1 Introduction
In recent years, the synthesis of combinatorial libraries has emerged as a valuable tool in the search for novel lead structures [1–5]. The success of combinatorial chemistry in drug discovery is dependent, in part, on further advances in solid-phase organic synthesis (SPOS) [6–9]. This offers the opportunity of synthesizing molecules via novel routes, which may be difficult or impossible using traditional solution-phase methods, and offers also the possibility for rapidly synthesizing drug-like molecules without tedious and time-consuming purification. At present, the increasing interest into combinatorial chemistry drives the development of efficient and reliable chemistry for the solid phase, which in turn requires new strategies of attachment and release of molecules from the solid support. The utilization of functionalized polymers as reagents and catalysts has recently appeared on this scene after an incubation time of more then 25 years. Here, it is not the substrate that remains attached to the solid support during a multistep synthesis, but the polymer-bound reagent or catalyst promotes a chemical transformation of a substrate that is present in solution [10–12].
One advantage of this polymer-assisted solution-phase synthesis (PASP) is the possibility to monitor the reaction using known analytical techniques. This hybrid technique that combines the concept of solid-phase synthesis with the idea of polymer-supported scavenging reagents has seen increased interest in polymer-assisted synthesis. Often, this method, which is only one example among other polymer-assisted combinations, has been termed resin ‘catch & release’. Safety-catch linkers are a particularly sophisticated form of attachment, whereby a linker present throughout the library assembly in a relatively inert form is activated and thereby made labile at the end of the synthesis to release the products from resin.
In contrast to supports designed for synthesis in which interactions between proximal sites can be problematic, these, ideally, should possess a high effective concentration of capturing group to maximize the efficiency of process. Similarly, whereas a range of swelling profiles is desirable for synthetic supports, a broad solvent tolerance is required in the polymer-assisted organic synthesis to enable use without requiring additional solvent manipulations.
As part of a general program looking at alternative base matrixes to polystyrene– and PEG-based resins commonly used in SPOS and PASP applications, we have examined the use of cellulose support to use in resin-aided capture-release strategy. Recently, most problems related to the use of the old fibrous and powdered cellulose have been solved by introducing a novel form, which is both porous and spherical [13]. Beaded cellulose can be widely used as functional polymer and starting material for the preparation of functional polymers. These cellulose supports, notably beads, can offer considerably higher loading level than that obtained with planar support [14]. Furthermore, beaded cellulose supports show different solvent swelling profiles relative to those exhibited by the standard organic polymers and are biodegradable too [15]. Cellulose shows good swelling properties in polar and aqueous solvents. Since ‘one-bead’screening for biological activity is typically carried out in aqueous solvents, the use of polar solvents in solid-phase synthesis should be a requirement for many of today’s supports. Surprisingly, to the best of our knowledge, only a single example has been published. In our report, Steel et al. describe the use of a cellulose-based resin as scavenger support to remove the excess bromine or acylating agent [16].
2 Results and discussion
Although a wide variety of functional groups can be rapidly introduced into cellulose, in this communication, we wish to report the novel use of a commercial aniline-functionalized cellulose in solid phase synthesis. The cellulose employed was a modified bead-form cellulose containing aminoaryl-ethylsulfone groups in flexible chains (Fig. 1). Cellulose beads were obtained from Iontosorb, Czech Republic. The content of amino aryl groups in Iontosorb AV can be regulated according to customer’s demands in the range 0.1 to 2.8 mmol g–1.

Aniline-functionalized cellulose.
As suitable transformation to illustrate the application of aniline cellulose as a solid support, we have chosen the preparation of a library of pyrazoles and isoxazoles via polymer-bound enaminone (Fig. 2). Differently substituted β-enamino ketones can be prepared by condensation reaction between the methylene group of β-keto-ester with N,N-dimethylformamide dimethyl acetal (DMF-DMA) and derivatives (Fig. 2) [17–18].
