstrated in roots [5] and leaf segments [19] treated by excess of copper. In fact, it is well known that copper initiates the lipoperoxidation process, which generates free radicals and is recognized to affect membrane integrity [1], leading to the alteration of ion transports [20].
These damaging membrane effects could explain, in part, the reduction of water content in treated sunflower roots (Fig. 1C) affecting cellular turgor and thereafter cell enlargement. So, growth delay could be related to the inhibition of cellular turgor, the reduction of total protein amount and to the generated free radicals by lipoperoxidation.
Generally, it is known that growth inhibition, noted in plants under heavy metals uptake, is related to some physiological process alterations owing to generated oxidative stress. Such damages could be mitigated and repaired by antioxidative enzymes like CAT, SOD and peroxidases.
In fact, our results show a stimulation of CAT and guaiacol peroxidase activities in treated roots. De Vos et al. [5] and Wecks and Clijsters [2] also reported an increase in the capacity of CAT by toxic concentrations of Cu in roots. Copper-induced guaiacol peroxidase activity was also shown by Mazhoudi et al. [7] and Chen et al. [21]. In the case of SOD, the inhibition of its activity after copper treatment (Fig. 3C) is confirmed by Palma et al. [22] and Wecks and Clijsters [2]. Thus, it seems that in sunflower treated roots CAT and guaiacol peroxidase take part in defence mechanisms against oxidative stress caused by copper, while SOD does not.
Finally, we have examined the effect of cupric stress on lignifying peroxidases (coniferyl alcohol and syringaldazine peroxidases) and PAL. Lignifying peroxidases were assayed using specific electron donors, coniferyl alcohol and syringaldazine. It appears that copper enhances coniferyl alcohol peroxidase activity (Fig. 3B). Qualitative and quantitative changes in isoenzymatic profile of syringaldazine peroxidase were investigated (Fig. 5). Our findings show a stimulation of isoform A2 and an induction of isoform A1. In the same way, Chen et al. [21] reported that copper excess induced lignifying peroxidase activities in radish roots, which are correlated with growth reduction. Thus, we can also propose that, in sunflower roots, growth reduction could be caused by Cu-enhanced lignifying peroxidase activities.
PAL, a key intermediate of phenylpropanoid pathway, is also activated by cupric stress (Fig. 4B). It was shown that PAL is generally stimulated in plant tissues exposed to several environmental stresses [6]. The same authors indicated that PAL enhancement in these stress conditions is due to H2O2 generation, which occurs as primary reaction in response to stress. So, it seems that, in sunflower roots, the enhancement of PAL activity could be related to the implication of this enzyme in the plant response to cupric stress.
In conclusion, activities of antioxidant enzymes, lignifying peroxidases and PAL increased when sunflower roots were treated with 50 μM CuSO4. This indicates that sunflower roots mobilize several enzymatic defence processes in order to mitigate Cu-stress damages.