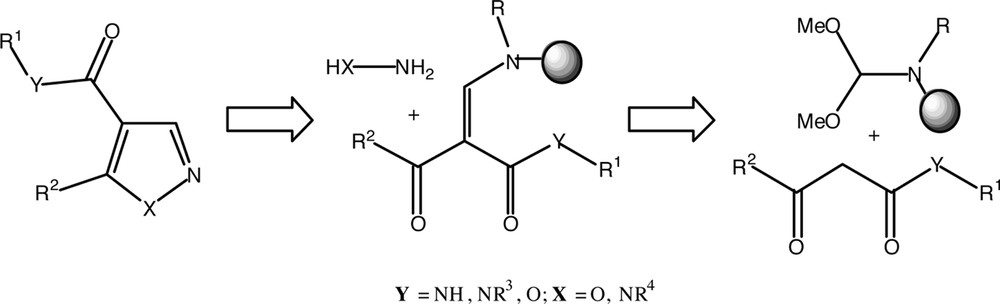
Retrosynthetic analysis.
In order to adapt this methodology to solid phase, it was necessary to prepare the β-enamino ketone on polymeric support. We were attracted to such a strategy because these enaminones are important building blocks for the generation of several heterocyclic privileged scaffolds, such as pyrimidines, pyridines, benzopyrones, pyrazolones, etc. [19–24]. Different strategic approaches were conceivable and a very attractive solution was the preparation on solid support of the formamide acetal 2 (Fig. 3). Therefore, we decided to explore this last strategy using a modified Bredereck’s reagent 1 [25] to convert the amino group of cellulose into N-formamide dialkylyacetal 2 (Fig. 3). Supported aniline reacted cleanly with a large excess of formamide acetal 1 in DMF under acid-catalyzed conditions (CSA) at ambient temperature for 5 h to afford the desired functionalized support 2 in quantitative yield. Yields were determined by a colorimetric assay. The diaziazonium salt of aniline give a coloured red compound when it is treated with β-naphtol under basic condition.

Synthesis of cellulose N-formamide dialkylacetal functionalized.
Treatment of functionalised cellulose 2 with an excess of β-dicarbonyl compounds in DMF at 80 °C for 48 h gave the corresponding solid-supported β-enaminodiones, which are purified from starting materials by simply filtering. Encouraged by these results, we further envisioned the possibility to carry out a one-pot reaction using the same reaction condition. This was accomplished by heating a mixture of aniline cellulose, N-formylimidazole dimethylacetal 1 and a β-keto ester 3 in DMF, at 80 °C for 48 h in presence of 10% camphorsulphonic acid (CSA) as catalyst (Fig. 4).
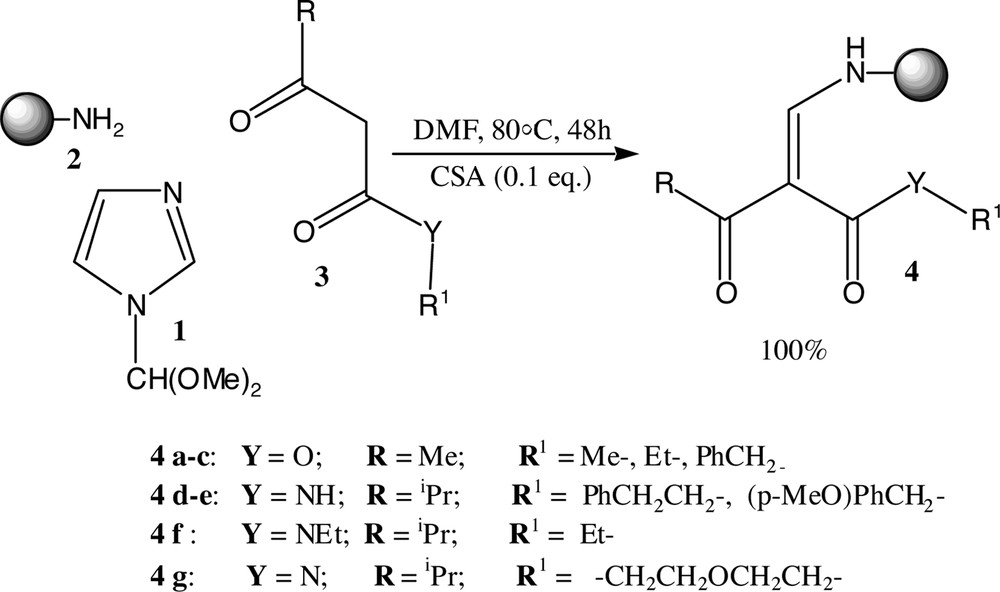
Preparation of cellulose-bound enaminone.
Interestingly, this approach allowed us to prepare the desired β-enaminodione 4 in about 48 h, in quantitative yield and in only one step. Surprisingly, for analogous reactions on solution-phase, both primary amine and aniline was found unreactive [25].
The synthetic strategy has been initially optimised on commercial β-keto esters and subsequently extended to β-keto esters and β-keto amides, prepared on solution phase according to classical malonic ester method [26].
Treatment of the cellulose-bound enaminones 4a–g, obtained by the described procedures with phenylhydrazine hydrochloride or hydroxylamine hydrochloride in the presence of NEt3 as base afforded the corresponding isomerically pure pyrazole or isoxazole on solution restoring the starting resin (Fig. 5). Four to six equivalents of hydroxylamine or hydrazines as hydrochloride salts were usually employed for the cyclisation. No attempt has been made to determine the limiting amount of nucleophiles.
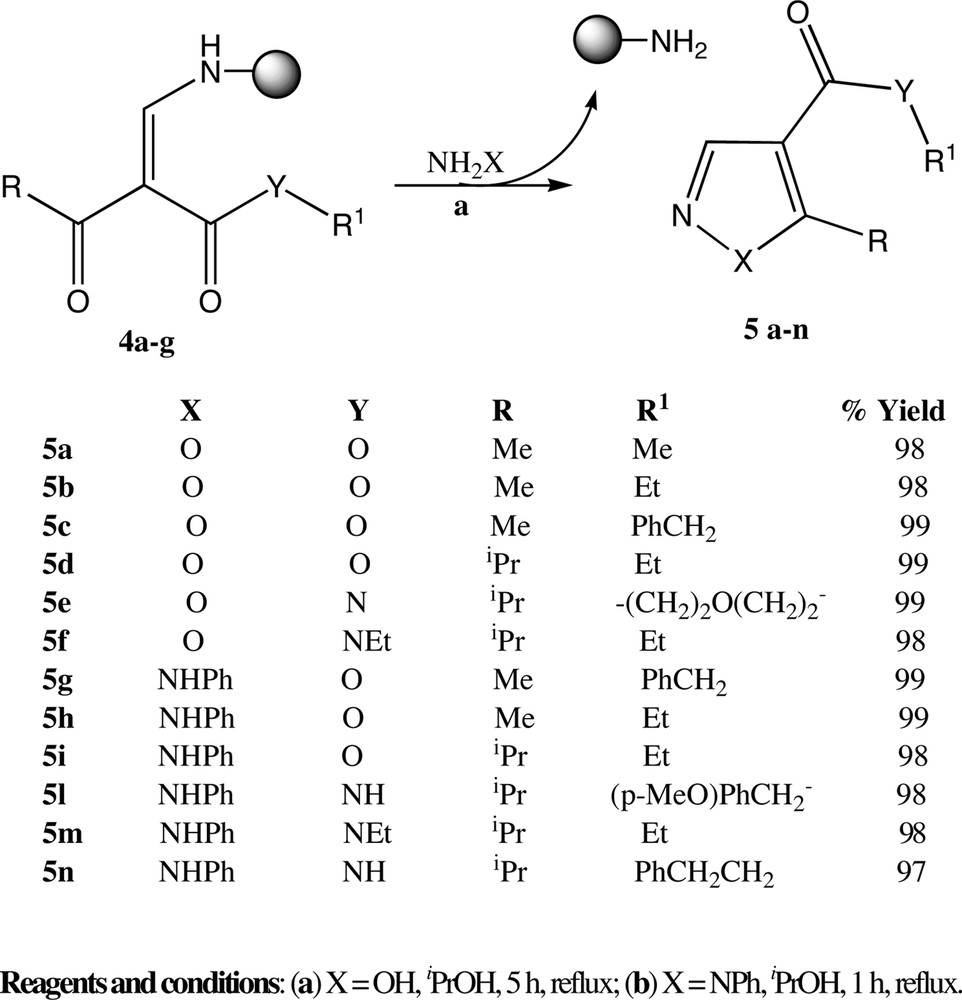
Regiospecific synthesis of 1,4,5-trisubstituted pyrazoles and 4,5-disubstituted isoxazoles using cellulose-bound enaminones 4.
The heterocycle cyclisation was carried out in refluxing iPrOH in about 1h for pyrazoles and in about 5 h for isoxazoles. In all cases no purification of final product was required after treatment of filtered solution with acid ion exchange cellulose resin or a simple aqueous acid work-up to remove hydrazines or hydroxylamine excess. Pyrazoles and isoxazoles were achieved in high yield and excellent purity (> 95%) independently of the structure of β-keto ester or β-ketoamide employed.
The cellulose used can be regenerated in turnover cycle. The procedure described was in fact repeated using recycled cellulose and always we obtained high yields of the desired products with high level of purity.
Various methods have been developed for analyses of support-bound intermediates as alternative to the cleavage of intermediates from the support and their characterization in solution. One of the most common analytical tools to check the reaction progress is the colorimetric assay for specific functional groups. To check the detection of free aromatic amine function a β-naphtol test has been used. The aniline cellulose support was first treated with a acid aqueous solution of NaNO2 than with a basic aqueous solution of β-naphtol. The cellulose bead immediately changed colour from white to red. This rather sensitive assay enables the detection of even small amounts of primary aromatic amine bounded on the support, and thereby the monitoring of the enamine synthesis.
However, in all cases, a cellulose sample was analysed by IR (KBr pellet) and showed strong absorption bands at 1690, 1640 and 1575 cm–1 that were absent in the parent aniline-cellulose. This IR spectrum indicated the presence of a carbonyl-containing compound bound to solid support 4. Also the formation of the heterocycles was easily monitored both by a colorimetric assay on cellulose bead and checking the solution by TLC. Iron (III) was able to do a intensely coloured complex (rust-brown) with enolizable ketones. Therefore, this old colorimetric assay is suitable and sensitive to detect the presence (absence) of β-dicarbonyl compounds on solid support. Cellulose-bounded aniline and N-methyl-aminomethylated polystyrene gave comparable results, whereas using the piperazinomethyl polystyrene, no reactivity has been observed. (Ring closure to form a heterocycle was tested successful with hydrazine and hydroxylamine). (Note: positive to β-naphtol test and negative to FeCl3 test).
Post-cyclisation IR analysis of the cellulose sample showed that the carbonyl bands have disappeared.
3 Conclusion
In summary, we have described the synthesis of libraries of substituted pyrazoles and isoxazoles starting from different β-keto-esters or β-keto-amides. Through the use of a modified Bredereck’s reagent in an ‘one-pot’ reaction, we have prepared a enaminone cellulose bound support, intermediate for the solid phase synthesis of heterocycles using ‘catch & release’ approach. In addition, this method uses a new low-cost and versatile biopolymer, under very mild condition.
Acknowledgements
The work was financially supported by the University of Sassari (Fondi MIUR ex 40% 2001).



